Abstract
Objective
Assess the relationship between spinal cord T2 hyperintense lesions and clinical status in multiple sclerosis (MS) with 1.5 and 3T MRI.
Methods
Whole cord T2-weighted fast spin-echo MRI was performed in 32 MS patients [Expanded Disability Status Scale (EDSS) score (mean±SD: 2±1.9), range 0–6.5]. Protocols at 1.5T and 3T were optimized and matched on voxel size.
Results
Moderate correlations were found between whole cord lesion volume and EDSS score at 1.5T (rs =.36, p=0.04), but not at 3T (rs =0.13, p=0.46). Pyramidal Functional System Score (FSS) correlated with thoracic T2 lesion number (rs=.46, p=0.01) and total spinal cord lesion number (rs=0.37, p=0.04) and volume (rs=0.37, p=0.04) at 1.5T. Bowel/bladder FSS correlated with T2 lesion volume and number in the cervical, thoracic, and total spine at 1.5T (rs 0.400.57, all p<0.05). These MRI-FSS correlations were non-significant at 3T. However, these correlation coefficients did not differ significantly between platforms (Choi’s test p>0.05). Correlations between whole cord lesion volume and timed 25-foot walk were non-significant at 1.5T and 3T (p>0.05). Lesion number and volume did not differ between MRI platforms in the MS group (p>0.05).
Conclusions
Despite the use of higher field MRI strength, the link between spinal lesions and MS disability remains weak. The 1.5T and 3T protocols yielded similar results for many comparisons.
Keywords: [41] Multiple sclerosis, [120] MRI, [252] all spinal cord
INTRODUCTION
A stronger concordance between MRI-visualized MS pathology and observed disability would allow clinicians and researchers to better monitor disease progression and efficacy of therapy. Only weak to moderate correlations have been observed between brain conventional MRI findings such as T1-gadolinium enhancement, T2 hyperintense lesion volume (T2LV), or T1 hypointense lesion volume and disability measures such as Expanded Disability Scale Score (EDSS) in patients with MS,1 however. One potential explanation for this discrepancy is that spinal cord lesions can substantially contribute to disability. Both pathologic and neuroimaging studies demonstrate that spinal cord damage can be appreciated in upwards of three quarters of MS patients.2, 3 Unfortunately, reported correlations between spinal T2LV and EDSS score have been relatively poor.3–5 The weak link between T2LV and disability could potentially be addressed by increasing magnet field strength.
With expansion of recent Food and Drug Administration guidelines, 3T MRI6 is increasingly being used in both clinical and research investigations.7 3T MRI has increased the sensitivity in the detection of MS related brain lesions.8–13 A post-mortem study of the spinal cord of MS patients found areas of partial demyelination not well visualized with low field MRI could be seen employing a high field scanner.14 It is not currently established whether a 3T platform will more accurately capture MS related spinal cord pathology and offer a better link with disability in a clinical setting. In this study, we examined a cohort of MS patients with both 1.5T and 3T whole spinal cord MRI to assess T2 hyperintense lesions and their relationship to ambulatory impairment and overall physical disability.
METHODS
Patients
We studied 32 patients who met the International Panel criteria for either MS or a clinically isolated demyelinating syndrome (CIS).15 Of these, 26 patients had relapsing remitting MS (RRMS) (median age 40, range 21–52 years) median disease duration 6, range 2 months-23 years, median EDSS score 1, range0–2.5, median timed 25 foot walk [T25FW] 5, range3–7 seconds,). Four patients had secondary progressive MS (SPMS) (median age 50, range 47–54 years; median disease duration 14, range 5–29 years; median EDSS score 6, range 6–6.5; median T25FW 8 range 5–29 seconds.) One patient each had primary progressive MS and CIS. Our combined population median age was 42 (range 21–54 years), median disease duration was 5.8 (range=0.16–29 years), and median T25FW was 5 (range 3–8 seconds). The MS cohort included eight men and twenty-four women. We also studied 6 normal volunteers to reduce bias in the analysis of lesions – median age 33 (range30–47 years), four women and two men. Patients were enrolled consecutively from a community-based, university-affiliated MS clinic. Our Institutional Review Board approved this study and informed consent was obtained on all subjects. MS disease course16 and clinical measures including EDSS score17 and T25FW18 were assigned by a treating neurologist blind to the spinal cord MRI findings within one month of MRI. In the addition to testing correlations between spinal MRI and overall EDSS score, we also assessed relationships to Functional System Score (FSS) subscales comprising EDSS for those that were pertinent to spinal cord function (pyramidal FSS, sensory FSS, and bowel/bladder FSS).17 The present study was part of a larger ongoing study in which patients are being recruited to assess the relationship between MRI findings and the development of sustained disability three years later. Thus, patients were included only if they had “active disease”. This was defined as a clinical relapse, new or enlarging MRI-defined CNS lesion, or an increase in EDSS score of at least 0.5 in the year prior to recruitment. Only patients aged 18–55 years were included to minimize confounding effects from age-related phenomena. Patients with compressive spinal cord degenerative disease or other major medical co-morbidities were excluded. At the time of MRI, 16 patients were receiving monotherapy with either beta-interferon or glatiramer acetate, 14 patients were on no disease-modifying treatment, one patient was receiving beta-interferon and daclizumab in combination, and another was receiving beta-interferon and cyclophosphamide in combination.
