Abstract
Central serotonergic signaling influences many physiological processes but a requirement for reproductive success has not been demonstrated. Using dams with a specific disruption in serotonin neuron development we show that serotonergic function is required for nurturing and survival of offspring. Full rescue of survival depends on the mother's expression level of the upstream serotonergic transcriptional cascade. Thus, intrinsic transcriptional programming of maternal serotonergic activity determines the quality of nurturing and organism survival.
Serotonergic signaling in the CNS is critical for proper maturation and homeostatic modulation of neural circuits that shape emotions and many physiological responses 1, 2. Perturbations in the level of serotonergic gene expression have significant impact on behavior and have been implicated in the pathogenesis of several neuropsychiatric diseases including disorders of anxiety, mood and appetite 1, 2. Although serotonin (5HT) has been implicated in the regulation of female sexual behavior 3, present evidence does not demonstrate an essential role for the central serotonergic transmitter system in reproductive success.
Expression of the Pet-1 ETS transcription factor in the brain is restricted to 5HT neurons 4. Normal numbers of serotonergic precursors are generated in Pet-1−/− midbrain but expression of serotonergic gene expression and 5HT synthesis are greatly reduced 5. We used Pet-1 deficient dams to investigate the impact of their arrested 5HT neuron development on offspring viability. Virgin wild type and Pet-1−/− females were bred with wild type or Pet-1+/− males. Birth rates and offspring body weights were normal for primiparous Pet-1−/− dams (Supplementary Table 1, online; Supplementary Fig. 1, online). Yet, while 99% of pups survived when born and nurtured by wild type dams, no pups survived when born to Pet-1−/− dams and the majority of these offspring were found dead without placentas and not cannibalized 3-4 days after birth (Fig. 1a). Cross fostering at postnatal day 1 showed that pups born to Pet-1−/− dams survived when nurtured by wild type dams (Fig. 1a, Supplementary Table 1 online).
Figure 1. Pet-1 is required for reproductive success.

(a) Survival of litters born to dams with genotypes and conditions: wild type, n=13; Pet-1−/− n=9; litters born to Pet-1−/− dams cross fostered to wild type dams (+/+, CF), n=4; Pet-1−/− dams with pre-huddled pups, n=4. (b) Representative views of nests and huddles for wild type and Pet-1−/− dams. Scale bar=4cm. Inset, pup on postnatal day 1 with milk band, scale bar=1cm. (c) Nest quality of wild type versus Pet-1−/− dams. (d) Litters of Pet-1−/− dams were never seen grouped in huddles. (c-d) dams observed: wild type, n=13; Pet-1−/−, n=6. (e) Pet-1−/− dams retrieved fewer pups than wild type dams when new bedding was added (n=6 for each genotype, P<0.0001) and when no new bedding was added (n=4 for each genotype, P<0.005). Wild type dams retrieved all six pups in all tests. Error bars represent s.e.m.
The profound deficit in survival of offspring born to Pet-1−/− dams suggested deficient maternal behavior. Successful nurturing requires the coordinate expression of several discrete behaviors including nest building, pup retrieval, cleaning, and nursing 6. Pups born to Pet-1−/− dams were consistently observed to have milk in their stomachs each postnatal day before death suggesting normal lactation (Fig. 1b, inset). However, the percent time crouching was significantly less for Pet-1−/− dams compared to wild type dams (Pet-1−/− dams': 53%, stdev 8.7%, n=6; wild type dams': 73%, stdev 10.1%, n=13; p<0.05). Moreover, Pet-1−/− dams often would not build suitable nests (Fig. 1b, c). Organizing pups in a huddle is essential for neonate survival as it facilitates feeding and maintains pup body temperature. Pups born to wild type dams were always found organized in huddles (Fig 1b, d) as were cross-fostered pups. In striking contrast, offspring of Pet-1−/− dams were never organized in huddles (Fig. 1b, d). To carefully evaluate maternal behavior we continuously video monitored dams in the home cage (Supplementary Methods online). Both wild type and Pet-1−/− dams gave birth within the nesting area. Immediately postpartum wild type dams were crouched over their pups and actively maintained them in an organized huddle within a well-constructed nest. Pet-1−/− dams were also present in the vicinity of nesting material during much of the night and did not appear hyperactive. However, the offspring of Pet-1−/− dams were not huddled underneath their mother and were often seen buried beneath disheveled bedding material or completely exposed at a distance from the material. Pup death occurred near the birth location or at some distance from this site as a result of the pups spontaneous tossing and turning. When we pre-huddled the pups in the evening we found that Pet-1−/− dams failed to maintain the huddle through the night and pups were scattered in as little as thirty minutes after pre-huddling (Supplemental Fig. 2 online). Additionally, providing Pet-1−/− dams with a pre-huddle did not increase offspring survival (Fig. 1a, Supplementary Table 1 online).
