Abstract
Cage change and gavage are routine procedures in animal facilities, yet little is known about whether housing modifications change responses to these procedures. Telemetric activity and cardiovascular parameters were assessed in this experiment. BN and F344 male rats were housed in open or individually ventilated cages, each containing 3 rats, 1 of which had a transponder. A crossover design was used, in which 2 groups were given dividers made of 2 intersecting boards (1 form contained holes loaded with food pellets; the other did not) and 1 group was given a rectangular tunnel. On day 8 of each 2-wk period, the cages were changed; on day 11, rats were gavaged. The parameters were evaluated for the first hour and for the following 17 h. Baseline values for each rat were subtracted from the corresponding response values. The presence of objects did not affect the responses of F344 rats to cage changing or gavage. In BN rats with IVCs, the presence of the plain divider modified the response to both procedures. Responses to procedures appeared to be dependent on both the strain and the cage object, thus complicating the establishment of valid general recommendations.
Abbreviations: bpm, beats per minute; HR, heart rate; IVC, individually ventilated cage; MAP, mean arterial pressure
The new European regulations on laboratory rodents12,15 mandate the provision of sufficient nest material to build a complete, covered nest or, if doing so is not possible, providing a nest box. Because rats are poor nest-builders,19 they must be provided with objects for this purpose. Moreover, objects that provide cover or divide the cage area may allow the rats to initiate or avoid contact with cagemates.35
All these regulations are rather specific, but generally the same for all laboratory rodents, that is, rats, mice, gerbils, hamsters, and guinea pigs. However, all rodent species and even strains and stocks within a species may have different needs. These differences raise the question of whether general guidelines, which may be valid for 1 species, may have a negative effect on welfare in other species and strains.
Cage change is a frequent routine procedure in animal facilities that induces temporary, but significant, cardiovascular and behavioral changes in rats.9,10,13,27,29,31-33 Similarly the frequency9,10 and time29 of changing, type of the bedding material,9 light intensity, and length of the dark period3 all modify the intensity of the response to cage changing. The effects on physiologic parameters, such as blood pressure and heart rate, after the cage change seem to be a consequence of the transfer procedure itself and of the novel environment.
Two features of rats suggest potential advantages of placing objects in the cage. First, rats are known to have a good sense of smell—1493 specific olfactory receptor genes have been identified on the cilia of the olfactory neurons—and smell is their primary sense for monitoring their environment.24 Second, rats have dominance hierarchies in which fighting is essentially territorial, rather than for any specific object.4 The term ‘skirmishing’ has been used to describe a pattern of behaviors often assumed to be aggressive in rats; in 1 study,10 the frequency of skirmishing was increased during the first 15 min after a cage change. Consequently, cage objects may retain a familiar odor cue during cage change; the presence of the old item in the new cage reduces the aggressive behavior of rats that is triggered by regrouping.10
Gavage is a method widely used to administer test compounds into the stomach of laboratory rats. Rats display increased blood pressure and heart rate (HR) immediately after gavage, and these increases may persist for 30 to 60 min after the procedure.7,40 Furthermore, elevations in plasma corticosterone levels have been measured in rats after gavage.8 The selection of the correct administration volume2,7,8,40 and a suitable probe material40 are important to performing this procedure properly, but whether housing can be advantageous is unknown.