MRI
Imaging of the whole spinal cord was performed in all subjects at 1.5T and 3T. Mean scan time interval between platforms was (mean±SD) 12 ± 11 (range 0–49) days. One hundred fifty to 200 axial slices without gaps were acquired on each subject to cover the whole spinal cord. Only slices extending from the foramen magnum through the inferior extent of the conus medullaris were analyzed. Table 1 shows technical details of the MRI units and scan parameters. The primary goal of this study was to determine correlation between 3T lesion burden and clinical measures rather than to directly compare 1.5 and 3T platforms. Attention was paid to achieving feasible scan time with optimized image quality on both platforms. Because of the potential at 3T to exceed specific absorption rate (SAR) patient safety limitations19 and scanning time considerations, TR, TE and echo train length varied between the two platforms, although voxel size was nearly equivalent. Fast spin echo T2-weighted sequences were employed to minimize scan time and maximize lesion detectability. The 1.5T scanner system took advantage of a multi-station scanning system (MobiTrak) with a large field of view coil, which served to reduce scanning times. Signal and contract-to-noise were not formally evaluated.
Table 1.
Scanner information and parameters
| 1.5T | 3T | |
|---|---|---|
| Coil: | Spinal phased array | Spinal phased array |
| Gradient strength (max) | 23 mT/m | 20 mT/m |
| Model Number | 5351 | 9900983 |
| Number of channels | 4 | 8 |
| Manufacturer | Philips | General Electric |
| Interleaving | No | Yes |
| Cardiac gating | No | No |
| Motion compensation | Yes | Yes |
| Sequence | T2 fast spin echo | T2 fast spin echo |
| Field of view (cm) | 24 × 24 | 24 × 19 |
| Matrix size | 256 × 256 | 256 × 256 |
| Slice thickness (mm) | 3 (no gap) | 3 (no gap) |
| Repetition time (TR) (milliseconds) | 9189 | 6116.66 |
| Echo time (TE) (milliseconds) | 100 | 110.24 |
| Echo train length | 40 | 12 |
| Number of signal averages | 2 | 2 |
| Flip angle (degrees) | 90 | 90 |
| Pixel size (mm) | .898 × .898 | .937 × .937 |
| Total acquisition time (range) | 18.5 – 20 minutes | 22.5 – 32 minutes |
Spinal cord lesions analysis
Image analysis was performed using the software package Jim (Version 3.0., Xinapse Systems, Northants, UK, http://www.xinapse.com). Scans of normal controls and MS patients were pooled and all images were anonymized, intermixed, and randomized for analysis. T2 hyperintense lesions were identified by the consensus of two trained observers (J.S., M.N.) and confirmed by an experienced observer (R.B.) to resolve any discrepancies. Image analysis was meant to replicate the clinical setting. Thus, images were optimized for clinical review by adjusting window width and level but not normalized in a standardized way. Number and volume of T2 hyperintense lesions was assessed. An edge-finding tool based on local thresholding was applied to each axial slice to identify the spinal cord lesion contour. Manual adjustments were applied where necessary.
Statistical Analysis
The Spearman rank correlation test assessed associations between T2 lesion number or T2LV and clinical measures including EDSS score, T25FW, and disease duration. Wheelchair bound patients were excluded from T25FW analysis. T25FW data was unavailable for a single patient. Spearman rank correlations were employed for all factors due to data skew and limited sample size. Correlation coefficients were compared between platforms using Choi’s test.20 A Wilcoxon signed rank test assessed the difference between the number and volume of lesions detected on the 1.5 and 3T platforms. Group differences were assessed by Wilcoxon rank sum tests. McNemar’s test was used to assess if the presence/absence of lesions differed on the two platforms.21 All comparisons were also assessed after T2LV data were log transformed and yielded results similar to those obtained with raw T2LV data. Thus, only the results with raw T2LV are reported below. A p value less than 0.05 was considered significant. Since this was an exploratory study, no corrections for multiple comparisons were performed. All statistical analyses were performed in R (R Development Core Team RR Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org).