We then asked whether a deficit in retrieval might account for the dispersion of pups. Pet-1−/− dams failed to retrieve the majority of their pups in assays in which new bedding material was supplied (Fig. 1e, Supplementary methods online). Instead, Pet-1−/− dams alternated between frequent digging behavior and active traversal of the cage (Supplementary Video online) and therefore appeared inattentive to their young. To determine whether the addition of new bedding material contributed to the retrieval deficits we performed retrieval assays without disturbing the bedding material for at least 5 days prior to the assay. While retrieval improved slightly, the number of pups retrieved was still lower than the number retrieved by wild type dams (Fig. 1e). Additionally, the average time to retrieve pups was three times longer for Pet-1−/− dams (wild type dams: 22.1s, stdev 12.9s; Pet-1−/− dams: 72.17s, stdev 2.6s; n=4 for each genotype, p<0.005). Elevated-plus maze activity was not different from wild type indicating normal anxiety-like and locomotor activity in Pet-1−/− dams (Supplementary Fig. 3 online). Additionally, Pet-1−/− dams exhibited normal latencies to detect various odors, indicating normal olfaction (Supplementary Fig. 4 online).
We next investigated whether Pet-1 loss of function could be corrected with a bacterial artificial chromosome (BAC) encoding Fev, the human orthologue of Pet-1 (Supplementary Fig. 5 online). Fev expression in several Fev BAC founder lines, Tg(Fev)/Pet-1−/−, was restricted to raphe nuclei (Supplementary Fig. 5a online), consistent with exclusive serotonergic expression of Pet-1 in the brain 4. The number of 5HT+ cells and fibers in the various rescue lines were not different from wild type (Supplementary Fig. 5b-f online). All of the classic membrane properties of 5HT neurons (Supplementary Table 2 online) as well as anxiety and home cage locomotor behavior (Supplementary Fig. 6 online) were the same as wild type, indicating no demonstrable disruption of these characteristics by BAC insertion.
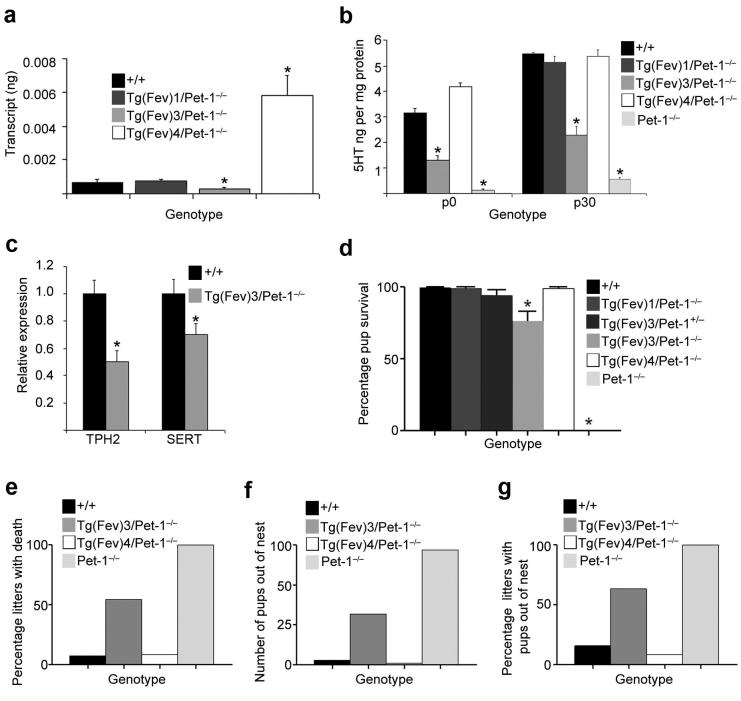
We then quantitated absolute levels of Fev transcripts in the dorsal raphe of the Tg(Fev)/Pet-1−/− lines. This analysis revealed significantly different transcript levels above and below the level of wild type Pet-1 transcripts in the postnatal period (Fig. 2a, Supplementary Table 3 online). Therefore, we sought to determine whether reduced Fev expression in Tg(Fev)3/Pet-1−/− or increased expression in Tg(Fev)4/Pet-1−/− would program early postnatal changes in the levels of brain serotonergic characteristics. 