Abstract
Inherited arrhythmias and conduction system diseases are known causes of sudden cardiac death and are responsible for significant mortality and morbidity in patients with congenital heart disease and electrical disorders. Knowledge derived from human genetics and studies in animal models have led to the discovery of multiple molecular defects responsible for arrhythmogenesis. This review summarizes the molecular basis of inherited arrhythmias in structurally normal and altered hearts.
On the cellular and molecular levels, minor disturbances can provoke severe arrhythmias. Ion channels are responsible for the initiation and propagation of the action potential within the cardiomyocyte. Structural heart diseases, such as hypertrophic or dilated cardiomyopathies, increase the likelihood of cardiac electrical abnormalities. Ion channels can also be up- or down-regulated in congenital heart disease, altering action potential cellular properties and therefore triggering arrhythmias. Conduction velocities may be inhomogeneously altered if connexin function, density or distribution changes.
Another important group of electrophysiologic diseases is the heterogeneous category of inherited arrhythmias in the structurally normal heart, with a propensity to sudden cardiac death. There have been many recent relevant discoveries that help explain the molecular and functional mechanisms of long QT syndrome, Brugada syndrome, catecholaminergic polymorphic ventricular tachycardia, and other electrical myopathies. Identification of molecular pathways allows the identification of new therapeutic targets, for both disease palliation and cure. As more disease-causing mutations are identified and genotypic-phenotypic correlation is defined, families can be screened prior to symptom-onset and patients may potentially be treated in a genotype-specific manner, opening the doors of cardiac electrophysiology to the emerging field of pharmacogenomics.
INTRODUCTION
Inherited arrhythmias can be life threatening, and are major cause of mortality and morbidity in developed nations. Identification of molecular pathways that increase susceptibility to arrhythmia is necessary to prevent disease occurrence, to improve current therapies and to target new drug development. In recent years, the discovery of pathogenic mutations in inherited arrhythmia syndromes has provided novel insights for the understanding and treatment of diseases predisposing to sudden cardiac death. In patients with the long QT syndromes (LQTS), genotype-phenotype relation studies [1] and genetic testing have influenced patient risk stratification [2] and refined treatment strategies [3].
Arrhythmia mechanisms include abnormal automaticity, triggered activity, and re-entrant excitation. Each of these mechanisms can occur in any type of myocardial disease or in inherited cardiac arrhythmias. The current article focuses on molecular mechanisms of arrhythmias in the structurally abnormal and normal heart. Hypertrophic and dilated cardiomyopathies, as well as arrhythmogenic right ventricular dysplasia/cardiomyopathy are common substrates of inherited arrhythmias in the structurally abnormal heart. Genetic diseases causing arrhythmias in the structural normal heart, also called electrical myopathies, include the long QT syndromes, Brugada syndrome, catecholaminergic polymorphic ventricular tachycardia (CPVT), and non-defined familiar idiopathic ventricular fibrillation. Most, but not all of these disorders are caused by mutations in genes encoding cardiac ion-channel proteins. Among family members carrying an identical mutation in a single gene, remarkable phenotypic variability and expressivity may be observed, suggesting both environmental [4] and genetic modifiers [5].
BACKGROUND
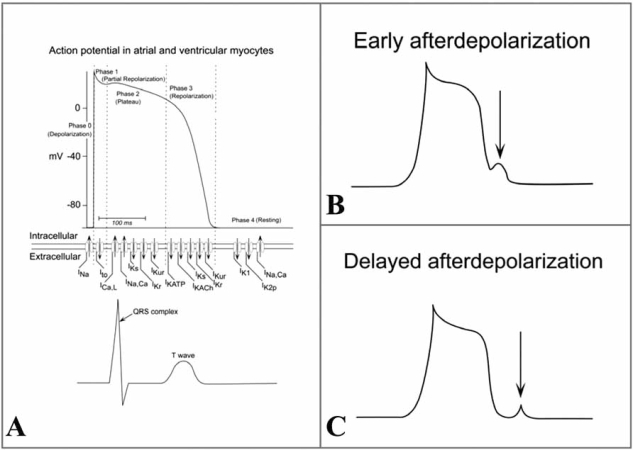
Fig. (1A) summarizes the action potential in a non-pacemaker cardiomyocyte and the contribution of distinct ion channels to the action potential shape. Voltage-gated Na+ channels are essential for the amplitude and upstroke velocity of the cardiac action potential, which are important determinants for impulse propagation and conduction velocity throughout the fast conducting conduction system and the working myocardium [6]. Potassium (K+) channels control resting potentials, action potential waveforms, automaticity, and refractory periods [7]. Calcium (Ca2+) channels contribute to the plateau phase of the action potential. In the past decade, numerous genes encoding various ion channels have been cloned and their structural biophysical properties, subunit stoichiometry, channel assembly and modulation by intracellular second messengers and ligands have been characterized [8] (see Fig. 2).
Fig. (1). Action potential in non-pacemaker cardiomyocytes.
A). The action potential of atrial and ventricular myocytes consists of 4 phases (upper part), to which specific ion flows contribute (middle). The long plateau phase (3) and a stable resting phase (4) are characteristic for myocyte action potential. The corresponding electrocardiographic ventricular activity is shown below. Early afterdepolarizations occur during phase 2 / 3 of the action potential, before the ongoing action potential has reached phase 0 (B). Delayed afterdepolarizations occur during phase 4 of the action potential, before the action potential has reached phase 0 (C).
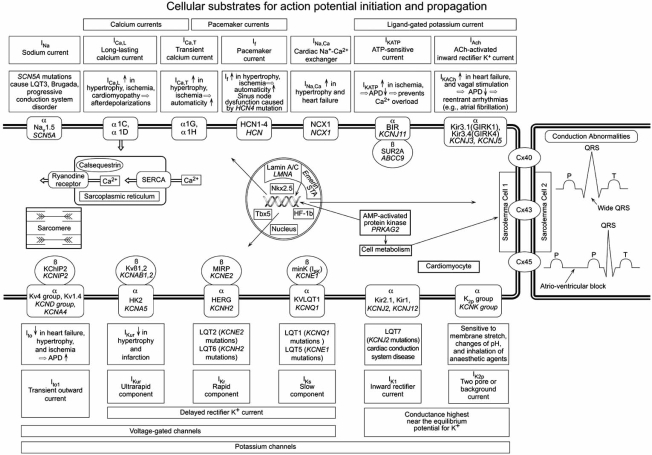
Fig. (2). Cellular substrates for action potential initiation and propagation.