Housing refinements have not been assessed in regard to their effect on refining the performance of procedures in rats, although housing modifications can alter their physiology and behavior. Rats have lower blood pressure and HR when housed on bedding compared with a grid or plastic floor.22 Rats also prefer a cage with shelter to a barren environment,36 perhaps because they prefer to spend most of the light phase inside the shelter.14 Rats with a furnished environment are more active than those which lack such objects,38 and the presence of a shelter in the cage decreases fearfulness.36 Finally, the availability of cage objects may allow the rats to exhibit species-specific behaviors.11
In many previous studies of the effects of cage changing or gavage,7,8,13,27,29,31-33 the presence of objects in the cages was not described. However, 1 study of both Sprague–Dawley and spontaneously hypertensive male rats showed that providing a multifaceted enrichment program over a week did not affect HR or systolic blood pressure responses to placement in a standard rodent restrainer for 60 min.34 However, after removal from the restrainer, the rats showed a secondary increase in HR and systolic blood pressure that was significantly attenuated in enriched compared with nonenriched rats of both strains. Moreover, enriched rats of both strains had lower HR and systolic blood pressure responses to a variety of procedures, including removal of a cagemate, tail-vein injection, and exposure to the odor of urine and feces of stressed male or female rats.34
We hypothesized that cage objects would alter the effect of cage change and gavage on telemetrically recorded cardiovascular parameters and locomotor activity. This study was designed to evaluate the effect of an aspen wall divider with or without restricted feeding and of the presence of an aspen tunnel in the cage on these measures after routine cage changing and gavage of laboratory rats in both open-top cages and IVCs.
Materials and Methods
The study was done in the Laboratory Animal Centre, University of Helsinki. The study protocol was reviewed and approved by the Animal Ethics Committee of the University of Helsinki.
Animals.
A total of 12 BN (BN/RijHsd) and 12 Fischer 344 (F344/NHsd) male rats, all supplied from Harlan (Horst, The Netherlands) were used in this study. The rats were 25 wk old and weighed 280 to 370 g (BN) or 350 to 460 g (F344) at the beginning of the experiment.
Animal housing and care.
For the first 8 wk, all rats were housed (3 rats per cage) in the same room in polysulfone IVCs (Tecniplast, Buguggiate, Italy) and then for 8 wk in open-top polysulfone cages (Tecniplast). The caging (48.0 × 37.5 × 21.0 cm; floor area, 1500 cm2) had a solid bottom and stainless steel wire lid; each IVC had its own double lid. The cage floor was covered with 3.0 L aspen chip bedding (4 × 4 × 1 mm; 4HP, Tapvei Oy, Kaavi, Finland). The cages were changed weekly. The room temperature was 21.2 ± 0.3 °C and relative humidity was 53.5% ± 7.7%, but the temperature was 1 to 4 °C higher and relative humidity was 2% to 3% higher in IVCs than in open cages and the room. Artificial lighting with fluorescent tubes (light color, warm white) was on from 0600 to 1800, and the light intensity at 1 m above floor in the open cages was 16 to 18 lx compared with 6 to 9 lx in the IVCs. The sound level adjusted with R-weighting (adjusted for the hearing sensitivity of rats6) in empty IVC cages was 20 to 25 dB(R) compared with 12 to 18 dB(R) in empty open cages, with the corresponding adjusted A-weighting (adjusted for the hearing sensitivity of humans) being 45 to 47 dB(A) and 46 to 49 dB(A), respectively. Tap water was provided in polycarbonate bottles, changed once weekly, and refilled once between the cage changes. A more thorough description of husbandry has been published previously.20
Experimental procedure.
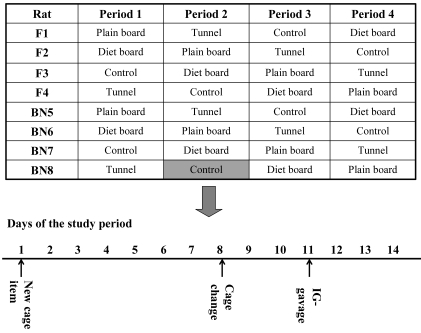
The experiment used a crossover design with 2-wk periods and a rotational order; the first item provided to each group was allocated randomly (Figure 2). Within both strains, variables included 2 kinds of dividers made of 2 intersecting aspen boards (34.0 × 14.7 × 3.2 cm; 21.1 × 14.7 × 3.2 cm) and a rectangular aspen tunnel (20.0 × 12.0 × 12.0 cm); control groups lacked these cage additions (Figure 1). One divider included round holes for food pellets, from which the rats had to gnaw wood to obtain food (diet board, Figure 1 A), whereas the other had no holes or food (plain board, Figure 1 B). The feed was added once weekly and weighed. Data on food consumption are presented elsewhere.21
Figure 2.
