Abstract
Significant efforts are being devoted towards the development of effective therapeutic vaccines against cancer. Specifically, well-characterized subunit vaccines, which are designed to generate anti-tumor cytotoxic CD8 T cell responses. Since CD4 T cells participate at various stages of CD8 T cell responses, it is important to study the role of CD4 T cells in the induction and persistence of anti-tumor CD8 T cell responses by these vaccines. Recent evidence points to the requirement of CD4 T cells for the long-term persistence of memory CD8 T cells, which in the case of cancer immunotherapy would be critical for the prevention of tumor recurrences. The purpose of the present study was to assess whether CD4 T cells are necessary for the generation and maintenance of antigen-specific CD8 T cells induced by subunit (peptide or DNA) vaccines. We have utilized a vaccination strategy that combines synthetic peptides representing CD8 T cell epitopes, a costimulatory anti-CD40 antibody and a Toll-like receptor agonist (TriVax) to generate large numbers of antigen-specific CD8 T cell responses. Our results show that the rate of decline (clonal contraction) of the antigen-specific CD8 T cells and their functional state is not affected by the presence or absence of CD4 T cells throughout the immune response generated by TriVax. We believe that these results bear importance for the design of effective vaccination strategies against cancer.
Introduction
Antigen mediated CD8 T cell responses undergo various phases, starting with activation, followed by extensive proliferation and subsequent differentiation to effector cells capable of killing (i.e., cytotoxic T lymphocytes) and/or secreting lymphokines capable of inhibiting tumor growth. Another important phase of typical CD8 T cell responses involves the contraction phase, which not only shuts down the response when it is no longer needed but also allows to generate and maintain a small pool of memory cells that could help provide long term protection against tumor recurrences (1-3). Some controversy exists regarding the role of CD4 T helper cells in the generation of effector and memory CD8 T cell responses. Initial studies suggested that CD4 T-cell help was essential for generating primary CD8 T cell responses, which depended mainly on CD40-CD40L interactions with professional antigen presenting cells (APCs) such as dendritic cells (DCs) (4-6). In addition, some of these studies showed that this “licensing” of APCs could be bypassed by the administration of agonistic anti-CD40 antibodies. Nonetheless, some studies using infectious disease models reported that primary CD8 T cell responses could be generated independently of CD4 T-cell help (7-9).
In addition, several apparently contradictory studies on the role of CD4 T cells in the induction and maintenance of memory CD8 T cell responses have recently emerged, further complicating this issue. On one hand, some investigators assert that CD4 T-cell help is essential for the generation of memory CD8 T cell responses and that in its absence, CD8 T cells are unable to undergo a second round of proliferation upon antigen re-encounter (10). These “helpless” memory CD8 T cells may become non-functional and/or fail to perform their cytolytic function (11). In contrast, other studies suggest that functional memory CD8 T cells can be generated in the absence of CD4 T-cell help (9, 12-14). Furthermore, in one of these studies it was demonstrated that although CD4 T cells were not be required for the generation of functional memory CD8 T cells, the presence of CD4 T cells was critical for the persistence of memory CD8 T cells (12). It should be noted that in this study and many others that assess the role of CD4 T cell help for the generation and maintenance of memory CD8 T cells are based on infectious disease models and rely on the use of MHC class II deficient mice, which concomitantly lack CD4 T cells.
For some time the goal of our laboratory has been to develop efficient peptide-based vaccines for various types of cancer (15, 16). We believe that the efficiency of these vaccines will depend not only on the generation of large numbers of effector tumor-reactive CD8 T cells but also on the induction of long-lasting memory CD8 responses that will help prevent tumor recurrences. Here we describe that a recently reported vaccination strategy (17), which we refer to as TriVax, consisting of synthetic peptides corresponding to an MHC class I restricted T cell epitopes, agonistic anti-CD40 antibodies and toll like receptor ligand (TLRL) results in the induction of large numbers of antigen specific T cells that persist in vivo for long time periods. Using TriVax, we have investigated whether CD4 T cells are necessary for the generation, maintenance and subsequent effector function of antigen-specific memory CD8 T cells. Using several antigen systems we report that CD8 T cell responses generated by the TriVax approach are able to persist in vivo for a large period of time irrespective of the presence of CD4 T cells. These results may be important for the design of novel vaccination strategies especially in circumstances where CD4 T cells are inoperative such as those occurring in HIV infections.
Materials and Methods
Mice
Six- to eight-week-old female BALB/c and C57BL/6 (B6) were obtained through the National Cancer Institute/Charles River program (Wilmington, MA). MHC class II knockout (MHC-II-KO) mice on the C57BL/6 background (strain ABBN12) were purchased from Taconic (Hudson, NY). Mice were allowed to acclimate to our animal facility for one week before beginning experiments. Our institutional animal care and use committee approved all experimental procedures done in mice.
Cell lines
EL4, EG7 (Ovalbumin-transfected EL4), 4T1 breast cancer cells, B16-F1 melanoma and A20 B cell lymphoma cell lines were purchased from the American Type Culture Collection (ATCC; Manassas, VA). The ovalbumin trasnfected B16 melanoma cell line, B16-Ova was obtained from Drs. Edith Lord and John Frelinger (University of Rochester Medical Center, Rochester, NY). The TUBO (Turin-Bologna) tumor is a cloned cell line established in vitro from a lobular carcinoma that arose spontaneously in a BALB-neuT mouse (18). 4T1-neu cells were produced by transfecting the 4T1 cells with a retrovirus encoding the rat neu (rNEU) gene (19) and were a generous gift from Dr. Zhaoyang You (University of Pittsburgh Cancer Institute, Pittsburgh, PA).