Displayed are transverse tubules of two neighboring cardiomyocytes with the ion channels localized on the sarcolemma of cell 1 and the connecting gap junction connexins to the sarcolemma of cell 2. Shown are the pore regions of the ion channels (α-subunits) through which ions flow across the plasma membrane, and the cytoplasmic β-subunits. Within each subunit, the encoding gene is displayed in italics, the protein in normal font. Differences in disease status are indicated in a box next to the currents. Intranuclear proteins and genes might also interact with ion channels and/or gap junction proteins. Disturbed calcium handling within the sarcomere or sarcoplasmic reticulum underlies distinct arrhythmia causing diseases.
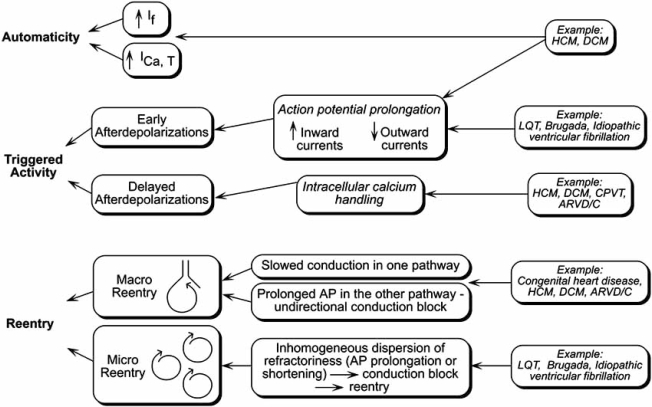
There are three different mechanisms that lead to tachyarrhythmias: Increased automaticity of myocytes, triggered activity arising from early and delayed afterdepolarizations, and re-entry (see Fig. 3). Increased automaticity and triggered activity are the typical mechanisms of non-re-entrant tachycardias [9].
Fig. (3). Cellular mechanisms of arrhythmias.
On the cellular level, three distinct entities causing tachyarrhythmias can be distinguished: automaticity, triggered activity and reentry. One or a combination of these mechanisms occurs in inherited arrhythmias. AP action potential, HCM hypertrophic cardiomyopathy, DCM dilated cardiomyopathy, ARVD/C arrhythmogenic right ventricular dysplasia/cardiomyopathy.
Increased automaticity of a myocyte denotes that the myocyte is able to depolarize itself, without an external stimulus. Normally, there is a stable resting membrane potential in a cardiomyocyte. Only pacemaker cardiomyocytes depolarize in the absence of extrinsic stimulation. Those automatic cells spontaneously depolarize during phase 4 of the cardiac action potential, reach threshold, and initiate an action potential.
In contrary to increased automaticity where focal activity starts spontaneously, “triggered” rhythms typically require a preceding action potential. Thus, triggered activity means that arrhythmias originate from afterdepolarizations. An afterdepolarization is a premature depolarization of the membrane potential before the ongoing action potential has reached the next phase 0. Afterdepolarizations arise as a consequence of intracellular calcium oscillations.
Early afterdepolarizations occur during phase 2 or 3 (repolarization phase), whereas delayed afterdepolarizations occur during phase 4 (resting phase) (see Fig. 1). If an afterdepolarization is large enough, it can engage rapid sodium influx and thus initiate a premature action potential, which is clinically observed as a premature contraction.
Another common arrhythmia mechanism is re-entrant excitation. Re-entry contributes to the development of ventricular tachycardia, a rapid, organized process in which the excitation travels a relatively well-defined circuit. Micro-re-entrant circuits involve small regions of heart tissue such as seen in LQT and Brugada syndrome. Here, intramural reentry is caused by a transmural dispersion of refractoriness. Macro-re-entry involves large regions of heart tissue and is most commonly seen in hypertrophied or stretched myocardium as seen in cardiomyopathies or in congenital heart disease (see Fig. 3).
Re-entry can degenerate to atrial or ventricular fibrillation, a chaotic electrical activity resulting from random propagation of multiple independent wavelets through the muscle. Fibrillation results in unorganized impulse propagation and absent contraction.
ARRHYTHMIA MECHANISMS IN THE STRUCTURALLY ALTERED HEART
Hypertrophic and Dilated Cardiomyopathies
Both hypertrophic cardiomyopathies (HCM) and dilated cardiomyopathies (DCM) are inherited disorders with a high incidence of lethal arrhythmias occurring at a young age. In both HCM and DCM, arrhythmias may occur unrelated to ischemia or heart failure.
HCM is a disease of the sarcomere and is the most common inherited cardiovascular disorder with a prevalence of 0.2-0.5% in the general population [10-12]. Sudden death, at least partially due to malignant arrhythmias, accounts for at least 50% of deaths among patients with HCM [13].
The actual mechanisms linking contractile dysfunction and arrhythmias remain poorly understood [14]. Multifactorial biological substrates including genetic, functional, environmental and hemodynamic features contribute to a high incidence of lethal ventricular tachycardias in cardiomyopathies. Histopathologic changes, such as myocardial fibrosis or myocyte disorganization and hypertrophy, might attribute to re-entry and thus engender arrhythmia susceptibility [15, 16]. However, only ventricular hypertrophy and not histopathologic changes, correlated with increased arrhythmia susceptibility in a HCM mouse model [17]. Increased automaticity by upregulation of pacemaker ion channel genes, which has been shown to occur in cardiac hypertrophy [18], could be another arrhythmia mechanism in HCM. Furthermore, sarcomere gene mutations alter cytosolic cardiomyocyte Ca2+ homeostasis [19] which promotes afterdepolarizations triggering arrhythmias [20] (see Fig. 3).
DCM is characterized by an increase in size of the ventricular chambers and frequently is associated with arrhythmias. Numerous mutations affecting the sarcomere and the cytoskeleton have been identified in DCM [21, 22]. The etiology of arrhythmias occurring in DCM is poorly understood, but it is known that stretch of the myocardium per se influences the shape and amplitude of the intracellular Ca2+ transient, therefore favoring afterdepolarizations [23].
DCM caused by mutations in the LMNA gene encoding for the inner nuclear membrane protein lamin A/C is associated with a high risk for arrhythmias and sudden death [24-26] and sometimes involves progressive atrioventricular nodal disease [26-28]. Both arrhythmias and conduction system disease can precede cardiac dilatation and presumably are a direct consequence of intrinsic changes of the myocyte secondary to lamin A/C deficiency [29].
Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy (ARVD/C)
ARVD/C refers to a genetically heterogeneous group of cardiomyopathies characterized by progressive degeneration and fibrofatty infiltration of the right ventricular myocardium [30]. Patients are prone to ventricular tachycardia, right heart failure, and sudden death [31, 32]. Mutations in genes encoding for the desmosomal proteins plakoglobin [33], desmoplakin [34, 35], and plakophilin-2 [36] have been shown to cause ARVD/C. The molecular mechanisms of arrhythmias in ARVD/C are poorly understood. ARVD2, caused by mutations in the ryanodine receptor (RyR2), is clinically different from other forms of ARVD/C in that ventricular arrhythmias are stereotypically effort-induced [30]. In ARVD2 the leaky ryanodine receptor results in cytosolic Ca2+ overload and, consequently, in delayed afterdepolarizations triggering arrhythmias, and may overlap with CPVT.