Cage item order in F344 and BN rats in 1 cage type (the order was the same in both IVCs and the open cages) and 1 crossover round, with the timing of the procedures performed within each round.
Figure 1.
Study groups. (A) Diet board. (B) Plain board. (C) Tunnel. (D) Control. Both rat strains (BN and F344) in both IVCs and open cages had an additional object for 2 wk.
Irradiated (25 kGy) pelleted feed (2016 Global Rodent Maintenance, Harlan Teklad, Bicester, UK) was offered to 3 groups (plain board, tunnel, and control groups) ad libitum, whereas the diet board group had the food pellets embedded snugly into drilled holes (12 mm) in the aspen board. The diet board reduces food consumption by 12% to 18% in both F344 and BN rats.21 The transition to the diet board was carried out without acclimation, as for the other items.
Eight rats, 4 BN and 4 F344, were implanted with radiotelemetry transmitters (model TA11PA-C40, Data Sciences International, St Paul, MN). The cylinder-shaped transmitter body (length, 3.0 cm; diameter, 1.5 cm) monitors blood pressure and locomotor activity by means of a fluid-filled catheter (length, 8 cm), which transmits signals to the electronic control module. The electronic module translates the signals into a digitized form and transmits them to the receiver plate located under the cage. The receiver detects the transmitted signal and converts it to a computer-readable form. For locomotor activity measurements, the telemetric receiver has 2 antennas, located at 90º to one another, and the receiver records the difference in signal strength when an animal is moving in relation to these antennas.
For implantation of the transmitters, the rats were anesthetized with the combination of fentanyl–fluanisone [Hypnorm (fentanyl citrate, 0.315 mg/mL; fluanisone, 10 mg/mL), Janssen Pharmaceutica, Beerse, Belgium] and midazolam [Dormicum (midazolam, 5 mg/mL), Hoffmann–La Roche AG, Grenzach-Wyhlen, Germany] at a dose of 0.15 to 0.20 mL per 100 g SC (1 part Hypnorm, 1 part Dormicum, and 2 parts sterile water). The abdominal area was clipped, scrubbed with 1% triclosan solution (MediScrub, Medichem International, Sevenoaks, UK) solution, and disinfected with chlorhexidine solution (5 mg/mL; Klorohexol, Leiras, Turku, Finland), and an ocular lubricant (Viscotears, Novartis Healthcare, Copenhagen, Denmark) was applied on both corneas. A sterile drape was placed over the surgical area, and a small area was cut away to enable a 3 cm incision to be made through the skin along the abdominal midline. The sterile transmitter was presoaked in sterile saline for at least 20 min before surgery and then placed into the abdominal cavity; the catheter was inserted into the abdominal aorta. The transmitter was sutured onto the abdominal wall with 4-0 absorbable suture (Ethilon II, Johnson and Johnson International, St Stevens Woluwe, Belgium) and the abdominal and skin incisions were closed with 5-0 suture (Vicryl, Johnson and Johnson International). After the surgery, the animals were given buprenorphine twice daily (0.01 to 0.05 mg/kg SC; Temgesic, Schering–Plough Europe, Brussels, Belgium), carprofen daily (5 mg/kg SC; Rimadyl, Vericore, Dundee, UK), and parenteral fluids for 3 d. The pain medication for each rat was titrated according to individual response. All rats initially were given buprenorphine at the highest dose for at least 2 d and carprofen medication for at least 3 d. The animals were allowed to recover for 10 d before the experiment was started.
After 1 wk of each 2-wk period, the rats were transferred to a clean cage between 11:00 and 13:00 by lifting the rats by the body with encircled fingers. Cage cleaning involved replacing the cage, all of the bedding and the water bottle; the cage lid and feed were retained. The tunnel and plain board were moved to the new cage, the diet board was changed. In the ad libitum feeding groups, more feed was added after weighing.