Peptides and antibodies
The synthetic peptides p66 (TYVPANASL), defined as one of the dominant epitopes of rNEU (20) and Ova257-264 (SIINFEKL), from ovalbumin, were purchased from A&A Labs (San Diego, CA). The purity (>95%) and identity of peptides were determined by analytic high-performance liquid chromatography and mass spectrometry analysis. Monoclonal antibodies used for in vivo cell depletion (anti-CD4, clone GK1.5) and vaccination (anti-CD40, clone FGK4.5) were prepared from hybridoma culture supernatants (cells obtained from ATTC).
Plasmid DNA and immunizations
Plasmid encoding for complete chicken ovalbumin and the amino terminal of rNEU have been previously described (21). Both constructs were in a pcDNA3 backbone and were confirmed by DNA sequencing. Plasmids were purified in an endotoxin-free formulation for vaccination using an EndoFree Plasmid Mega Kit (Qiagen, Valencia, CA). Mice were immunized with a total of 100 μg of plasmid DNA resuspended in normal saline and injected into 2 sites in the quadriceps femoris muscles. Immunization was followed immediately by electroporation of the injected area (95 V, four pulses of 65 ms with re-poling) using an Electro Square Porator device (BTX, model TX830; San Diego, CA). For TriVax immunization, 100μg of synthetic peptide, TLR ligand (50μg of poly-IC or 100μg of CpG 1826) and 50μg of agonistic anti-CD40 were injected intravenously (IV) as a cocktail mixture. CpG 1826 was prepared using phosphorothioate backbone as described (20). Poly-IC (a stabilized formulation containing poly-L-lysine and carboxymethyl cellulose, Poly-ICLC or Hiltonol™) was a kind gift from Dr. Andres Salazar (Oncovir, Inc. Washington, DC). For CD4 T cell depletion, mice were injected with 300 μg of GK1.5 antibody intraperitoneally (IP), twice a week throughout the entire experiment. CD4 depletion was monitored by flowcytometry using a different clone of anti-mouse CD4 (RM 4-4), which does not compete with the depleting antibody.
Flowcytometry
For assessing antigen-specific T cell responses, peripheral blood was collected from the sub-mandibular vein, and the red blood cells were lysed by brief treatment with ammonium chloride buffer, followed by staining with peptide/MHC tetramer. Phycoerythrin (PE)-conjugated-H-2Kb Ova257-264 (Ova257-263/Kb) tetramer was purchased from Beckman Coulter. Phycoerythrin (PE)-conjugated-H-2Kd/rNEUp66 (p66/Kd) tetramer was provided by the National Institute of Allergy and Infectious Disease Tetramer Facility (Emory University Vaccine Center, Atlanta, GA). Cells were stained with Fluorescein isothiocyanate (FITC)-conjugated anti-MHC class II, PerCP Cy5.5-conjugated CD8a (eBioscience, San Diego, CA), and PE-conjugated tetramers (p66/Kd for BALB/c or Ova257-263/Kb for B6) for 30 min. After washing three times with 1% BSA/PBS solution, the fluorescence intensity was evaluated using FACS Calibur (BD Biosciences, San Jose, CA, USA) flowcytometer and analyzed using FlowJo software.
In vivo cytotoxicity assays
B6 splenocytes, unpulsed or pulsed with 10 μM Ova257-264 peptide for 3 h at 37° C in a humidified incubator containing 5% CO2, were labeled with low (0.5 μM) and high (5 μM) concentrations of CFSE, respectively. Labeled splenocytes were co-injected IV in a 1:1 ratio (1 × 107 total cells in 100 μl of PBS). Mice were euthanatized 18 h later and the presence of CFSEhigh to CFSElow cells in splenocytes was determined by flowcytometry.
ELISPOT assays
For detection of CD8 T cells secreting IFN-γ, enzyme-linked immunosorbent spot (ELISPOT) assays were performed as described previously (20, 21). Briefly, CD8 T cells were purified from splenocytes of vaccinated mice by positive selection using antibody-coated magnetic beads (Miltenyi Biotec, Auburn, CA). Responder (CD8-purified) cells were incubated at different concentrations per well, together with 5 × 104 stimulator cells (peptide pulsed, unpulsed or tumor cells). Anti-mouse IFN-γ antibodies (capture and detection) were purchased from Mabtech, Inc. (Mariemont, OH). Cultures were incubated at 37°C for 20 h, and spots (IFN-γ producing cells) were developed.). Spot counting was done with an AID EliSpot Reader System (Autoimmun Diagnostika GmbH, Strassberg, Germany).
Tumor challenge experiments
Mice were vaccinated and boosted as mentioned above, in the presence or absence of CD4 T cells and monitored over a period of 3 months. Following this, BALB/c mice were challenged with 3 × 105 TUBO cells subcutaneously. Mice were monitored for tumor progression and sacrificed when tumor size reached an area of 200 mm2.