Arrhythmia mechanisms in the structurally normal heart
The occurrence of arrhythmias in the absence of any structural heart disease is classified as a “primary electrical disease”. Ion channelopathies are examples of arrhythmogenic mechanisms generated primarily at the cellular level. It is still unclear how arrhythmias are triggered at a multicellular level, but the presumption is that delayed repolarization increases the spatial voltage gradients sufficiently to trigger repetitive excitation of the ventricle. A defect in an ion channel alone is not sufficient to induce arrhythmias. Extensive phenotypic variability can be seen among family members carrying mutations in these channel genes. While some family members harboring the mutations suffer from sudden cardiac death, other members carrying the same primary genetic mutations may never develop any arrhythmias. The underlying cause for the onset of lethal arrhythmias must be therefore multifactorial.
Long QT Syndromes
The long QT syndrome is characterized by the electrocardiographic finding of QT interval prolongation and T wave abnormalities in conjunction with a clinical presentation and/or a family history of syncope, ventricular arrhythmias, and unexpected sudden death. This genetic disorder of cardiac electrical repolarization is caused by mutations of genes encoding for cardiac potassium and sodium channels, usually inherited in an autosomal-dominant fashion. The altered ion channel function produces prolongation of the action potential and a propensity to torsades de pointes ventricular tachycardia. “Torsades de Pointes” means twisting around the point, an allusion to the alternating axis of the QRS complex around the isoelectric line of the ECG during this arrhythmia.
To date, more than ten different types of LQTS have been characterized according to their underlying monogenic defect (see Table 1 and Fig. 2).
Table 1. Long QT Syndromes.
More than ten distinct types of congenital long QT (LQT) syndromes due to different gene mutations have been defined to date. Most LQT syndromes (LQT1, 2, 5, 6 and 7) are caused by loss-of-function mutations in potassium channels. LQT3 and LQT10 involve mutations in the sodium channel. LQT8 is caused by mutations in the L-type calcium channel. LQT4 and LQT9 are caused by structural proteins.
| Gene Mutations | Affected Protein/Subunit | Ion Channel | |
|---|---|---|---|
| LQT1 | KCNQ1 | KVLQT1 Kv7.1α |
IKs |
| LQT2 | KCNH2 | HERG Kv11.1α |
IKr |
| LQT3 | SCN5A | Nav1.5α | INa |
| LQT4 | Ank2 | Ankyrin-B | - |
| LQT5 | KCNE1 | MinKβ | IKs |
| LQT6 | KCNE2 | MiRP1β | IKr |
| LQT7 (Andersen syndrome) | KCNJ2 | Kir2.1α | IK1 |
| LQT8 (Timothy syndrome) | CACNA1c | Cav1.2α1c | ICa,L |
| LQT9 | CAV3 | Caveolin-3 | - |
| LQT10 | SCN4B | Nav1.5β4 | INa |
LQT1 (42%) and LQT2 (45%) account for about 87% of identified mutations, and LQT3 (8%), LQT5 (3%), and LQT6 (2%) for the other 13% [37]. Missense mutations in the transmembrane and pore domains are the most common [37]. Mutant subunits lead to reduction of IKs or IKr by a loss-of-function mechanism, often with dominant-negative effect [38-41].
LQT syndrome-associated mutations in SCN5A cause a gain-of-function leading to prolonged opening of the sodium channel [42, 43].
In the heart, reduced IKs or IKr or increased INa leads to prolongation of the cardiac action potential, lengthening of the QT interval, and increased risk of ventricular arrhythmia (see Fig. 2).
Genes such as KVLQT1, HERG, SCN5A, KCNE1, and KCNE2 have been implicated in the autosomal-dominant Romano-Ward syndrome, which is associated with long QT syndrome in the absence of other phenotypic abnormalities [44]. Mutations in KVLQT1 and KCNE1 also cause the less-common autosomal recessive Jervell and Lange-Nielsen syndrome [45, 46], characterized by congenital bilateral deafness associated with marked QT prolongation on the electrocardiogram, syncopal attacks due to ventricular arrhythmias and a high risk for sudden death.
Recent finding of a mutation in the ANK2 gene encoding for ankyrin-B [47] at the LQT4 locus reveals that the phenotype of LQTS can also be caused by abnormal proteins other that cardiac ion channels. ANK2 mutations modify ankyrin-B binding proteins and alter the sodium pump [48], inositol-1,4,5-triphosphate receptors, and the sodium/calcium exchanger function, thereby increasing arrhythmia susceptibility [47].
LQT7, or Andersen syndrome (And1), describes a clinical disorder consisting of potassium-sensitive periodic paralysis, ventricular arrhythmias, and dysmorphic features [49], caused by a missense mutation in the KCNJ2 gene [50].
Timothy's syndrome presents LQT8 and is due to mutations in the calcium channel Cav1.2 encoded by the gene CACNA1c. Since the Calcium channel Cav1.2 is abundant in many tissues, patients with Timothy's syndrome have many clinical manifestations including congenital heart disease, autism, syndactyly and immune deficiency [51].
Most recently mutations in the gene CAV3 encoding for Caveolin-3, a structural protein, have been described to cause LQT9 [52]. Caveolin-3 is the major scaffolding protein forming specific membrane domains in the heart called caveolae and. Caveolin-3 co-localizes and interacts with the SCN5A sodium channel, thereby altering ion channel function and presumably increasing arrhythmia vulnerability [52].
Last, the Na+ channel ß4 subunit encoded by SCN4B has been identified as a novel and rare LQT-susceptibility gene (LQT10). A missense mutation in SCN4B was found to cause a secondary gain of function on the Na+ channel mimicking that of classic LQT3-associated mutations in SCN5A [53].
Understanding the molecular differences between the different LQT types is important for risk stratification and has clinical implications for the management of LQT patients. Genetic screening using mutational analysis can improve presymptomatic diagnosis and has recently become commercially available.
A large phenotype-genotype correlation study showed that LQT1 patients are more prone to arrhythmias during exercise and swimming, whereas LQT2 and LQT3 patients experience more lethal and non-lethal events during rest and sleep [1]. The response to medical therapy differs between underlying LQT mutation: LQT1 patients seem to respond better to beta-blocker therapy than LQT2 and LQT3 patients [1, 3].
However, clinical heterogeneity among patients with long-QT syndrome sharing the same disease-causing mutation challenges their medical management. Some recognized modifiers of the clinical phenotype include gender, age, drugs, metabolic derangements or hypothermia. Additionally, recent studies provided more evidence for genetic modulation altering arrhythmia susceptibility in primary arrhythmia syndromes, such as the presence of compound mutations [54] or the coexistence of modifier alleles or single nucleotide polymorphisms [55].