The implanted rats were gavaged 3 d after cage change between 09:00 and 10:00 h; they were lifted from the cage gently by grasping the chest. The grip was changed to the scruff of the neck when the rats were in the holder's lap.5 The rat was gently stroked twice from chin to the base of the tail, and a stainless steel gavage tube (length, 84 mm; shaft diameter, 1.2 mm) was passed through the esophagus into the stomach and maintained in that position for 3 s before being retracted slowly; nothing was administered. The time line of the study is illustrated in Figure 2.
Data processing and statistical analysis.
Means of locomotor activity, mean arterial pressure (MAP), and HR were processed at 5-min periods for the first hour after the procedure and for 17 h thereafter at 30-min periods, separately for the light and dark periods and for the 2 cage types. Mean baseline values of MAP and HR for each rat for the dark and light periods and both cage types were calculated from recordings obtained on day 7, the day before the procedures; these values were subtracted from the corresponding response values. Mixed-model repeated measures ANOVA (SPSS Windows, version 14.0, SPSS, Chicago, IL, USA) was used to assess means and parameter responses, and Bonferroni correction was used for posthoc comparisons. For activity calculation, group was used as a main effect and age as the covariate. For the MAP and HR calculation, activity and parameter baseline values were added into the covariates.
Response duration for both MAP and HR was calculated as described previously.5 The resulting durations were compared by mixed-model repeated measures ANOVA using group, strain, and cage type as main effects, and age as a covariate.
Individual mean values of control group on day 7 were calculated separately for light and dark periods; these values were processed to cage type- and strain-specific average night–day difference values.
Results
Cage change.
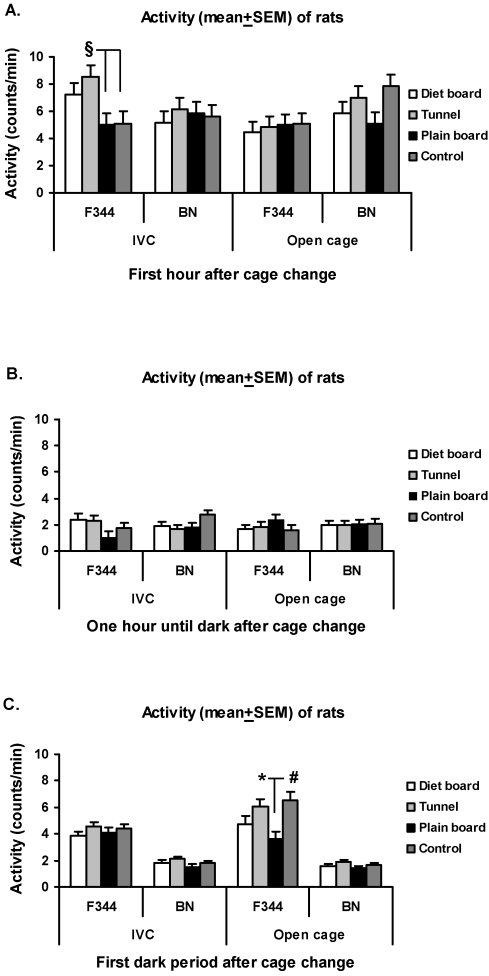
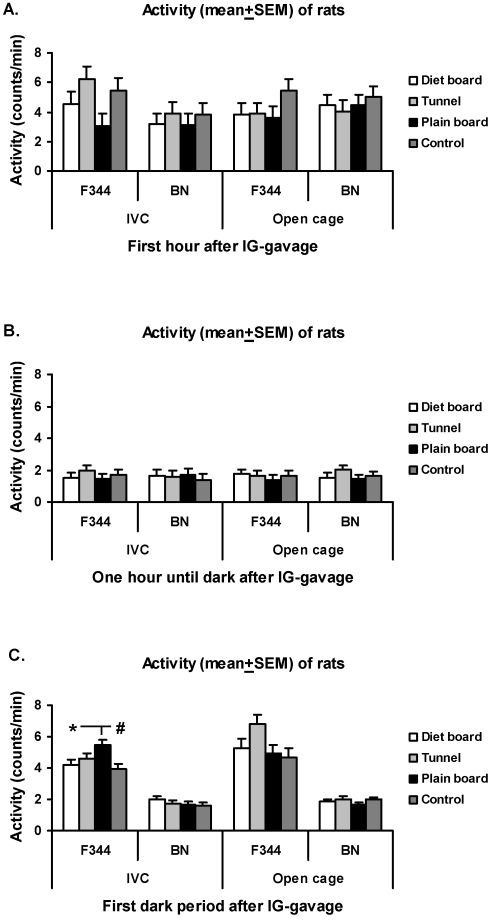
The locomotor activity response during the first hour after IVC cage change was higher (P < 0.001) in the tunnel group of F344 rats than in the control and in the plain board groups (Figure 3A). In the open-top cages during the first dark period, the F344 rats in the plain board group were significantly less active as compared with both control (P < 0.01) and tunnel (P < 0.05) groups (Figure 3C). Overall, the locomotor activity response decreased (P < 0.001) in both strains after the first hour (Figure 3). MAP and HR response durations exhibited no differences between the cage items. The night–day difference values for the MAP and HR in the control group before the procedures are illustrated in Table 1.