Results
TriVax immunization induces large numbers of long-lasting antigen specific CD8 T cells
First we examined the induction of immune responses to peptide vaccination using the immunodominant CD8 T cell epitope Ova257-264, which was administered using the TriVax formulation (mixture of soluble peptide, anti-CD40 antibody and TLR-L, administered intravenously as described in Materials and Methods) using either a TLR3-L (Poly-IC) or a TLR9-L (CpG). Mice that were primed with Ova257-264 TriVax and boosted 3 weeks later with the same vaccine formulation were studied at various time points for the frequency of antigen-specific CD8 T cells in peripheral blood using tetramer analysis. As shown in Figure 1, both TriVax/Poly-IC and TriVax/CpG generated large numbers of antigen specific T cells that subsequently contracted after the vaccine prime. Although the primary responses and degree of contraction appeared to be similar in the TriVax/Poly-IC and TriVax/CpG vaccines, only the TriVax/Poly-IC boost was able to generate a robust secondary CD8 T cell response (Fig. 1A). In addition, the booster response of TriVax/PolyIC lasted for several months and showed a slow rate of contraction. At the end of the experiment (day 85 after the prime), the effector function of the Ova257-264-specific CD8 T cells was evaluated using an in vivo cytotoxicity assay (as described in Materials and Methods). At the same time, the numbers of antigen specific CD8 T cells present in the spleens were assessed by tetramer analysis. The results presented in Figure 1B demonstrate that long after receiving the booster immunization, the mice vaccinated with TriVax/Poly-IC had much higher levels of tetramer positive cells in their spleen than the TriVax/CpG immunized mice. Notwithstanding, these results show that the CD8 T cell responses elicited by either TriVax/Poly-IC or TriVax/CpG resulted in similar levels of effector function since in all cases the target cells pulsed with the Ova257-264 peptide (CFSEhigh) were eliminated in the vaccinated mice (Fig. 1B). It should be noted that without the inclusion of a TLR-L in TriVax, the primary response was substantially lower and that no booster effect was obtained (data not presented). Since TriVax/Poly-IC was the most potent vaccine, this formulation was selected for all the subsequent experiments in the present study, which were designed to evaluate the role of CD4 T cells in CD8 T cell responses to TriVax immunization.
Figure 1. TriVax immunization induces of large numbers of antigen-specific CD8 T cells.
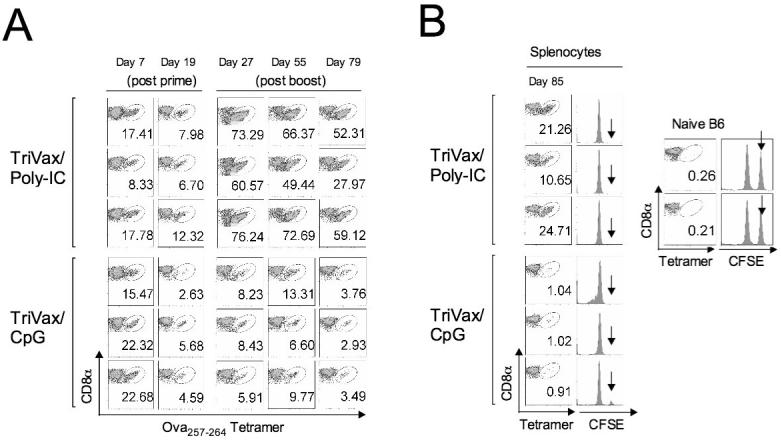
B6 mice (n = 3 per group) received 2 consecutive immunizations on days 0 and 21 (prime-boost) with TriVax/Poly-IC or TriVax/CpG prime-containing the Ova257-264 peptide. A, Antigen specific CD8 T cell responses were measured in peripheral blood by tetramer analysis at various time points. Dot plots for each animal are shown where the numbers in each plot represent the % tetramer positive cells (oval gates) of all CD8 positive cells. B, On day 84 (post prime), the in vivo antigen-specific effector cytolytic activity was estimated by injecting CFSE-labelled target cells and one day later the mice were euthanatized and tetramer positive cells were also measured in the spleens. Results obtained with naïve B6 mice show the absence of tetramer positive cells and persistence of Ova257-264-pulsed, CFSEhigh (arrow) target cells. On the other hand, all the vaccinated mice show tetramer positive spleen cells and almost complete absence of the peptide-pulsed CFSEhigh target cells. CFSElow target cells, which were not pulsed with peptide serve as an internal control were present in all the animals.
Effect of CD4 T cell depletion in CD8 T cell responses to TriVax
Administration of agonistic anti-CD40 antibody has been shown to bypass the need for CD4 T cell help for the generation of primary CD8 T cell responses (4, 6). In addition, TLR-Ls can directly activate DCs, without CD4 T cell help (22). However, as previously noted, CD4 T cells may play a role in the long term persistence of CD8 T cells. Hence, we investigated whether continuous CD4 T cell depletion (using biweekly anti-CD4 antibody administrations) would affect the generation, but more importantly, the maintenance of antigen-specific CD8 T cell responses induced by TriVax immunization. Normal or continuously CD4 T cell depleted C57BL/6 mice were primed and boosted with Ova257-264TriVax/Poly-IC and the numbers of antigen specific CD8 T cells were measured at various time points in peripheral blood. In a similar experiment, persistently CD4 depleted or untreated mice received an intramuscular vaccine prime with a plasmid encoding the ovalbumin cDNA and then were boosted with Ova257-264-peptide TriVax/Poly-IC. As shown in Figure 2, high numbers of Ova257-264-specific CD8 T cells were induced in both immunization protocols, and these numbers were not significantly different with respect to the presence or absence of CD4 T cells. Notably, priming with DNA and boosting with peptide TriVax (Fig. 2B) generated a more robust and long lasting response as compared to the peptide-prime, peptide-boost protocol (Fig. 2A). In these experiments, CD4 depletion was started 2 days before the vaccine prime and the anti-CD4 antibody-treated mice were monitored for their CD4 cell numbers in blood throughout the study and depletion was shown to be >98% effective (data not shown). These results indicate that the large numbers of CD8 T cells generated with the TriVax immunization protocols are long-lived, even in the absence of CD4 T cells.