Modifier genes also play a significant role in drug-induced LQT syndrome. There are differences in responses of patients to medication and knowing the gene variants that cause differences among patients allow ‘personalized’ drug therapy [56]. This is of specific interest regarding drugs that are known IKr blockers, such as certain antibiotics, antihistamines, antipsychotics and antiarrhythmic agents [57].
Brugada Syndrome
Brugada and Brugada described an autosomal-dominant life-threatening disease occurring in the structurally normal heart and characterized by ST segment elevation in the right precordial leads (V1 to V3), right bundle branch block, and susceptibility to ventricular tachyarrhythmias [58]. The cellular basis for the Brugada syndrome is thought to be due to an outward shift in the ionic current active during phase 1 of the right ventricular epicardial action potential [59] in which Ito is prominent, exaggerating shortening of action potential duration in epicardium versus endocardium [60].
Mutations in the SCN5A gene account for less than 25% of cases [61]. In contrast to LQT3, these mutations result in a loss-of-function of INa [62] (see Fig. 2).
Recently, a novel mutation in the glycerol-3-phosphate dehydrogenase 1–like gene (GPD1-L) was identified to cause Brugada syndrome. By disrupting trafficking of SCN5A, GPD1-L mutations decrease SCN5A surface membrane expression and reduces INa [63].
Catecholaminergic Polymorphic Ventricular Tachycardia
Arrhythmias in catecholaminergic polymorphic ventricular tachycardia (CPVT) are typically triggered by catecholamine surges as occurs with exercise and emotion [64]. An alternating QRS axis on a beat-to-beat basis, the so-called bidirectional VT, is often the distinguishing pathognomonic presentation of CPVT-related arrhythmias [65]. Supraventricular arrhythmias are also frequently observed during exercise among CPVT patients.
Mutations in the ryanodine receptor gene (RyR2) [65, 66] and the calsequestrin gene CASQ2 [67], both involved in intracellular calcium homeostasis and excitation-contraction coupling, are two of the genetic causes of CPVT (see Fig. 2).
The underlying pathogenic mechanism of arrhythmias in both ryanodine [68] as well as calsequestrin gene mutations [69] is a leakiness of the ryanodine receptor causing intracellular Ca2+ disturbances. Catecholamine surges caused by stress or exercise furthermore increase intracellular calcium overload and thus trigger afterdepolarizations, resulting in polymorphic ventricular tachycardia.
Idiopathic Ventricular Fibrillation
Variable penetrance and distinct modifiers in allelic disorders make disease definitions challenging. If sudden unexpected cardiac death occurs and no electrophysiological abnormality is recognized, this patient group is categorized as having idiopathic ventricular fibrillation. The terminology acknowledges the current inability to identify a specific cause [70]. Patients who survive an episode of ventricular fibrillation are at a high risk of recurrence for life-threatening events [71].
In some patients with idiopathic ventricular fibrillation, mutations in the sodium channel encoding gene SCN5A have been revealed [72, 73], but a subset of these also present a Brugada or LQT like phenotype [74].
Sudden unexplained death syndrome (“SUDS”, or “Lai-Tai” in Thai) is more prevalent in the Asian as compared to the western population. The cause of this disorder is widely unknown. Most patients suffering from SUDS show Brugada-like ECG pattern, but mutations in the gene were found in a minority of them [75]. Therefore, SCN5A mutations different from previously reported ones or other candidate genes might be responsible for SUDS and remain to be investigated.
Recently, genome-wide association studies have found a common variant in the nitric oxide synthase 1 adaptor protein (NOS1AP) to be associated with QT-interval variation [76] but increased risk for ventricular fibrillation and sudden death could not yet be established [77].
FUTURE
Understanding of the molecular and ionic mechanisms underlying cardiac electrophysiology is essential for the appreciation of the pathogenesis of cardiac arrhythmias in structurally normal and altered hearts. These fundamental principles and concepts allow incorporation of genetic factors in addition to clinical parameters to ultimately improve personalized risk assessment and determine optimal therapy [14].
One area in which technologies are rapidly evolving is that of polymorphism identification. These data will be used to identify single nucleotide polymorphisms, or groups of polymorphisms, that predict particular diseases or individualized responses to drug therapies.
Another new therapeutic field is the gene therapy approach. Gene delivery and cell-based therapies are currently being explored for treating the substrate for re-entry after myocardial infarction or for replacing electronic pacemakers with biological ones [78, 79]. In the experimental setting, over-expression of β2-adrenergic receptors [80], use of a dominant-negative construct to suppress inward rectifier current when expressed together with the wild-type Kir2.1 [81], and implantation of vectors carrying the pacemaker gene HCN2 into atrium [82] or bundle branches [83] have been studied. A common problem inherent in these approaches is the use of viruses to deliver the necessary genes. Although the vectors are replication-deficient adenoviruses that have little infectious potential, there is concern related to the possibility of only a transient improvement in pacemaker function as well as potential inflammatory responses [84].
The identification of molecular pathways allows development of new therapeutic targets, for both disease palliation and cure. Increasing knowledge about disease causing mutations and genotypic-phenotypic correlation opens the doors of cardiac electrophysiology to the emerging field of pharmacogenomics.
ACKNOWLEDGEMENTS
The authors are very grateful to Emily McIntosh, Emily Harris and Bill McIntosh for excellent graphical design of the figures.