RESULTS
Correlation between MRI and clinical status
Correlations between spinal cord lesions and clinical data are reported in Table 2 and Figure 1. Moderate but significant correlations were obtained between whole cord T2LV and EDSS score at 1.5T (rs=0.36, p=0.04). On the other hand, whole cord 3T derived T2LV did not correlate significantly with EDSS score (rs=0.13, p=0.46). However, the correlation coefficients did not differ significantly (p=0.42, Choi’s test). When considering regional characterization of lesions at 1.5T, cervical T2LV was significant (p=0.045) while thoracic (p=0.07) T2LV displayed a trend toward significance with EDSS score. Neither cervical nor thoracic T2LV correlated significantly with EDSS at 3T. Correlation coefficients for T2LV and EDSS between field strengths did not differ in the cervical spine (p=0.93, Choi’s test) while a difference in favor of the 1.5T platform was observed in the thoracic spine in part due to the negative correlation between the T2LV at 3T (p=0.04, Choi’s test). Whole cord T2LV did not correlate significantly with T25FW at either 1.5T (rs=0.30, p=0.12) or 3T (rs=0.17, p=0.38). T25FW did not correlate significantly with cervical spinal T2LV at either field strength. T25FW correlated moderately and significantly with thoracic T2LV (rs=0.40, p=0.03) at 1.5T but not at 3T (rs=0.15, p=0.44). The strength of correlation between T2LV and T25FW did not differ in any of the regions (p>0.1, Choi’s test). Regardless of MRI field strength, disease duration did not significantly correlate with T2LV (Table 2, Figure 1). T2 lesion number (T2LN) analysis showed results similar to T2LV for all of the above comparisons (Table 2), except that a trend observed between thoracic 1.5T T2LV and EDSS (rs=0.32, p=0.07) was significant between thoracic 1.5T T2LN and EDSS (rs=0.55, p=0.001). Significant correlations (rs range=0.40–0.57, p <0.05) between bowel/bladder FSS and T2LV or T2LN were obtained in the cervical, thoracic, and total spinal cord at 1.5T, while correlations at 3T were non-significant. Pyramidal FSS correlated with total T2LV (rs=0.37, p=0.04) and both total (rs=0.37, p=0.04) and thoracic (rs=0.46. p=0.009) T2LN at 1.5T, though not at 3T. Sensory FSS correlations with T2LN and T2LV were non-significant on both platforms. When the strength of correlation between MRI and FSS scores was compared between platforms using Choi’s test, no statistical differences could be demonstrated (p=0.07–0.97). Correlations between MRI and clinical status in the MS subgroups were not assessed due to the small sample size.
Table 2.
Correlation between T2 hyperintense spinal cord lesion volume and clinical status in patients with multiple sclerosis at 1.5T and 3T
| Cervical spinal cord | Thoracic spinal cord | Total spinal cord | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.5T T2LV | 1.5T T2LN | 3T T2LV | 3T T2LN | 1.5T T2LV | 1.5T T2LN | 3T T2LV | 3T T2LN | 1.5T T2LV | 1.5T T2LN | 3T T2LV | 3T T2LN | |
| EDSS score | ||||||||||||
| r value | 0.36 | 0.29 | 0.20 | 0.10 | 0.32 | 0.55 | −0.01 | 0.03 | 0.36 | 0.46 | 0.13 | 0.06 |
| p value | 0.05* | 0.11 | 0.26 | 0.57 | 0.07 | 0.001* | 0.96 | 0.86 | 0.04* | 0.008* | 0.46 | 0.76 |
| Timed 25 foot walk | ||||||||||||
| r value | 0.20 | 0.12 | 0.08 | −0.001 | 0.40 | 0.40 | 0.15 | 0.12 | 0.30 | 0.31 | 0.17 | 0.08 |
| p value | 0.30 | 0.52 | 0.68 | 0.99 | 0.03* | 0.03* | 0.44 | 0.52 | 0.12 | 0.11 | 0.38 | 0.70 |
| Disease duration | ||||||||||||
| r value | 0.09 | 0.10 | 0.01 | 0.04 | 0.08 | 0.11 | −0.22 | −0.31 | 0.10 | 0.11 | 0.01 | −0.18 |
| p value | 0.63 | 0.59 | 0.94 | 0.83 | 0.67 | 0.56 | 0.22 | 0.09 | 0.60 | 0.54 | 0.96 | 0.33 |
| Sensory FSS | ||||||||||||
| r value | 0.09 | 0.04 | 0.02 | −0.07 | 0.04 | 0.13 | −0.20 | −0.18 | 0.05 | 0.10 | −0.08 | −0.17 |
| p value | 0.64 | 0.83 | 0.91 | 0.69 | 0.82 | 0.49 | 0.26 | 0.33 | 0.8 | 0.58 | 0.68 | 0.36 |
| Pyramidal FSS | ||||||||||||
| r value | 0.31 | 0.2 | 0.18 | 0.03 | 0.29 | 0.46 | 0.14 | 0.13 | 0.37 | 0.37 | 0.19 | 0.04 |
| p value | 0.09 | 0.27 | 0.33 | 0.88 | 0.10 | 0.009* | 0.44 | 0.49 | 0.04* | 0.04* | 0.29 | 0.81 |
| Bowel/bladder FSS | ||||||||||||
| r value | 0.44 | 0.40 | 0.26 | 0.13 | 0.42 | 0.57 | 0.21 | 0.20 | 0.46 | 0.54 | 0.31 | 0.19 |
| p value | 0.01* | 0.02* | 0.15 | 0.47 | 0.02* | 0.001* | 0.25 | 0.26 | 0.007* | 0.001* | 0.08 | 0.31 |
Key: T2LV=T2 hyperintense lesion volume; EDSS=Expanded Disability Status Scale; FSS=Functional System Score; Spearman rank correlation results are reported for EDSS, disease duration, and timed 25 foot walk, and FSS.