Figure 3.
Activity (mean ± SEM) of rats after cage change in IVCs and open-top cages. (A) First hour after cage change. (B) From 1 h after cage change to start of dark period. (C) First dark period after cage change. Overall there are 16 experimental units, 4 animals in 4 rounds. *, P < 0.05; #, P < 0.01; §, P < 0.001.
Table 1.
The night and day baseline values and night–day differences of MAP (mmHg) and HR (bpm) of control F344 and BN rats in 2 different cage types (IVC and open top).
| IVC |
Open top |
|||||||
| F344 |
BN |
F344 |
BN |
|||||
| MAP | HR | MAP | HR | MAP | HR | MAP | HR | |
| Night | 115.3 | 376.4 | 93.8 | 309.0 | 113.1 | 395.1 | 92.4 | 301.3 |
| Day | 108.9 | 320.9 | 92.0 | 277.6 | 105.2 | 327.1 | 91.1 | 282.8 |
| Night – day | 6.4 | 55.5 | 1.8 | 31.4 | 7.9 | 68.0 | 1.3 | 18.5 |
F344 rats and MAP response.
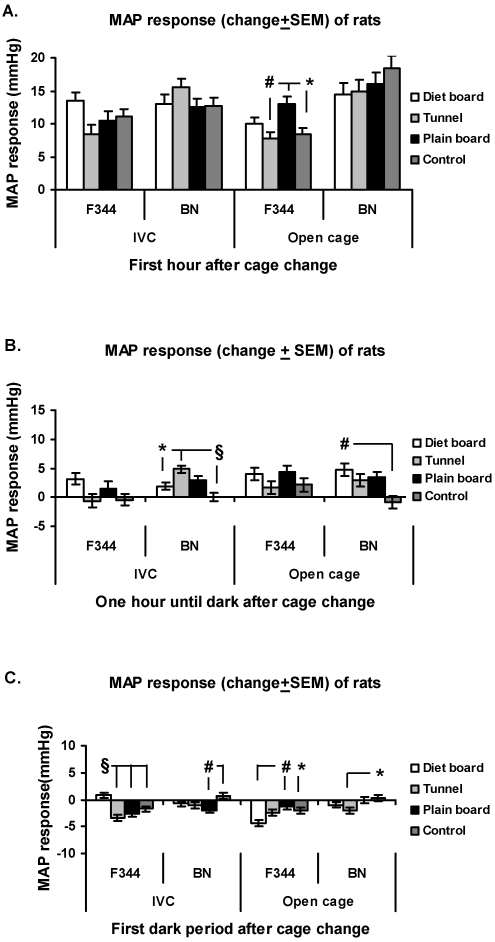
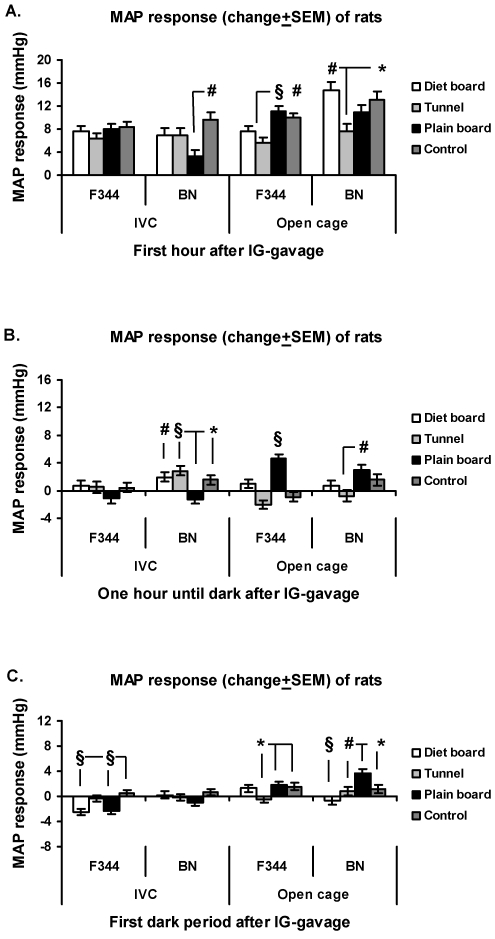
In the IVCs, the F344 rats displayed a significant (P < 0.001) difference between item MAP responses only during the subsequent dark period, during which the MAP of the diet board group was higher than that of the controls and the other 2 groups (Figure 4C). In the open cages during the first hour, the MAP responses experienced by the plain board F344 rats were significantly higher than those of the control group (P < 0.05) and the tunnel group (P < 0.01; Figure 4 A). Later, during the dark period, the MAP response was lower in the diet board group compared with the control (P < 0.05) and plain board (P < 0.01) F344 groups (Figure 4 C).