REFERENCES
- 1.Schwartz PJ, Priori SG, Spazzolini C, Moss AJ, Vincent GM, Napolitano C, Denjoy I, Guicheney P, Breithardt G, Keating MT, Towbin JA, Beggs AH, Brink P, Wilde AA, Toivonen L, Zareba W, Robinson JL, Timothy KW, Corfield V, Wattanasirichaigoon D, Corbett C, Haverkamp W, Schulze-Bahr E, Lehmann MH, Schwartz K, Coumel P, Bloise R. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 2.Priori SG, Schwartz PJ, Napolitano C, Bloise R, Ronchetti E, Grillo M, Vicentini A, Spazzolini C, Nastoli J, Bottelli G, Folli R, Cappelletti D. Risk stratification in the long-QT syndrome. N. Engl. J. Med. 2003;348:1866–1874. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- 3.Priori SG, Napolitano C, Schwartz PJ, Grillo M, Bloise R, Ronchetti E, Moncalvo C, Tulipani C, Veia A, Bottelli G, Nastoli J. Association of long QT syndrome loci and cardiac events among patients treated with beta-blockers. JAMA. 2004;292:1341–1344. doi: 10.1001/jama.292.11.1341. [DOI] [PubMed] [Google Scholar]
- 4.Priori SG. Inherited arrhythmogenic diseases: the complexity beyond monogenic disorders. Circ. Res. 2004;94:140–145. doi: 10.1161/01.RES.0000115750.12807.7E. [DOI] [PubMed] [Google Scholar]
- 5.Brink PA, Crotti L, Corfield V, Goosen A, Durrheim G, Hedley P, Heradien M, Geldenhuys G, Vanoli E, Bacchini S, Spazzolini C, Lundquist AL, Roden DM, George AL Jr, Schwartz PJ. Phenotypic variability and unusual clinical severity of congenital long-QT syndrome in a founder population. Circulation. 2005;112:2602–2610. doi: 10.1161/CIRCULATIONAHA.105.572453. [DOI] [PubMed] [Google Scholar]
- 6.Herfst LJ, Rook MB, Jongsma HJ. Trafficking and functional expression of cardiac Na+ channels. J. Mol. Cell. Cardiol. 2004;36:185–193. doi: 10.1016/j.yjmcc.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Nerbonne JM, Nichols CG, Schwarz TL, Escande D. Genetic manipulation of cardiac K(+) channel function in mice: what have we learned, and where do we go from here? Circ. Res. 2001;89:944–956. doi: 10.1161/hh2301.100349. [DOI] [PubMed] [Google Scholar]
- 8.Tamargo J, Caballero R, Gomez R, Valenzuela C, Delpon E. Pharmacology of cardiac potassium channels. Cardiovasc. Res. 2004;62:9–33. doi: 10.1016/j.cardiores.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 9.Pogwizd SM. Nonreentrant mechanisms underlying spontaneous ventricular arrhythmias in a model of nonischemic heart failure in rabbits. Circulation. 1995;92:1034–1048. doi: 10.1161/01.cir.92.4.1034. [DOI] [PubMed] [Google Scholar]
- 10.Seidman JG, Seidman C. The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell. 2001;104:557–567. doi: 10.1016/s0092-8674(01)00242-2. [DOI] [PubMed] [Google Scholar]
- 11.Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation. 1995;92:785–789. doi: 10.1161/01.cir.92.4.785. [DOI] [PubMed] [Google Scholar]
- 12.Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287:1308–1320. doi: 10.1001/jama.287.10.1308. [DOI] [PubMed] [Google Scholar]
- 13.Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol. Rev. 1999;79:215–262. doi: 10.1152/physrev.1999.79.1.215. [DOI] [PubMed] [Google Scholar]
- 14.Knollmann BC, Roden DM. A genetic framework for improving arrhythmia therapy. Nature. 2008;451:929–936. doi: 10.1038/nature06799. [DOI] [PubMed] [Google Scholar]
- 15.Wu TJ, Ong JJ, Hwang C, Lee JJ, Fishbein MC, Czer L, Trento A, Blanche C, Kass RM, Mandel WJ, Karagueuzian HS, Chen PS. Characteristics of wave fronts during ventricular fibrillation in human hearts with dilated cardiomyopathy: role of increased fibrosis in the generation of reentry. J. Am. Coll. Cardiol. 1998;32:187–196. doi: 10.1016/s0735-1097(98)00184-3. [DOI] [PubMed] [Google Scholar]
- 16.Spirito P, Seidman CE, McKenna WJ, Maron BJ. The management of hypertrophic cardiomyopathy. N. Engl. J. Med. 1997;336:775–785. doi: 10.1056/NEJM199703133361107. [DOI] [PubMed] [Google Scholar]
- 17.Wolf CM, Moskowitz IP, Arno S, Branco DM, Semsarian C, Bernstein SA, Peterson M, Maida M, Morley GE, Fishman G, Berul CI, Seidman CE, Seidman JG. Somatic events modify hypertrophic cardiomyopathy pathology and link hypertrophy to arrhythmia. Proc. Natl. Acad. Sci. USA. 2005;102:18123–18128. doi: 10.1073/pnas.0509145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swynghedauw B. Remodeling of the heart in chronic pressure overload. Basic. Res. Cardiol. 1991;86((Suppl 1)):99–105. [PubMed] [Google Scholar]
- 19.Fatkin D, McConnell BK, Mudd JO, Semsarian C, Moskowitz IG, Schoen FJ, Giewat M, Seidman CE, Seidman JG. An abnormal Ca(2+) response in mutant sarcomere protein-mediated familial hypertrophic cardiomyopathy. J. Clin. Invest. 2000;106:1351–1359. doi: 10.1172/JCI11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knollmann BC, Kirchhof P, Sirenko SG, Degen H, Greene AE, Schober T, Mackow JC, Fabritz L, Potter JD, Morad M. Familial hypertrophic cardiomyopathy-linked mutant troponin T causes stress-induced ventricular tachycardia and Ca2+-dependent action potential remodeling. Circ. Res. 2003;92:428–436. doi: 10.1161/01.RES.0000059562.91384.1A. [DOI] [PubMed] [Google Scholar]
- 21.Towbin JA, Bowles NE. The failing heart. Nature. 2002;415:227–233. doi: 10.1038/415227a. [DOI] [PubMed] [Google Scholar]
- 22.Li D, Tapscoft T, Gonzalez O, Burch PE, Quinones MA, Zoghbi WA, Hill R, Bachinski LL, Mann DL, Roberts R. Desmin mutation responsible for idiopathic dilated cardiomyopathy. Circulation. 1999;100:461–464. doi: 10.1161/01.cir.100.5.461. [DOI] [PubMed] [Google Scholar]
- 23.Calaghan SC, Belus A, White E. Do stretch-induced changes in intracellular calcium modify the electrical activity of cardiac muscle? Prog. Biophys. Mol. Biol. 2003;82:81–95. doi: 10.1016/s0079-6107(03)00007-5. [DOI] [PubMed] [Google Scholar]