Significant (p<0.05)
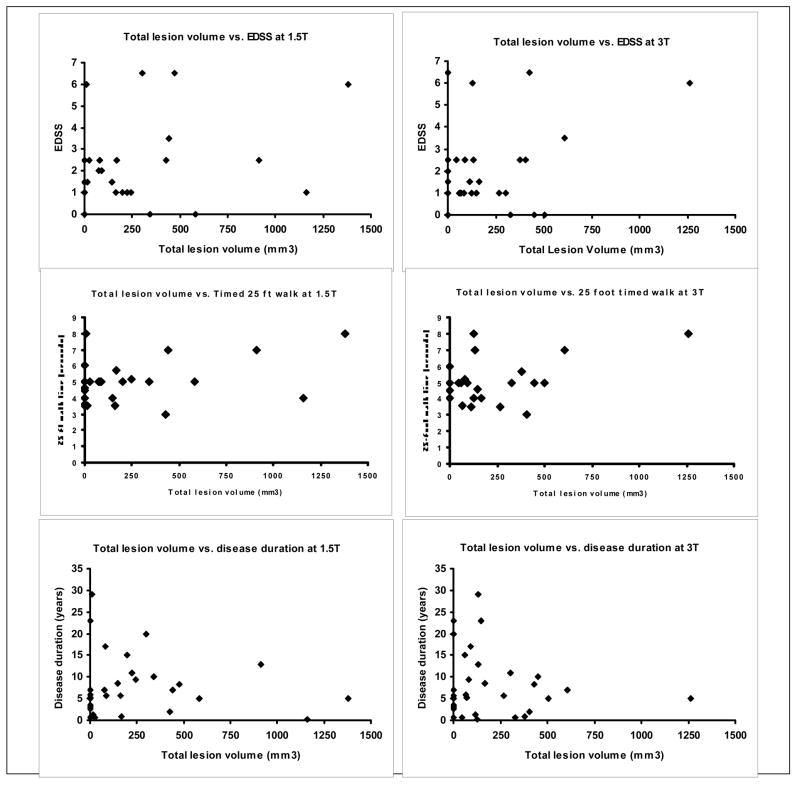
FIGURE 1. Relationship between total spinal lesion volume and clinical measures at 1.5 and 3T.
. Scatterplots show the relationship between total spinal cord T2 lesion volume (T2LV) and clinical measures at 1.5 and 3T in the MS group (n=32). Each diamond represents a patient with MS. At 1.5T, total spinal T2LV (r=0.36, p=0.04) correlated with Expanded Disability Status Scale (EDSS) score, while at 3T, it did not (r=0.13, p=0.46). Neither 1.5T T2LV (r=0.30, p=0.12) nor 3T T2LV (r=0.17, p=0.38) correlated with timed 25 foot walk. Disease duration was not related to T2LV at either 1.5T (r=0.10, p=0.60) or 3T (r=−0.01, p=0.96).
Relationship between MRI and clinical phenotype
Examples of images obtained at 1.5T and 3T are shown in figure 2. No lesions were found in normal controls in any region of the spinal cord. A summary of T2LV data obtained on both platforms in the MS group is shown in Table 3. Regional or whole cord T2LV or T2LN in the MS group did not significantly differ between 1.5T and 3T (all p>0.05, Table 3). No lesions were seen in the conus medullaris. When characterizing patients as either having at least one spinal cord lesion or having no lesions, the number of patients without lesions did not differ between 1.5T [(total n=11 (34%), cervical n=15 (47%), thoracic n=12 (38%)] and 3T [(total n=10 (31%), cervical n=15 (47%), thoracic n=13 (41%)] (p>0.5 for all regions).
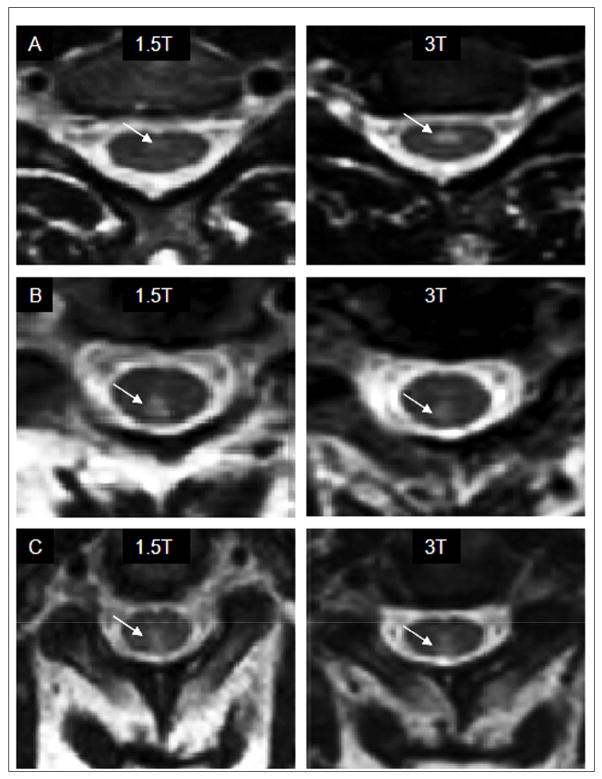
FIGURE 2. Comparison of cervical cord T2 hyperintensities at 1.5T and 3T in patients with MS.