5HT was at wild type levels in the Tg(Fev)1/Pet-1−/− and Tg(Fev)4/Pet-1−/− lines, but the level of 5HT in Tg(Fev)3/Pet-1−/− line was intermediate to the levels in wild type and Pet-1−/− brain (Fig. 2b). Overexpression of mouse Pet-1 in wild type brain did not result in changes in brain 5HT (Supplementary Fig 7 online). Consistent with reduced 5HT levels in Tg(Fev)3/Pet-1−/− mice, the level of tryptophan hydroxylase 2 (TPH2) RNA as well as that of the 5HT transporter (SERT) in the midbrain raphe of Tg(Fev)3/Pet-1−/− were reduced relative to their levels in wild type mice (Fig. 2c). In contrast, their levels were not different from wild type in either the Tg(Fev)1/Pet-1−/− and Tg(Fev)4/Pet-1−/− lines (Supplementary Fig. 8 online). Dopamine levels did not differ (Supplementary Fig. 9 online). Together, these findings show that Fev can rescue serotonergic defects in Pet-1−/− mice but full rescue of 5HT levels and serotonergic gene expression depends on the level of expression of the upstream transcriptional cascade directed by Fev. However, increased Fev or Pet-1 expression above levels of endogenous Pet-1 had no impact on 5HT indicating a possible homeostatic transcriptional constraint on serotonergic character.
Figure 2. Full rescue of 5HT neuron phenotype, nurturing, and offspring survival depends on maternal Fev expression level.
(a) Absolute quantification of Fev and Pet-1 transcript levels by qPCR. Asterisks indicate significantly (P<0.05) different Fev transcript levels in Tg(Fev)/Pet-1−/− rescue lines compared to wild type Pet-1 transcript levels. Wild type, n=8; Tg(Fev)3/ Pet-1−/−, n=8; Tg(Fev)1/ Pet-1−/−, n=7; Tg(Fev)4/ Pet-1−/−, n=5. (b) Level of brain 5HT at postnatal day 0 and 30 in Tg(Fev)3/Pet-1−/− mice was intermediate between the level in wild type, Tg(Fev)1/Pet-1−/− and Tg(Fev)4/Pet-1−/−, and Pet-1−/−mice. *P<0.05, n=8 for each genotype. (c) TPH2 and SERT RNA were reduced in Tg(Fev)3/Pet-1−/− brain relative to wild type level. Wild type n=11; Tg(Fev)3/Pet-1−/− n=14. P<0.05 (d) Percentage of surviving pups born to wild type (n=13), Tg(Fev)1/Pet-1−/− (n=10), Tg(Fev)3/Pet-1+/− (n=9), Tg(Fev)3/Pet-1−/− (n=11), Tg(Fev)4/Pet-1−/− (n=12), and Pet-1−/− (n=13) dams. ANOVA with Dunnett’s post test, P<0.05. (e) Percentage of litters with offspring death. (f) Number of alive and dead pups found outside of the nest. (g) Percentage of litters with alive or dead pups out of the nest. (e-g): Number of litters observed: wild type, n=13; Tg(Fev)3/Pet-1−/−, n=11; Tg(Fev)4/Pet-1−/−, n=12; Pet-1−/−, n=6. Error bars represent s.e.m.