Figure 2. TriVax induced antigen specific CD8 T cells expand and persist in vivo in the absence of CD4 T cells.
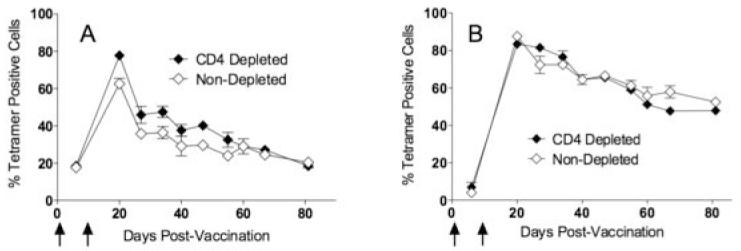
Untreated or continuously CD4 depleted B6 mice were primed either with Ova257-264-peptide TriVax (A) or ovalbumin DNA (B), followed 2 weeks later with a booster immunization with Ova257-264-peptide TriVax. CD4 depletion was initiated 3 days before the first vaccine administration and was continued throughout the entire experiment. Vertical arrows depict the vaccination time points. Antigen-specific CD8 T cell responses and CD4 T cell numbers in blood, were evaluated at various time points using Ova257-264-specific tetramers and anti-CD4 staining, respectively.
Effector function of CD8 T cells induced in the absence of CD4 T cells
There have been some reports indicating that although CD8 responses can occur and persist in the absence of CD4 T cells, the effector function (killing, cytokine production and subsequent antigen-driven expansion) of these cells is somewhat compromised. Thus, we assessed whether the antigen-specific CD8 T cells elicited by TriVax could be maintained in a functional state for a long time period in the absence of CD4 T cells. For this determination, in vivo cytotoxicity assays were performed in vaccinated CD4 depleted and control mice three months after the boost. As shown in Figure 3A, the antigen-expressing target cells (CFSEHI) disappeared 18 hours after their injection into the TriVax immunized mice, irrespective of the presence or absence of CD4 T cells. In addition, the persistence of CD8 T cell effector function in the absence of CD4 T cells was also confirmed in these mice by an in vitro ELISPOT assay, where antigen-induced effector cytokine production (IFN-γ) was measured (Fig. 3B). In these studies, the CD8 T cells maintained in the absence of CD4 T cells not only demonstrated recognition of peptide pulsed target cells but also EG7 and B16-Ova tumor cells, which endogenously process and express ovalbumin epitope. These results demonstrate that TriVax generates high levels of antigen specific CD8 T cell responses that can be maintained in a functional state in the absence of CD4 T cells.
Figure 3. TriVax expanded antigen specific CD8 T cells exhibit effector activity in the absence of CD4 T cells.
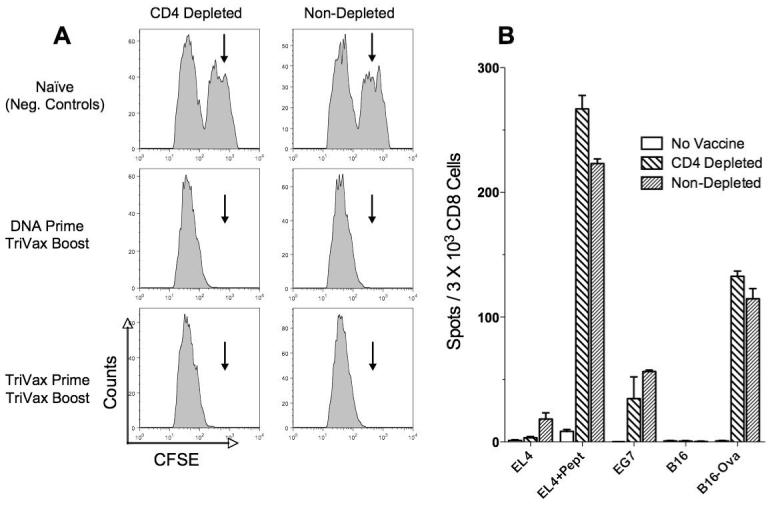
TriVax Ova257-264 immunized mice that were depleted or not of CD4 T cells were evaluated for the presence of effector CD8 T cells 3 months after the initial immunization. A, Mice from the experiment presented in Figure 2 were used for an in vivo cytotoxicity assay as described in Materials and Methods. Results obtained with naïve B6 mice (top panels) show persistence of Ova257-264-pulsed, CFSEhigh (arrows) target cells. On the other hand, the vaccinated mice show an almost complete absence of the peptide-pulsed CFSEhigh target cells. CFSElow target cells, which were not pulsed with peptide, serve as an internal control and were present in all the animals. B, IFN-γ ELISPOT assay using purified splenic CD8 T cells from Ova257-264 TriVax primed and boosted untreated and CD4-depleted mice against peptide pulsed tumor cells as well as ovalbumin-transfected tumors that naturally present the Ova257-264 epitope (EG7 and B16-Ova).