. (A and B): These paired images were acquired from a 49 year-old woman with relapsing-remitting multiple sclerosis and mild physical disability [Expanded Disability Status Scale (EDSS) score of 1, and timed 25 foot walk of 3.5 seconds]. (C) These paired images were acquired from a 45 year-old man with primary progressive multiple sclerosis and moderate physical disability [EDSS score of 3.5 and timed 25 foot walk of 7 seconds]. Paired images (A) taken from the same level in the same patient show better spinal cord lesion definition (arrows) on the 3T platform, while paired images (B) show T2 hyperintensity (arrows) better on the 1.5T (left) platform. Paired images (C) show T2 hyperintensity (arrows) about equally well on both platforms.
Table 3.
T2 hyperintense spinal cord lesion volume in patients with multiple sclerosis at 1.5T vs. 3T
| 1.5T | 3T | p value | |
|---|---|---|---|
| Cervical spinal cord T2LV | |||
| Mean ± SD | 126.5 ± 200.37 | 118.8 ± 207.3 | 0.68 |
| Median (IQR) | 47.0 (0, 184.6) | 25.9 (0, 177.4) | |
| Cervical spinal cord T2LN | |||
| Mean ± SD | 1.06 ± 1.37 | 1.03 ± 1.23 | 0.91 |
| Median (IQR) | 1 (0, 2) | 1 (0, 2) | |
| Thoracic spinal cord T2LV | |||
| Mean ± SD | 106.4 ± 164.3 | 73.7 ± 100.8 | 0.30 |
| Median (IQR) | 18.2 (0, 151.8) | 41.2 (0, 94.1) | |
| Thoracic spinal cord T2LN | |||
| Mean ± SD | 1.53 ± 2.34 | 2 ± 1.93 | 0.23 |
| Median (IQR) | 1 (0, 1) | 1 (0, 2) | |
| Total spinal cord T2LV | |||
| Mean ± SD | 232.9 ± 347.2 | 192.6 ± 262.2 | 0.94 |
| Median (IQR) | 84.4 (0, 311.1) | 103.2 (0, 308.9) | |
| Total spinal cord T2LN | |||
| Mean ± SD | 2.59 ± 3.51 | 2 ± 1.93 | 0.67 |
| Median (IQR) | 2 (0, 3) | 2 (0, 3) |
T2LV=T2 hyperintense lesion volume (mm3); IQR=interquartile range; SD=standard deviation; p values are from paired Wilcoxon tests of 1.5T vs. 3T data.
We compared MRI in patients with progressive disease (PPMS and SPMS, n=5) to patients with relapsing disease (CIS/RRMS, n=27). For total spinal cord T2LV, significantly higher T2LV was observed in the progressive group at 1.5T [SPMS mean=520 ± 514 mm3, median=440 IQR (301, 474) mm3] vs. the relapsing group [mean=180 ± 290 mm3, median 74 IQR (0, 211) mm3, p=0.03] while a trend toward higher lesion volume was seen in the progressive [mean=485 ± 495 mm3, median=428 IQR (130, 608) mm3] vs. the relapsing group [mean=138 ± 157 mm3, median=82 IQR (0, 216) mm3, p=0.08] at 3T. The progressive group had significantly higher T2LV in the cervical cord at both 1.5T [mean 312 ± 277 mm3, median 293, IQR (193, 317) mm3] vs. relapsing [mean=92.3 ± 168 mm3, median 0, IQR (0, 112) mm3, p=0.03] and 3T [SPMS mean 397 ± 396 mm3, median 372, IQR (104,503) vs. RRMS mean 67.3 ± 97 mm3, median=0, IQR (0,120) mm3, p=0.03]. Thoracic cord T2LV did not differ between groups at either field strength (data not shown). Between relapsing and progressive groups, 1.5T T2LN was significantly higher in the progressive group in the thoracic (p=0.01) and total spine (p=0.04) but not cervical spine. At 3T T2LN differences were not significant (data not shown).
DISCUSSION
The major finding of this study was that clinical-MRI correlations using 3T MRI to detect overt spinal cord T2 hyperintense lesions remained weak. Both total and regional measurements of MS related lesions in the spinal cord obtained at 3T did not significantly differ from that obtained on a 1.5T platform and did not improve correlations with clinical measures such as EDSS or T25FW. Though the magnitude of correlation obtained both in total and regionally was at times greater with the 1.5T platform, statistical testing indicates that this was not a major difference. A small subgroup analysis revealed higher total T2LV and T2LN in the progressive group when compared to the relapsing group at 1.5T. At 3T progressive patients trended towards a higher lesion volume, but lesion number was not-significantly different. At 3T, we were unable to detect significantly more spinal cord T2 lesions in the patients with MS than at 1.5T.