We then investigated survival of offspring born to Tg(Fev)3/Pet-1−/− dams. Strikingly, these offspring showed reduced survival compared to the nearly 100% survival of pups born to wild type, Tg(Fev)1/Pet-1−/− and Tg(Fev)4/Pet-1−/− (Fig 2d, Supplementary Table 1 online). Yet overall survival of Tg(Fev)3/Pet-1−/− offspring was better than survival seen for offspring born to Pet-1−/− dams. Decreased survival was observed in about 50% of litters born to Tg(Fev)3/Pet-1−/− dams (Fig. 2e). Pup survival was normal in litters born to Tg(Fev)3/Pet-1+/− dams in which one intact Pet-1 allele was present, showing that poor survival could be corrected in the Tg(Fev)3/Pet-1−/− line by increasing serotonergic characteristics (Fig. 2d and Supplementary Table 1 online). The partial restoration of Tg(Fev)3/Pet-1−/− offspring survival suggested a partial rescue of maternal care. Indeed, the number of offspring born to Tg(Fev)3/Pet-1−/− dams found outside of the nest was greatly increased over the numbers seen for pups born to wild type dams, but was less than the number of offspring seen out of the nest that were born to Pet-1−/− dams (Fig. 2f, Supplementary Fig. 10 online). Pups were found outside of nests in nearly two-thirds of litters born to Tg(Fev)3/Pet-1−/− dams (Fig. 2g). The number of pups born to Tg(Fev)4/Pet-1−/− mothers that were found outside of the nest was not different from the number of pups out of the nest that were born to and nurtured by wild type dams. A Kendall Tau-b statistical analysis (tau-b = −0.66, p<0.0001; Supplementary Methods and Table 4 online), suggested an intermediate level of maternal behavior in the Tg(Fev)3/Pet-1−/− line.
Despite the prevalence of postpartum depression and the frequent use of selective 5HT reuptake inhibitors to treat this disorder 7, a requirement for maternal serotonergic function in the mother's behavior toward her offspring or offspring survival has not been convincingly demonstrated with either pharmacological depletion of 5HT or targeting of 5HT receptors 8, 9. The findings reported here directly demonstrate a critical role for the serotonergic system in reproductive fitness by showing that a specific disruption of maternal 5HT neuron differentiation causes pup mortality, the likely consequence of pup exposure caused by a failure of the dam to maintain pups in an organized huddle within a properly constructed nest. Although Pet-1 deficient adults not in a reproductive period exhibited increased anxiety-like behavior 5, Pet-1 deficient dams did not. This difference in emotional state is consistent with the known reduction in anxiety and fear that accompanies lactation in mice and rats 6 and suggests that the deficit in Pet-1−/− maternal behavior is not the result of a generalized anxiety phenotype.
The neural circuitry that gives rise to the various behavioral components of nurturing comprise numerous nuclei and their interconnections situated in many different brain regions such as the medial preoptic area (MPA) and the bed nucleus of the stria terminalis (BST) 6. Dopaminergic modulation arising within the ventral tegmental area has been implicated in maternal retrieval of pups 9 and studies of dopamine beta hydroxylase deficient mice have demonstrated a critical role for noradrenergic modulation of pup retrieval, nursing and offspring survival 10. Several transcription factor genes have been implicated in maternal behavior but unlike Pet-1, in most instances the specific CNS neuronal cell types in which these factors are needed intrinsically to enable nurturing have not yet been determined 6.