TriVax immunization in MHC class II deficient mice
Taking into account that many studies exploring the role CD4 T cells play in generating and maintaining memory CD8 T cell responses have been conducted in MHC class II knockout mice (MHC-II-KO, which are deficient in CD4 T cells), we considered the possibility that antibody-mediated CD4 T cell depleted mice and MHC class II KO mice could behave differently. It is possible that MHC-II-KO mice could have other immunological defects affecting memory CD8 T cell responses, which could be different from the mere absence of CD4 T cells. Thus, we compared simultaneously the CD8 T cell responses induced by TriVax in antibody-CD4 depleted, MHC-II-KO and wild type (untreated) mice and followed the persistence of antigen-specific CD8 T cells over several months. The results from these experiments showed that CD8 T cell responses in MHC-II-KO mice were generated and persisted in a manner similar to the antibody-CD4 T cell depleted mice. In these experiments, mice received a TriVax prime - TriVax boost (Fig. 4A) or a DNA prime - TriVax boost (Fig. 4B) with nearly identical results. In agreement with previous studies, few CD4 T cells were detected in the peripheral blood of MHC-II-KO mice and these numbers were consistently higher than those observed in the antibody CD4 T cell depleted mice (data not shown). These results validate that the persistence of CD8 T cell responses triggered by TriVax occurs independently of the presence of CD4 T cells in at least 2 model systems where CD4 T cells were absent in the vaccinated individuals.
Figure 4. TriVax immunization in MHC-II-KO and CD4 T cell depleted mice yield similar results regarding the persistence of long-term CD8 T cell responses.
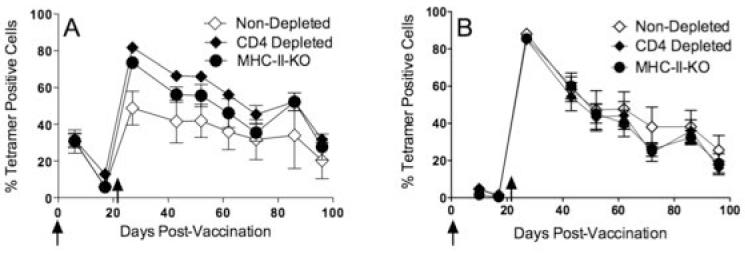
Normal (untreated) B6, CD4 depleted and MHC-II-KO mice were primed with either TriVax Ova257-264 (A) or ovalbumin DNA (B) followed by a TriVax boost 3 weeks later. The vaccination time points are depicted by the vertical arrows. Peripheral blood lymphocytes were evaluated for the presence of antigen-specific CD8 T cells and CD4 positive periodically for a 3-month period.
Tumor-specific CD8 T cell responses can be generated and maintained in the absence of CD4 T cells
Ovalbumin is a highly immunogenic antigen and hence has been most often used as a proof of principle for numerous vaccination strategies. Furthermore, some studies suggest that CD8 T cell responses to strong-foreign antigens may occur more independently of CD4 T cells as compared to weaker-self antigens, such as tumor-associated antigens (TAAs). Since a major goal of our laboratory is to develop effective peptide cancer vaccines, we investigated whether TriVax would be able to generate sustained CD8 T cell responses against epitopes derived from TAAs antigens, even in the absence of CD4 T cells. For these experiments, we targeted the breast TAA rNEU (the rat ortholog of human HER2/neu), which functions as an oncogene in mice that develop autochthonous breast tumors (23). In our hands, the TriVax prime-boost immunization strategy in BALB/c mice has been shown to be inefficient in generating CD8 T cell responses to several peptide epitopes unless CD4 T cells are deleted (data not shown). To overcome this limitation for the present studies, we first vaccinated the mice with a plasmid encoding rNEU cDNA, followed by a TriVax boost using a peptide corresponding to the immunodominant CD8 T cell epitope p66 (20). Following this approach, we investigated whether CD4 T cell depletion would affect generation and persistence of p66-specific CD8 T cells. Depletion of CD4 T cell before the DNA prime slightly decreased the number of antigen specific T cells that were generated after the TriVax boost (27% in non depleted vs. 20% in depleted; Fig. 5A). However, the contraction of the CD8 T cell response after the boost (reduction of antigen-specific T cell numbers throughout time) followed similar kinetics in mice that lack CD4 T cells as compared to the untreated controls. At the end of the experiment, the effector function (cytokine release) of the rNEU-specific CD8 T cells was evaluated for both groups. As shown in Figure 5B, both the CD4 T cell-depleted and the control mice exhibited significant effector T cell function against peptide-pulsed targets and rNEU-expressing tumor cells (4T1-neu). However, the non-depleted mice had approximately 2-3 times higher numbers of antigen specific CD8 T cells as compared to the CD4 T cell-depleted animals, which correlated with the numbers of tetramer positive cells (Fig. 5A). The differences in the magnitude of the response observed in this experiment between the CD4-depleted mice and the control mice could be due to the differences in antigen-specific CD8 T cells that were generated by the DNA priming event (1week after the DNA prime the control mice had 1.75±0.94 p66 tetramer positive cells, while the CD4-depleted mice had 0.69±0.33 tetramer positive cells). In order to more precisely assess the role that CD4 T cells play in the persistence of TAA-specific CD8 T cells induced by TriVax, we compared the effect of CD4 T cell depletion before the DNA prime, before the TriVax boost and after the TriVax boost. As shown in Figure 5C, as with the prior experiment (Fig. 5A), CD4 depletion before the cDNA prime decreased slightly the magnitude of the response as compared to the non-depleted mice. On the other hand, CD4 depletion after the DNA prime, either before or after the TriVax boost had no significant effect in the overall numbers of antigen-specific CD8 T cells in blood. More importantly, the kinetics of the immune responses and clonal contraction of the CD4 depleted mice, regardless of the time of depletion were not significantly different as compared to the non-depleted mice. In addition, when the mice received a second TriVax booster approximately 100 days after the original cDNA immunization, all groups exhibited a marked expansion of TAA-specific CD8 T cells (Fig. 5C) indicating that these cells remained functional with respect to their capacity to proliferate in response to antigen. Moreover, one week later at the conclusion of this experiment, the effector function (cytokine production) of the CD8 T cells from all experimental groups appeared to be similar (Fig. 5D). These results indicate that the presence of CD4 T cells in this experimental model was not necessary for the persistence and function of TriVax-induced CD8 responses to a peptide epitope derived from a TAA.