- 24.Raffaele DB, Ricci E, Galluzzi G, Tonali P, Mora M, Morandi L, Romorini A, Voit T, Orstavik KH, Merlini L, Trevisan C, Biancalana V, Housmanowa-Petrusewicz I, Bione S, Ricotti R, Schwartz K, Bonne G, Toniolo D. Different mutations in the LMNA gene cause autosomal dominant and autosomal recessive Emery-Dreifuss muscular dystrophy. Am. J. Hum. Genet. 2000;66:1407–1412. doi: 10.1086/302869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacLeod HM, Culley MR, Huber JM, McNally EM. Lamin A/C truncation in dilated cardiomyopathy with conduction disease. BMC. Med. Genet. 2003;4:4. doi: 10.1186/1471-2350-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fatkin D, MacRae C, Sasaki T, Wolff MR, Porcu M, Frenneaux M, Atherton J, Vidaillet HJ Jr, Spudich S, De Girolami U, Seidman JG, Seidman C, Muntoni F, Muehle G, Johnson W, McDonough B. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N. Engl. J. Med. 1999;341:1715–1724. doi: 10.1056/NEJM199912023412302. [DOI] [PubMed] [Google Scholar]
- 27.Arbustini E, Pilotto A, Repetto A, Grasso M, Negri A, Diegoli M, Campana C, Scelsi L, Baldini E, Gavazzi A, Tavazzi L. Autosomal dominant dilated cardiomyopathy with atrioventricular block: a lamin A/C defect-related disease. J. Am. Coll. Cardiol. 2002;39:981–990. doi: 10.1016/s0735-1097(02)01724-2. [DOI] [PubMed] [Google Scholar]
- 28.Chen L, Lee L, Kudlow BA, Dos Santos HG, Sletvold O, Shafeghati Y, Botha EG, Garg A, Hanson NB, Martin GM, Mian IS, Kennedy BK, Oshima J. LMNA mutations in atypical Werner's syndrome. Lancet. 2003;362:440–445. doi: 10.1016/S0140-6736(03)14069-X. [DOI] [PubMed] [Google Scholar]
- 29.Wolf CM, Wang L, Alcalai R, Pizard A, Burgon PG, Ahmad F, Sherwood M, Branco DM, Wakimoto H, Fishman GI, See V, Stewart CL, Conner DA, Berul CI, Seidman CE, Seidman JG. Lamin A/C haploinsufficiency causes dilated cardiomyopathy and apoptosis-triggered cardiac conduction system disease. J. Mol. Cell. Cardiol. 2008;44:293–303. doi: 10.1016/j.yjmcc.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiso N, Stephan DA, Nava A, Bagattin A, Devaney JM, Stanchi F, Larderet G, Brahmbhatt B, Brown K, Bauce B, Muriago M, Basso C, Thiene G, Danieli GA, Rampazzo A. Identification of mutations in the cardiac ryanodine receptor gene in families affected with arrhythmogenic right ventricular cardiomyopathy type 2 (ARVD2) Hum. Mol. Genet. 2001;10:189–194. doi: 10.1093/hmg/10.3.189. [DOI] [PubMed] [Google Scholar]
- 31.Thiene G, Nava A, Corrado D, Rossi L, Pennelli N. Right ventricular cardiomyopathy and sudden death in young people. N. Engl. J. Med. 1988;318:129–133. doi: 10.1056/NEJM198801213180301. [DOI] [PubMed] [Google Scholar]
- 32.Paul M, Schulze-Bahr E, Breithardt G, Wichter T. Genetics of arrhythmogenic right ventricular cardiomyopathy--status quo and future perspectives. Z. Kardiol. 2003;92:128–136. doi: 10.1007/s00392-003-0892-9. [DOI] [PubMed] [Google Scholar]
- 33.McKoy G, Protonotarios N, Crosby A, Tsatsopoulou A, Anastasakis A, Coonar A, Norman M, Baboonian C, Jeffery S, McKenna WJ. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease) Lancet. 2000;355:2119–2124. doi: 10.1016/S0140-6736(00)02379-5. [DOI] [PubMed] [Google Scholar]
- 34.Rampazzo A, Nava A, Malacrida S, Beffagna G, Bauce B, Rossi V, Zimbello R, Simionati B, Basso C, Thiene G, Towbin JA, Danieli GA. Mutation in human desmoplakin domain binding to plakoglobin causes a dominant form of arrhythmogenic right ventricular cardiomyopathy. Am. J. Hum. Genet. 2002;71:1200–1206. doi: 10.1086/344208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norgett EE, Hatsell SJ, Carvajal-Huerta L, Cabezas JC, Common J, Purkis PE, Whittock N, Leigh IM, Stevens HP, Kelsell DP. Recessive mutation in desmoplakin disrupts desmoplakin-intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum. Mol. Genet. 2000;9:2761–2766. doi: 10.1093/hmg/9.18.2761. [DOI] [PubMed] [Google Scholar]
- 36.Dalal D, Molin LH, Piccini J, Tichnell C, James C, Bomma C, Prakasa K, Towbin JA, Marcus FI, Spevak PJ, Bluemke DA, Abraham T, Russell SD, Calkins H, Judge DP. Clinical features of arrhythmogenic right ventricular dysplasia/cardiomyopathy associated with mutations in plakophilin-2. Circulation. 2006;113:1641–1649. doi: 10.1161/CIRCULATIONAHA.105.568642. [DOI] [PubMed] [Google Scholar]
- 37.Splawski I, Shen J, Timothy KW, Lehmann MH, Priori S, Robinson JL, Moss AJ, Schwartz PJ, Towbin JA, Vincent GM, Keating MT. Spectrum of mutations in long-QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation. 2000;102:1178–1185. doi: 10.1161/01.cir.102.10.1178. [DOI] [PubMed] [Google Scholar]
- 38.Chouabe C, Neyroud N, Guicheney P, Lazdunski M, Romey G, Barhanin J. Properties of KvLQT1 K+ channel mutations in Romano-Ward and Jervell and Lange-Nielsen inherited cardiac arrhythmias. EMBO J. 1997;16:5472–5479. doi: 10.1093/emboj/16.17.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shalaby FY, Levesque PC, Yang WP, Little WA, Conder ML, Jenkins-West T, Blanar MA. Dominant-negative KvLQT1 mutations underlie the LQT1 form of long QT syndrome. Circulation. 1997;96:1733–1736. doi: 10.1161/01.cir.96.6.1733. [DOI] [PubMed] [Google Scholar]
- 40.Wollnik B, Schroeder BC, Kubisch C, Esperer HD, Wieacker P, Jentsch TJ. Pathophysiological mechanisms of dominant and recessive KVLQT1 K+ channel mutations found in inherited cardiac arrhythmias. Hum. Mol. Genet. 1997;6:1943–1949. doi: 10.1093/hmg/6.11.1943. [DOI] [PubMed] [Google Scholar]
- 41.Sanguinetti MC, Curran ME, Spector PS, Keating MT. Spectrum of HERG K+--channel dysfunction in an inherited cardiac arrhythmia. Proc. Natl. Acad. Sci. USA. 1996;93:2208–2212. doi: 10.1073/pnas.93.5.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bennett PB, Yazawa K, Makita N, George AL Jr. Molecular mechanism for an inherited cardiac arrhythmia. Nature. 1995;376:683–685. doi: 10.1038/376683a0. [DOI] [PubMed] [Google Scholar]