The correlations obtained between spinal cord lesions and EDSS score or T25FW at 1.5T and 3T demonstrate that overt spinal damage is only partly related to ambulatory impairment and physical disability in MS. In this paper we report, for the first time, correlations between thoracic spinal cord T2LV and T25FW. However, a lack of correlation between cervical spinal cord T2 hyperintensity and T25FW was observed on both platforms. Better clinical correlations in the thoracic spinal cord between T2LV measured at 1.5T relative to 3T may drive the enhanced correlations at 1.5T observed in total spinal T2LV. We believe that the poor clinical correlations obtained at 3T in the thoracic spinal cord may be due to respiratory and cardiac cycle artifact adversely impacting 3T more than 1.5T imaging.
The correlations we obtained between total spinal T2LV and EDSS score at 1.5T (r=.36, p=.04) are in accord with results published previously by Trop et al. (r = 0.42, p <0.05)5 but differ from non-significant correlations reported earlier by Nijeholt et al.4 and Kidd et al.3 The differences in the strength of correlation may related to differences in patient population. We had very few patients with high disability or SPMS. Our RRMS population, when compared with standard large cohorts was skewed to lower disability. This was based on the difficult in recruiting patients with higher EDSS scores due to limited patient mobility and inability to tolerate longer acquisition times. Though better correlations might have been obtained in a more disabled group, a previous study4 in a relatively unimpaired group (median EDSS=2) reported significant correlations between lesion measures and EDSS scores while another5, with a more impaired population (mean EDSS=5) did not. Our finding that disease duration did not correlate with either T2 lesions on either platform is in keeping with prior studies at 1.5T.22 Correlations between pyramidal and bowel/bladder FSS and T2LV or T2LN at 1.5T suggests a potential link between spinal damage and specific functional difficulties. Caution should be used when interpreting these results, however, because of the small sample size. Our observation that spinal cord lesions are more prominent in progressive vs. relapsing subgroups is in disagreement with reports by Nijeholt et al.14 that T2LVs were similar between RRMS and SPMS cohorts at 1.0T. This discrepancy might be attributed to higher field strengths in our study. Our results fit better with recent magnetization transfer (MT) studies that detect more spinal damage in SPMS patients than RRMS patients.23
It should be emphasized that this study did not seek to rigorously compare imaging quality at 1.5 and 3T. Fundamentally, our parameters at 3T could not mirror those at 1.5T due to limitations imposed by the specific absorption rate (SAR). This represents a greater challenge in spinal cord imaging than in the brain because of the smaller size and greater length of the spinal cord. In order to keep scanning times between platforms roughly equivalent given SAR limitations, echo train length, TR, and TE were adjusted. It is possible that these adjustments may have mitigated any advantage obtained by a favorable signal-to-noise ratio (SNR), interleaving, and increased MT effects at 3T. On the other hand, as field strength increases, T2 values diminish and T2 contrast between tissues decreases.24 Less than linear gains in SNR due to increased receiver bandwidth, and chemical shift artifacts arising on T2 sequences at 3T, might also reduce resolution.8 Increased non-uniform magnetic field artifacts seen within trabecular bone, or at bone-soft tissue interfaces while present at 1.5T25 are increased at 3T.26 However, it is doubtful that chemical shift or artifacts from trabecular bone substantially alter lesion detectability in the spinal cord. Parallel imaging has been proposed to reduce scan time and address SAR concerns when there is ample SNR.27 At the time of initiating our study, parallel imaging was unavailable. Cardiac gating might also have reduced artifacts but was not performed on either platform due to scan time concerns. We did not assess T2 decay curves, which might have helped distinguish underlying pathologies, such as myelin-specific damage.28 Lesion measurements at the two field strengths also may have differed due to technical differences because the machines were from different manufacturers.29
No study has directly compared 1.5T and 3T to visualize MS-related spinal lesions. However, our inability to detect an increased sensitivity to spinal cord lesions at 3T when compared to a 1.5T platform stands in contrast to the enhanced sensitivity reported at 3T in the brain.8–13 Many potential explanations for this discrepancy exist. Technically it is challenging to image the spinal cord because of its small size, motion, and close proximity to CSF related artifact CSF. Some of the discrepancy might also be due to limitations of the T2 sequence. Stronger correlations might have been obtained had the spinal cord lesions been classified into anterior, posterior, and lateral segments; but technical limitations did not permit such an analysis with reliability in our dataset. Such topographic determinations would await advances in hardware or pulse sequences. In addition, our data lend further support to findings that clinically relevant occult diffuse damage is present in the spinal cord of MS patients that extends beyond areas of overt lesions seen with conventional or high field strength MRI.30,31 Studies by Berger et al.32 and Bot et al.33 seem particularly pertinent. At 4.7T, both T2 hyperintense areas and normal appearing areas in MS patients showed evidence of demyelination and axonal loss. These and an additional 7T MRI study34 have shown that correlation between T2 hyperintensity and underlying pathology such as demyelination or axonal loss were unreliable, even though many of the challenges frequently inherent in clinical scanning such as physiologic and patient motion artifacts were absent. Diffusion tensor imaging (DTI), magnetic resonance spectroscopy, or MT could potentially capture spinal damage that is not detected by 3T conventional T2 lesion detection methods. Though MT has been able to demonstrate diffuse spinal cord damage in patients with MS and diffusion anisotropy showed a moderate correlation with disability,35 technical limitations remain, precluding current widespread use. Once optimized, these techniques either individually or in combination may help to improve the clinical-MRI correlations related to spinal cord damage in patients with MS.