Figure 5. DNA prime - TriVax boost CD8 T cell responses against an immunodominant tumor-reactive (p66) persist in vivo in the absence of CD4 T cells.
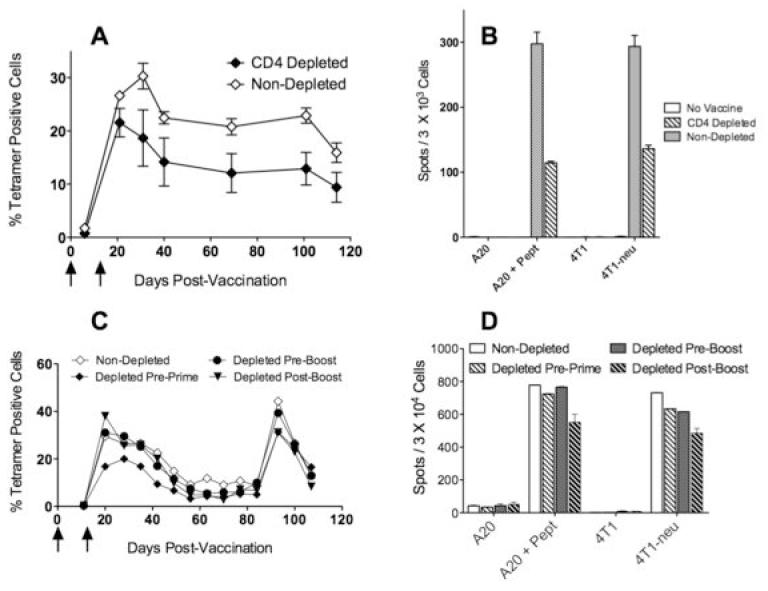
BALB/c mice (CD4 T cell depleted or not) were primed with a DNA plasmid encoding a fragment of rNEU and were boosted 3 weeks later with p66 peptide TriVax. A, Antigen specific responses were monitored in blood using p66/Kd tetramer analysis periodically for over 3 months in untreated and in mice that were CD4 T depleted before the prime. B, Mice from the experiment described in panel A, were studied for their CD8 responses using an IFN-γ ELISPOT assay against peptide pulsed tumor cells (A20) as well as rNEU-transfected tumors (4T1-neu) that naturally present the p66 epitope. C, CD8 T cell responses from untreated or CD4-depleted mice (at either before the prime, before the boost or after the boost) were measured by tetramer analysis in blood samples. In addition, these mice received a second TriVax boost at day 83 to assess the capacity of these cells to re-expand for a second consecutive time. D, Mice from the experiment described in panel C were studied for their CD8 responses using an IFN-γ ELISPOT assay as described in panel B. Vertical arrows depict the vaccination time points.
Anti-tumor effects of CD8 T lymphocytes induced and maintained in the absence of CD4 T cells
The ultimate evidence of functional CD8 T cells is their ability to exhibit anti-tumor effects in vivo. To assess this, both CD4 T cell-depleted and control (untreated) mice received the DNA prime - p66 TriVax boost. Three months after the boost, the mice with challenged subcutaneously with live breast tumor cells (TUBO), which express the rNEU TAA. All the control unvaccinated mice developed palpable tumors within 7-10 days after injection and had to be euthanatized by day 25 when the tumors reached a 20 mm diameter. On the other hand, all of the vaccinated mice, irrespective of the presence or absence of CD4 T cells, rejected the tumor challenge (Fig. 6). Thus, this experiment indicates that CD8 T cell induced and maintained in the absence of CD4 T cells exhibit potent in vivo anti-tumor effects.
Figure 6. Anti-tumor effector function of CD8 T cells expanded by TriVax immunization.
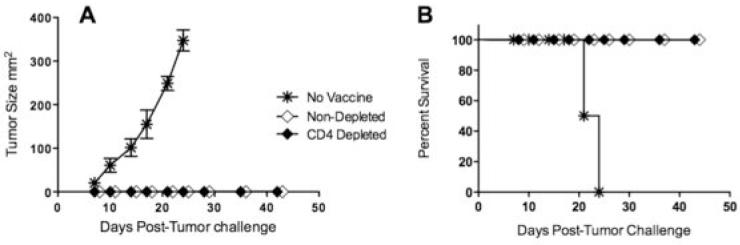
Several groups of BALB/c mice (from Figs. 5A and 5B) were challenged subcutaneously with 3 × 105 live TUBO breast cancer cells and monitored for tumor growth (A) and survival (B). A non-vaccinated group was added as control.