- 43.Dumaine R, Wang Q, Keating MT, Hartmann HA, Schwartz PJ, Brown AM, Kirsch GE. Multiple mechanisms of Na+ channel--linked long-QT syndrome. Circ. Res. 1996;78:916–924. doi: 10.1161/01.res.78.5.916. [DOI] [PubMed] [Google Scholar]
- 44.Romano C, Gemme G, Pongiglione R. Aritmiecardiache rare dell'eta pediatrica II: accessi sincopali per fibrillazione ventricolare parossitica. Clin. Pediatr. 1963;45:656–683. [PubMed] [Google Scholar]
- 45.Neyroud N, Tesson F, Denjoy I, Leibovici M, Donger C, Barhanin J, Faure S, Gary F, Coumel P, Petit C, Schwartz K, Guicheney P. A novel mutation in the potassium channel gene KVLQT1 causes the Jervell and Lange-Nielsen cardioauditory syndrome. Nat. Genet. 1997;15:186–189. doi: 10.1038/ng0297-186. [DOI] [PubMed] [Google Scholar]
- 46.Vetter DE, Mann JR, Wangemann P, Liu J, McLaughlin KJ, Lesage F, Marcus DC, Lazdunski M, Heinemann SF, Barhanin J. Inner ear defects induced by null mutation of the isk gene. Neuron. 1996;17:1251–1264. doi: 10.1016/s0896-6273(00)80255-x. [DOI] [PubMed] [Google Scholar]
- 47.Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, DuBell WH, Song LS, Haurogne K, Kyndt F, Ali ME, Rogers TB, Lederer WJ, Escande D, Le Marec H, Bennett V. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003;421:634–639. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 48.Chauhan VS, Tuvia S, Buhusi M, Bennett V, Grant AO. Abnormal cardiac Na(+) channel properties and QT heart rate adaptation in neonatal ankyrin(B) knockout mice. Circ. Res. 2000;86:441–447. doi: 10.1161/01.res.86.4.441. [DOI] [PubMed] [Google Scholar]
- 49.Tawil R, Ptacek LJ, Pavlakis SG, DeVivo DC, Penn AS, Ozdemir C, Griggs RC. Andersen's syndrome: potassium-sensitive periodic paralysis, ventricular ectopy, and dysmorphic features. Ann. Neurol. 1994;35:326–330. doi: 10.1002/ana.410350313. [DOI] [PubMed] [Google Scholar]
- 50.Plaster NM, Tawil R, Tristani-Firouzi M, Canun S, Bendahhou S, Tsunoda A, Donaldson MR, Iannaccone ST, Brunt E, Barohn R, Clark J, Deymeer F, George AL Jr, Fish FA, Hahn A, Nitu A, Ozdemir C, Serdaroglu P, Subramony SH, Wolfe G, Fu YH, Ptacek LJ. Mutations in Kir2. cause the developmental and episodic electrical phenotypes of Andersen's syndrome. Cell. 2001;105:511–519. doi: 10.1016/s0092-8674(01)00342-7. [DOI] [PubMed] [Google Scholar]
- 51.Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 52.Vatta M, Ackerman MJ, Ye B, Makielski JC, Ughanze EE, Taylor EW, Tester DJ, Balijepalli RC, Foell JD, Li Z, Kamp TJ, Towbin JA. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation. 2006;114:2104–2112. doi: 10.1161/CIRCULATIONAHA.106.635268. [DOI] [PubMed] [Google Scholar]
- 53.Medeiros-Domingo A, Kaku T, Tester DJ, Iturralde-Torres P, Itty A, Ye B, Valdivia C, Ueda K, Canizales-Quinteros S, Tusie-Luna MT, Makielski JC, Ackerman MJ. SCN4B-encoded sodium channel beta4 subunit in congenital long-QT syndrome. Circulation. 2007;116:134–142. doi: 10.1161/CIRCULATIONAHA.106.659086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Westenskow P, Splawski I, Timothy KW, Keating MT, Sanguinetti MC. Compound mutations: a common cause of severe long-QT syndrome. Circulation. 2004;109:1834–1841. doi: 10.1161/01.CIR.0000125524.34234.13. [DOI] [PubMed] [Google Scholar]
- 55.Crotti L, Lundquist AL, Insolia R, Pedrazzini M, Ferrandi C, De Ferrari GM, Vicentini A, Yang P, Roden DM, George AL Jr, Schwartz PJ. KCNH2-K897T is a genetic modifier of latent congenital long-QT syndrome. Circulation. 2005;112:1251–1258. doi: 10.1161/CIRCULATIONAHA.105.549071. [DOI] [PubMed] [Google Scholar]
- 56.Roden DM. Pharmacogenetics and drug-induced arrhythmias. Cardiovasc. Res. 2001;50:224–231. doi: 10.1016/s0008-6363(00)00302-3. [DOI] [PubMed] [Google Scholar]
- 57.Roden DM. Mechanisms and management of proarrhythmia. Am. J. Cardiol. 1998;82:49I–57I. doi: 10.1016/s0002-9149(98)00472-x. [DOI] [PubMed] [Google Scholar]
- 58.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J. Am. Coll. Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 59.Antzelevitch C. The Brugada syndrome diagnostic criteria and cellular mechanisms. Eur. Heart J. 2001;22:356–363. doi: 10.1053/euhj.2000.2461. [DOI] [PubMed] [Google Scholar]
- 60.Alings M, Wilde A. “Brugada” syndrome: clinical data and suggested pathophysiological mechanism. Circulation. 1999;99:666–673. doi: 10.1161/01.cir.99.5.666. [DOI] [PubMed] [Google Scholar]
- 61.Priori SG, Napolitano C, Gasparini M, Pappone C, Della BP, Giordano U, Bloise R, Giustetto C, De Nardis R, Grillo M, Ronchetti E, Faggiano G, Nastoli J. Natural history of Brugada syndrome: insights for risk stratification and management. Circulation. 2002;105:1342–1347. doi: 10.1161/hc1102.105288. [DOI] [PubMed] [Google Scholar]
- 62.Napolitano C, Rivolta I, Priori SG. Cardiac sodium channel diseases. Clin. Chem. Lab. Med. 2003;41:439–444. doi: 10.1515/CCLM.2003.066. [DOI] [PubMed] [Google Scholar]
- 63.London B, Michalec M, Mehdi H, Zhu X, Kerchner L, Sanyal S, Viswanathan PC, Pfahnl AE, Shang LL, Madhusudanan M, Baty CJ, Lagana S, Aleong R, Gutmann R, Ackerman MJ, McNamara DM, Weiss R, Dudley SC Jr. Mutation in glycerol-3-phosphate dehydrogenase 1 like gene (GPD1-L) decreases cardiac Na+ current and causes inherited arrhythmias. Circulation. 2007;116:2260–2268. doi: 10.1161/CIRCULATIONAHA.107.703330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation. 1995;91:1512–1519. doi: 10.1161/01.cir.91.5.1512. [DOI] [PubMed] [Google Scholar]