Our work should be regarded as preliminary due to sample size and the technical considerations outlined above. Further hardware and software optimization of 3T spinal MRI will likely improve future investigations. Development of better gradient coils, and sequences and techniques that are able to minimize SAR considerations will be important in future work.
Acknowledgments
This work was supported by research grants to Dr. Bakshi from the National Institutes of Health (1R01NS055083-01) and National Multiple Sclerosis Society (RG3705A1; RG3798A2). We thank Ms. Sophie Tamm for assistance with manuscript preparation.
Footnotes
Disclosures: Dr. Alsop GE personal compensation GE Healthcare technologies. Other authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barkhof F. MRI in multiple sclerosis: correlation with expanded disability status scale (EDSS) Mult Scler. 1999;5:283–286. doi: 10.1177/135245859900500415. [DOI] [PubMed] [Google Scholar]
- 2.Ikuta F, Zimmerman HM. Distribution of plaques in seventy autopsy cases of multiple sclerosis in the United States. Neurology. 1976;26:26–28. doi: 10.1212/wnl.26.6_part_2.26. [DOI] [PubMed] [Google Scholar]
- 3.Kidd D, Thorpe JW, Thompson AJ, et al. Spinal cord MRI using multi-array coils and fast spin echo. II. Findings in multiple sclerosis. Neurology. 1993;43:2632–2637. doi: 10.1212/wnl.43.12.2632. [DOI] [PubMed] [Google Scholar]
- 4.Nijeholt GJ, van Walderveen MA, Castelijns JA, et al. Brain and spinal cord abnormalities in multiple sclerosis. Correlation between MRI parameters, clinical subtypes and symptoms. Brain. 1998;121:687–697. doi: 10.1093/brain/121.4.687. [DOI] [PubMed] [Google Scholar]
- 5.Trop I, Bourgouin PM, Lapierre Y, et al. Multiple sclerosis of the spinal cord: diagnosis and follow-up with contrast-enhanced MR and correlation with clinical activity. AJNR Am J Neuroradiol. 1998;19:1025–1033. [PMC free article] [PubMed] [Google Scholar]
- 6.U.S. Department of Health and Human Services. [Accessed May 16, 2008];Criteria for Significant Risk Investigations of Magnetic Resonance Diagnostic Devices. 2003 Available at: http://www.fda.gov/cdrh/ode/guidance/793.pdf.
- 7.Neema M, Stankiewicz J, Arora A, Guss ZD, Bakshi R. MRI in multiple sclerosis: what’s inside the toolbox? Neurotherapeutics. 2007;4:602–617. doi: 10.1016/j.nurt.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sicotte NL, Voskuhl RR, Bouvier S, Klutch R, Cohen MS, Mazziotta JC. Comparison of multiple sclerosis lesions at 1.5 and 3.0. Tesla Invest Radiol. 2003;38:423–427. doi: 10.1097/01.RLI.0000065426.07178.f1. [DOI] [PubMed] [Google Scholar]
- 9.Keiper MD, Grossman RI, Hirsch JA, et al. MR identification of white matter abnormalities in multiple sclerosis: A comparison between 1.5 T and 4 T AJNR. Am J Neuroradiol. 1998;19:1489–1493. [PMC free article] [PubMed] [Google Scholar]
- 10.Kangarlu A, Bourekas EC, Ray-Chaudhury A, Rammohan KW. Cerebral cortical lesions in multiple sclerosis detected by MR imaging at 8 Tesla. AJNR Am J Neuroradiol. 2007;28:262–266. [PMC free article] [PubMed] [Google Scholar]
- 11.Wattjes MP, Harzheim M, Kuhl CK, et al. Does high-field MR imaging have an influence on the classification of patients with clinically isolated syndromes according to current diagnostic MR imaging criteria for multiple sclerosis? AJNR Am J Neuroradiol. 2006;27:1794 –1798. [PMC free article] [PubMed] [Google Scholar]
- 12.Wattjes MP, Lutterbey GG, Harzheim M, et al. Higher sensitivity in the detection of inflammatory brain lesions in patients with clinically isolated syndromes suggestive of multiple sclerosis using high field MRI: an intraindividual comparison of 1.5 T with 3.0 T. Eur Radiol. 2006;16:2067–2073. doi: 10.1007/s00330-006-0195-4. [DOI] [PubMed] [Google Scholar]
- 13.Bachmann R, Reilmann R, Schwindt W, Kugel H, Heindel W, Krämer S. FLAIR imaging for multiple sclerosis: a comparative MR study at 1.5 and 3.0 Tesla. Eur Radiol. 2006;16:915–921. doi: 10.1007/s00330-005-0070-8. [DOI] [PubMed] [Google Scholar]
- 14.Nijeholt GJ, Bergers E, Kamphorst W, et al. Post-mortem high-resolution MRI of the spinal cord in multiple sclerosis: a correlative study with conventional MRI, histopathology and clinical phenotype. Brain. 2001;124:154–166. doi: 10.1093/brain/124.1.154. [DOI] [PubMed] [Google Scholar]