Discussion
The persistence of CD8 T cell as memory cells determines the longevity of the immune response and prevention of disease recurrence (24). However, the mechanisms involved in the generation and persistence of memory CD8 T cells are still not clearly defined. Requirement for simultaneous CD4 T cell activation for efficient effector and memory CD8 T cell generation has been widely debated over a number of years. Recently, redundancy of CD4 T cell help in generation of effector CD8 T cells was described in several model systems, where TLR-Ls or CD40 agonists could substitute for CD4 T cell ‘help’ (9, 13, 14). Nevertheless, there is substantial evidence that CD4 T cell help is essential not only for the generation of an effective memory CD8 T cell response but also for its maintenance (10, 12, 25, 26). It has been shown that administration of CD40 agonists (anti-CD40 antibody) can substitute the role of CD4 T cells for the initial generation memory CD8 T cell responses (4, 6). Several recent studies have reported that “unhelped” CD8 T cells fail to generate memory cells, and the resulting cells are unable to proliferate to a secondary challenge due to TRAIL-induced apoptosis (10, 25). Nevertheless, Bevan and colleagues observed in MHC-II-KO mice that, in the absence of CD4 T cells, memory CD8 T cells could be generated against an infectious agent antigenic challenge but were unable to persist for long term in vivo (12). Although the exact mechanism involved in the requirement of CD4 T cells for the persistence of memory CD8 T cells was not uncovered, these authors hypothesized that cytokines continuously provided by CD4 T cells could be essential in the maintenance of memory CD8 cells. In the present study, we describe that using a potent vaccine combining MHC class I restricted peptide, a TLR3-L and a CD40 agonist, it was possible to generate high numbers of antigen specific CD8 T cells that differentiated into memory cells and later developed effector function upon subsequent antigen stimulation, in spite of being generated and maintained in the absence of CD4 T cells. Interestingly, while both a TLR9-L (CpG) and a TLR3-L (Poly-IC) were equally effective in priming a CD8 T cell response, only the TLR3-L demonstrated the capacity of boosting the response (Fig. 1). Additional experiments indicate that TriVax-CpG is able to boost very effectively a CD8 T cell response to a DNA prime or in mice that are depleted of CD4 T cells prior to receiving a TriVax-CpG prime (H. Cho, unpublished results). We hypothesize that TriVax-CpG when administered more than once, generates a CD4 T cell subset (TH2, TH3 or regulatory T cells) capable of inhibiting antigen-driven CD8 T cell proliferation. Further work is in progress to evaluate this possibility.
Interestingly, our results show that while the absence CD4 T cells during DNA priming using an ovalbumin cDNA plasmid did not affect the CD8 T cell response (Fig. 2B), the presence of CD4 T cells increased the CD8 T cell primary response (and subsequent expansion to TriVax boost) when the rNEU cDNA plasmid was used (Figs. 5A and 5C). These differences could be due to the intrinsic strength of the CD8 T cell epitope, where Ova257-264 represents a strong/foreign antigen and p66 rNEU is a weaker TAA. The differences could be due to genetic differences existing between the C57BL/6 and BALB/c strains of mice.
There are numerous ways that CD4 T cells could be involved in the generation and maintenance of CD8 T cell responses besides simply licensing of APC through CD40 crosslinking (27). Previous work by our laboratory demonstrated that CD4 T cells augmented CD8 T cell survival and function during the effector phase of immune responses (at the tumor site) by direct costimulatory interactions between CD4 and CD8 T cells (28), and by diminishing activation-induced cell death (29). However, under these specific circumstances the antigen recognized by both CD4 and CD8 T cells was present, so the CD8 T cells remained in an activated state. On the other hand, in the present experiments antigen-specific CD4 T cell responses were not deliberately induced by TriVax and the CD8 antigen used (soluble peptide), was most likely eliminated rapidly from the organism. Thus, our previous work and the present findings suggest that in the absence of antigen, the presence of CD4 T lymphocytes (antigen-speci fic or not) will not affect the longevity and function of resting memory CD8 T cells. However, in cases where there is a persistence of antigen such as during anti-tumor responses, chronic viral infections and during pathological autoimmune responses, antigen-specific CD4 T cells could play an important role for the survival and continued function of CD8 T cell responses. In the case of therapeutic anti-tumor responses, we previously reported (20) that administration of anti-CD4 antibodies with the purpose of depleting CD4/CD25 T regulatory (Treg) cells prior to vaccination with p66 peptide (administered in IFA and CpG), although increased the CD8 T cell response (as compared to non-depleted mice) and initial anti-tumor effects, the vaccinated mice ultimately developed tumors and succumbed to disease. On the other hand, Treg depletion using anti-CD25 increased both the anti-CD8 T cell response to the vaccine and provided long-term anti-tumor effects. If this hypothesis turns out to be correct, we predict that concurrent TriVax immunization using CD4 and CD8 TAA T cell epitopes will be more effective in the therapeutic setting (against established tumors) than TriVax immunization solely based on CD8 T cell epitopes. A crucial difference in the previous studies and the present one is the vaccination strategy utilized. Compared to other conventional vaccines, TriVax is able to generate massive CD8 T cell responses, which could be the reason why these cells become independent of CD4 T cell help for their maintenance. The results from these studies will likely impact the development of peptide vaccination strategies for human cancer patients.
Acknowledgments
The authors would like to thank the NIAID Tetramer Facility for providing valuable reagents. Supported in part by NIH grants R01CA80782 and R01CA103921.
Footnotes
Conflict of interest: The authors state that there is no financial interest in this work.