Maternal neglect exhibited by Pet-1 deficient dams may be caused by an acute insufficiency of 5HT or other serotonergic neuron derived signals in, for example the BST and MPA, which are normally innervated by serotonergic afferents arising in the dorsal raphe, median raphe and B9 nucleus 11. Alternatively, disruption of serotonergic signaling earlier in the life of Pet-1 deficient females might account for the neglect of offspring. Proper levels of the SERT 12, 13, TPH 13, 14, and 5HT 1a receptors 15 during the early postnatal period are likely to be required for normal maturation of circuitry governing emotional behaviors. This raises the intriguing possibility that abnormal nurturing exhibited by Pet-1 deficient dams is the result of altered formation of circuitry governing maternal motivation and reward.
Our findings illustrate a potential mechanism whereby genetically or environmentally directed decreases, but not increases, in the expression level of intrinsic transcriptional control genes, developmentally program graded changes in the levels of serotonergic neuron characteristics. These changes in turn may alter the quality of adult 5HT modulated behaviors such as those needed for offspring nurturing and survival.
Supplementary Material
Acknowledgements
We thank Stefan Herlitze for presubmission review of the manuscript. We thank RoxAnne Murphy for help with in situ hybridization, Kathy Lobur for assistance with behavioral testing, Christi Wylie for help gathering confocal images, Gemma Casadesus and Michele Ionno of the Case Western Reserve University Rodent Behavior Core and the Case Transgenic and Targeting Core for BAC transgenic production. This work was supported by grants from the National Institutes of Mental Health (NRSA Pre-doctoral fellowship MH073296 to JKL; RO1 MH062723 and P50 MH078028 to ESD and MH 0754047 to SGB).
Footnotes
Author Contributions: This study was designed, interpreted, and written by JKL and ESD. JKL performed all of the experiments except for the electrophysiology. DF advised and performed statistical analyses on maternal care data. LKC and SGB performed and analyzed the electrophysiological data.
References
- 1.Leonardo ED, Hen R. Annu Rev Psychol. 2006:117–137. doi: 10.1146/annurev.psych.57.102904.190118. [DOI] [PubMed] [Google Scholar]
- 2.Sodhi MS, Sanders-Bush E. Int Rev Neurobiol. 2004;59:111–174. doi: 10.1016/S0074-7742(04)59006-2. [DOI] [PubMed] [Google Scholar]
- 3.Uphouse L. Brain Res Brain Res Rev. 2000;33:242–257. doi: 10.1016/s0165-0173(00)00032-1. [DOI] [PubMed] [Google Scholar]
- 4.Hendricks T, Francis N, Fyodorov D, Deneris E. J. Neurosci. 1999;19:10348–10356. doi: 10.1523/JNEUROSCI.19-23-10348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendricks TJ, et al. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- 6.Gammie SC. Behav Cogn Neurosci Rev. 2005;4:119–135. doi: 10.1177/1534582305281086. [DOI] [PubMed] [Google Scholar]
- 7.Lattimore KA, et al. J Perinatol. 2005;25:595–604. doi: 10.1038/sj.jp.7211352. [DOI] [PubMed] [Google Scholar]
- 8.Brunner D, Buhot MC, Hen R, Hofer M. Behav Neurosci. 1999;113:587–601. doi: 10.1037//0735-7044.113.3.587. [DOI] [PubMed] [Google Scholar]
- 9.Numan M. In: The Neurobiology of Parental Behavior. Insel TR, editor. Springer; New York: 2003. [Google Scholar]
- 10.Thomas SA, Palmiter RD. Cell. 1997;91:583–592. doi: 10.1016/s0092-8674(00)80446-8. [DOI] [PubMed] [Google Scholar]
- 11.Simerly RB, Swanson LW, Gorski RA. J Comp Neurol. 1984;225:151–166. doi: 10.1002/cne.902250202. [DOI] [PubMed] [Google Scholar]
- 12.Ansorge MS, Morelli E, Gingrich JA. J Neurosci. 2008;28:199–207. doi: 10.1523/JNEUROSCI.3973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maciag D, et al. Neuropsychopharmacology. 2006;31:47–57. doi: 10.1038/sj.npp.1300823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura K, et al. Journal of molecular biology. 2006;363:345–354. doi: 10.1016/j.jmb.2006.08.051. [DOI] [PubMed] [Google Scholar]
- 15.Gross C, et al. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.