- 15.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 16.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46:907–911. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- 17.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 18.Fischer JS, Rudick RA, Cutter GR. The Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force Mult Scler. 1999;5:244–250. doi: 10.1177/135245859900500409. [DOI] [PubMed] [Google Scholar]
- 19.Boss A, Graf H, Berger A, et al. Tissue warming and regulatory responses induced by radio frequency energy deposition on a whole-body 3-Tesla magnetic resonance imager. J Magn Reson Imaging. 2007;26:1334–1339. doi: 10.1002/jmri.21156. [DOI] [PubMed] [Google Scholar]
- 20.Choi SC. Tests of equality of dependent correlation coefficients. Biometrika. 1977;64:645–647. [Google Scholar]
- 21.Hollander M, Wolfe DA. Nonparametric Statistical Methods. 2. New York: John Wiley & Sons; 1999. [Google Scholar]
- 22.Tartaglino LM, Friedman DP, Flanders AE, Lublin FD, Knobler RL, Liem M. Multiple sclerosis in the spinal cord: MR appearance and correlation with clinical parameters. Radiology. 1995;195:725–732. doi: 10.1148/radiology.195.3.7754002. [DOI] [PubMed] [Google Scholar]
- 23.Filippi M, Bozzali M, Horsfield MA, et al. A conventional and magnetization transfer MRI study of the cervical cord in patients with MS. Neurology. 2000;54:207–213. doi: 10.1212/wnl.54.1.207. [DOI] [PubMed] [Google Scholar]
- 24.Crémillieux Y, Ding S, Dunn JF. High-resolution in vivo measurements of transverse relaxation times in rats at 7 Tesla. Magn Reson Med. 1998;39:285–290. doi: 10.1002/mrm.1910390216. [DOI] [PubMed] [Google Scholar]
- 25.Taber KH, Herrick RC, Weathers SW, Kumar AJ, Schomer DF, Hayman LA. Pitfalls and artifacts encountered in clinical MR imaging of the spine. Radiographics. 1998;18:1499–1521. doi: 10.1148/radiographics.18.6.9821197. [DOI] [PubMed] [Google Scholar]
- 26.Schick F. Whole-body MRI at high field: technical limits and clinical potential. Eur Radiol. 2005;15:946–959. doi: 10.1007/s00330-005-2678-0. [DOI] [PubMed] [Google Scholar]
- 27.Katscher U, Börnert P. Parallel magnetic resonance imaging. Neurotherapeutics. 2007;4:499–510. doi: 10.1016/j.nurt.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore GR, Leung E, MacKay AL, et al. A pathology-MRI study of the short-T2 component in formalin-fixed multiple sclerosis brain. Neurology. 2000;55:1506–1510. doi: 10.1212/wnl.55.10.1506. [DOI] [PubMed] [Google Scholar]
- 29.Filippi M, Rocca MA, Gasperini C, et al. Interscanner variation in brain MR lesion load measurements in multiple sclerosis using conventional spin-echo, rapid relaxation-enhanced, and fast-FLAIR sequences. AJNR Am J Neuroradiol. 1999;20:133–137. [PubMed] [Google Scholar]
- 30.Hesseltine SM, Law M, Babb J, et al. Diffusion tensor imaging in multiple sclerosis: assessment of regional differences in the axial plane within normal-appearing cervical spinal cord. AJNR Am J Neuroradiol. 2006;27:1189–1193. [PMC free article] [PubMed] [Google Scholar]
- 31.Bjartmar C, Kidd G, Mörk S, Rudick R, Trapp BD. Neurological disability correlates with spinal cord axonal loss and reduced N-acetyl aspartate in chronic multiple sclerosis patients. Ann Neurol. 2000;48:893–901. [PubMed] [Google Scholar]
- 32.Bergers E, Bot JC, De Groot CJ, et al. Axonal damage in the spinal cord of MS patients occurs largely independent of T2 MRI lesions. Neurology. 2002;59:1766–1771. doi: 10.1212/01.wnl.0000036566.00866.26. [DOI] [PubMed] [Google Scholar]
- 33.Bot JC, Blezer EL, Kamphorst W, et al. The spinal cord in multiple sclerosis: relationship of high-spatial-resolution quantitative MR imaging findings to histopathologic results. Radiology. 2004;233:531–540. doi: 10.1148/radiol.2332031572. [DOI] [PubMed] [Google Scholar]
- 34.Mottershead JP, Schmierer K, Clemence M, et al. High field MRI correlates of myelin content and axonal density in multiple sclerosis—a post-mortem study of the spinal cord. J Neurol. 2003;250:1293–1301. doi: 10.1007/s00415-003-0192-3. [DOI] [PubMed] [Google Scholar]
- 35.Agosta F, Filippi M. MRI of spinal cord in multiple sclerosis. J Neuroimaging. 2007;17:46S–49S. doi: 10.1111/j.1552-6569.2007.00137.x. [DOI] [PubMed] [Google Scholar]