References
- 1.Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Current Opin Immunol. 2005;17:326–32. doi: 10.1016/j.coi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Prlic M, Williams MA, Bevan MJ. Requirements for CD8 T-cell priming, memory generation and maintenance. Current Opin Immunol. 2007;19:315–9. doi: 10.1016/j.coi.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Antia R, Ganusov VV, Ahmed R. The role of models in understanding CD8+ T-cell memory. Nat Rev Immunol. 2005;5:101–11. doi: 10.1038/nri1550. [DOI] [PubMed] [Google Scholar]
- 4.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–80. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 5.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–8. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 6.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–3. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 7.Belz GT, Wodarz D, Diaz G, Nowak MA, Doherty PC. Compromised influenza virus-specific CD8(+)-T-cell memory in CD4(+)-T-cell-deficient mice. J Virol. 2002;76:12388–93. doi: 10.1128/JVI.76.23.12388-12393.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansen P, Stamou P, Tascon RE, Lowrie DB, Stockinger B. CD4 T cells guarantee optimal competitive fitness of CD8 memory T cells. Eur J Immunol. 2004;34:91–7. doi: 10.1002/eji.200324231. [DOI] [PubMed] [Google Scholar]
- 9.Jones ND, Carvalho-Gaspar M, Luo S, Brook MO, Martin L, Wood KJ. Effector and memory CD8+ T cells can be generated in response to alloantigen independently of CD4+ T cell help. J Immunol. 2006;176:2316–23. doi: 10.4049/jimmunol.176.4.2316. [DOI] [PubMed] [Google Scholar]
- 10.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–6. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 11.Novy P, Quigley M, Huang X, Yang Y. CD4 T cells are required for CD8 T cell survival during both primary and memory recall responses. J Immunol. 2007;179:8243–51. doi: 10.4049/jimmunol.179.12.8243. [DOI] [PubMed] [Google Scholar]
- 12.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nature Immunol. 2004;5:927–33. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hervas-Stubbs S, Olivier A, Boisgerault F, Thieblemont N, Leclerc C. TLR3 ligand stimulates fully functional memory CD8+ T cells in the absence of CD4+ T-cell help. Blood. 2007;109:5318–26. doi: 10.1182/blood-2006-10-053256. [DOI] [PubMed] [Google Scholar]
- 14.Marzo AL, Vezys V, Klonowski KD, et al. Fully functional memory CD8 T cells in the absence of CD4 T cells. J Immunol. 2004;173:969–75. doi: 10.4049/jimmunol.173.2.969. [DOI] [PubMed] [Google Scholar]
- 15.Appella E, Loftus DJ, Sakaguchi K, Sette A, Celis E. Synthetic antigenic peptides as a new strategy for immunotherapy of cancer. Biomed Pept Proteins Nucleic Acids. 1995;1:177–84. [PubMed] [Google Scholar]
- 16.Buteau C, Markovic SN, Celis E. Challenges in the development of effective peptide vaccines for cancer. Mayo Clin Proc. 2002;77:339–49. doi: 10.4065/77.4.339. [DOI] [PubMed] [Google Scholar]
- 17.Ahonen CL, Doxsee CL, McGurran SM, et al. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J Exp Med. 2004;199:775–84. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boggio K, Nicoletti G, Di Carlo E, et al. Interleukin 12-mediated Prevention of Spontaneous Mammary Adenocarcinomas in Two Lines of Her-2/neu Transgenic Mice. J Exp Med. 1998;188:589–96. doi: 10.1084/jem.188.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JH, Majumder N, Lin H, Chen J, Falo LD, Jr., You Z. Enhanced immunity by NeuEDhsp70 DNA vaccine Is needed to combat an aggressive spontaneous metastatic breast cancer. Mol Ther. 2005;11:941–9. doi: 10.1016/j.ymthe.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Nava-Parada P, Forni G, Knutson KL, Pease LR, Celis E. Peptide vaccine given with a Toll-like receptor agonist is effective for the treatment and prevention of spontaneous breast tumors. Cancer Res. 2007;67:1326–34. doi: 10.1158/0008-5472.CAN-06-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho HI, Niu G, Bradley N, Celis E. Optimized DNA vaccines to specifically induce therapeutic CD8 T cell responses against autochthonous breast tumors. Cancer Immunol Immunother. 2008;57:1695–703. doi: 10.1007/s00262-008-0465-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nature Immunol. 2004;5:987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 23.Lucchini F, Sacco MG, Hu N, et al. Early and multifocal tumors in breast, salivary, harderian and epididymal tissues developed in MMTY-Neu transgenic mice. Cancer Lett. 1992;64:203–9. doi: 10.1016/0304-3835(92)90044-v. [DOI] [PubMed] [Google Scholar]
- 24.Mescher MF, Curtsinger JM, Agarwal P, et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 25.Janssen EM, Droin NM, Lemmens EE, et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 26.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–42. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy R, Celis E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol Rev. 2008;222:129–44. doi: 10.1111/j.1600-065X.2008.00616.x. [DOI] [PubMed] [Google Scholar]
- 28.Giuntoli RL, 2nd, Lu J, Kobayashi H, Kennedy R, Celis E. Direct costimulation of tumor-reactive CTL by helper T cells potentiate their proliferation, survival, and effector function. Clin Cancer Res. 2002;8:922–31. [PubMed] [Google Scholar]
- 29.Kennedy R, Celis E. T Helper Lymphocytes Rescue CTL from Activation-Induced Cell Death. J Immunol. 2006;177:2862–72. doi: 10.4049/jimmunol.177.5.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]