- 65.Priori SG, Napolitano C, Memmi M, Colombi B, Drago F, Gasparini M, DeSimone L, Coltorti F, Bloise R, Keegan R, Cruz Filho FE, Vignati G, Benatar A, DeLogu A. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74. doi: 10.1161/01.cir.0000020013.73106.d8. [DOI] [PubMed] [Google Scholar]
- 66.Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, Sorrentino V, Danieli GA. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 67.Lahat H, Pras E, Olender T, Avidan N, Ben Asher E, Man O, Levy-Nissenbaum E, Khoury A, Lorber A, Goldman B, Lancet D, Eldar M. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am. J. Hum. Genet. 2001;69:1378–1384. doi: 10.1086/324565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu N, Colombi B, Memmi M, Zissimopoulos S, Rizzi N, Negri S, Imbriani M, Napolitano C, Lai FA, Priori SG. Arrhythmogenesis in catecholaminergic polymorphic ventricular tachycardia: insights from a RyR2 R4496C knock-in mouse model. Circ. Res. 2006;99:292–298. doi: 10.1161/01.RES.0000235869.50747.e1. [DOI] [PubMed] [Google Scholar]
- 69.Song L, Alcalai R, Arad M, Wolf CM, Toka O, Conner DA, Berul CI, Eldar M, Seidman CE, Seidman JG. Calsequestrin 2 (CASQ2) mutations increase expression of calreticulin and ryanodine receptors, causing catecholaminergic polymorphic ventricular tachycardia. J. Clin. Invest. 2007;117:1814–1823. doi: 10.1172/JCI31080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wever EF, Robles de Medina EO. Sudden death in patients without structural heart disease. J. Am. Coll. Cardiol. 2004;43:1137–1144. doi: 10.1016/j.jacc.2003.10.053. [DOI] [PubMed] [Google Scholar]
- 71.Wever EF, Hauer RN, Oomen A, Peters RH, Bakker PF, Robles de Medina EO. Unfavorable outcome in patients with primary electrical disease who survived an episode of ventricular fibrillation. Circulation. 1993;88:1021–1029. doi: 10.1161/01.cir.88.3.1021. [DOI] [PubMed] [Google Scholar]
- 72.Chen Q, Kirsch GE, Zhang D, Brugada R, Brugada J, Brugada P, Potenza D, Moya A, Borggrefe M, Breithardt G, Ortiz-Lopez R, Wang Z, Antzelevitch C, O'Brien RE, Schulze-Bahr E, Keating MT, Towbin JA, Wang Q. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature. 1998;392:293–296. doi: 10.1038/32675. [DOI] [PubMed] [Google Scholar]
- 73.Moric-Janiszewska E, Herbert E, Cholewa K, Filipecki A, Trusz-Gluza M, Wilczok T. Mutational screening of SCN5A linked disorders in Polish patients and their family members. J. Appl. Genet. 2004;45:383–390. [PubMed] [Google Scholar]
- 74.Viskin S, Fish R, Eldar M, Zeltser D, Lesh MD, Glick A, Belhassen B. Prevalence of the Brugada sign in idiopathic ventricular fibrillation and healthy controls. Heart. 2000;84:31–36. doi: 10.1136/heart.84.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sangwatanaroj S, Yanatasneejit P, Sunsaneewitayakul B, Sitthisook S. Linkage analyses and SCN5A mutations screening in five sudden unexplained death syndrome (Lai-tai) families. J. Med. Assoc. Thai. 2002;85((Suppl 1)):S54–S61. [PubMed] [Google Scholar]
- 76.Arking DE, Pfeufer A, Post W, Kao WH, Newton-Cheh C, Ikeda M, West K, Kashuk C, Akyol M, Perz S, Jalilzadeh S, Illig T, Gieger C, Guo CY, Larson MG, Wichmann HE, Marban E, O'Donnell CJ, Hirschhorn JN, Kaab S, Spooner PM, Meitinger T, Chakravarti A. A common genetic variant in the NOS1 regulator NOS1AP modulates cardiac repolarization. Nat. Genet. 2006;38:644–651. doi: 10.1038/ng1790. [DOI] [PubMed] [Google Scholar]
- 77.Aarnoudse AJ, Newton-Cheh C, de Bakker PI, Straus SM, Kors JA, Hofman A, Uitterlinden AG, Witteman JC, Stricker BH. Common NOS1AP variants are associated with a prolonged QTc interval in the Rotterdam Study. Circulation. 2007;116:10–16. doi: 10.1161/CIRCULATIONAHA.106.676783. [DOI] [PubMed] [Google Scholar]
- 78.Sasano T, McDonald AD, Kikuchi K, Donahue JK. Molecular ablation of ventricular tachycardia after myocardial infarction. Nat. Med. 2006;12:1256–1258. doi: 10.1038/nm1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bucchi A, Plotnikov AN, Shlapakova I, Danilo P Jr, Kryukova Y, Qu J, Lu Z, Liu H, Pan Z, Potapova I, KenKnight B, Girouard S, Cohen IS, Brink PR, Robinson RB, Rosen MR. Wild-type and mutant HCN channels in a tandem biological-electronic cardiac pacemaker. Circulation. 2006;114:992–999. doi: 10.1161/CIRCULATIONAHA.106.617613. [DOI] [PubMed] [Google Scholar]
- 80.Edelberg JM, Aird WC, Rosenberg RD. Enhancement of murine cardiac chronotropy by the molecular transfer of the human beta2 adrenergic receptor cDNA. J. Clin. Invest. 1998;101:337–343. doi: 10.1172/JCI1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miake J, Marban E, Nuss HB. Biological pacemaker created by gene transfer. Nature. 2002;419:132–133. doi: 10.1038/419132b. [DOI] [PubMed] [Google Scholar]
- 82.Qu J, Plotnikov AN, Danilo P Jr, Shlapakova I, Cohen IS, Robinson RB, Rosen MR. Expression and function of a biological pacemaker in canine heart. Circulation. 2003;107:1106–1109. doi: 10.1161/01.cir.0000059939.97249.2c. [DOI] [PubMed] [Google Scholar]
- 83.Plotnikov AN, Sosunov EA, Qu J, Shlapakova IN, Anyukhovsky EP, Liu L, Janse MJ, Brink PR, Cohen IS, Robinson RB, Danilo P Jr, Rosen MR. Biological pacemaker implanted in canine left bundle branch provides ventricular escape rhythms that have physiologically acceptable rates. Circulation. 2004;109:506–512. doi: 10.1161/01.CIR.0000114527.10764.CC. [DOI] [PubMed] [Google Scholar]
- 84.Potapova I, Plotnikov A, Lu Z, Danilo P Jr, Valiunas V, Qu J, Doronin S, Zuckerman J, Shlapakova IN, Gao J, Pan Z, Herron AJ, Robinson RB, Brink PR, Rosen MR, Cohen IS. Human mesenchymal stem cells as a gene delivery system to create cardiac pacemakers. Circ. Res. 2004;94:952–959. doi: 10.1161/01.RES.0000123827.60210.72. [DOI] [PubMed] [Google Scholar]