Figure 4.
MAP response (change from baseline ± SEM) of rats to cage change in IVCs and open-top cages. (A) First hour after cage change. (B) From 1 h after cage change to start of dark period. (C) First dark period after cage change. The y axis scale is the same in all figures. Overall there are 16 experimental units, 4 animals in 4 rounds. *, P < 0.05; #, P < 0.01; §, P < 0.001.
BN rats and MAP response.
No differences between groups of BN rats were detected in either cage type during the first 60 min after cage change (Figure 4 A). During the remaining light period in the IVCs, the BN tunnel group displayed a significantly larger MAP response than did the control (P < 0.001) and diet board (P < 0.05) groups. In the open-top cages, the diet board BN animals exhibited a significantly (P < 0.01) higher MAP response compared with controls (Figure 4 B). During the subsequent dark phase in the IVCs, the plain board BN group expressed a significantly (P < 0.01) smaller MAP response than did the control group, and in the open-top cages, the tunnel group exhibited a significantly (P < 0.05) lower MAP response than did control animals (Figure 4 C).
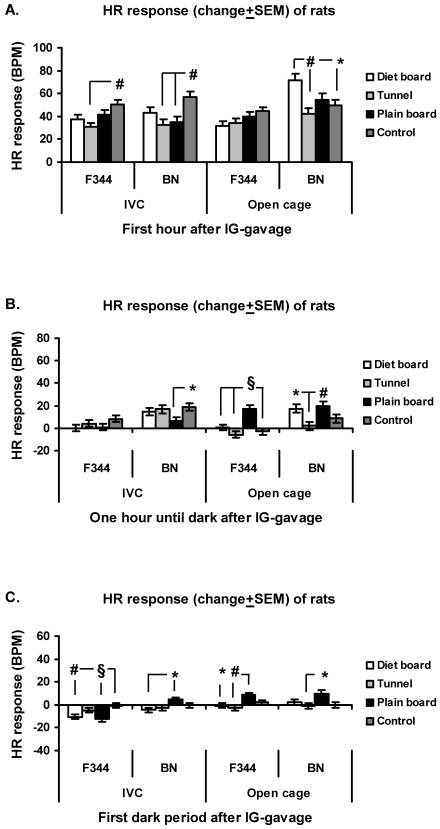
F344 rats and HR response.
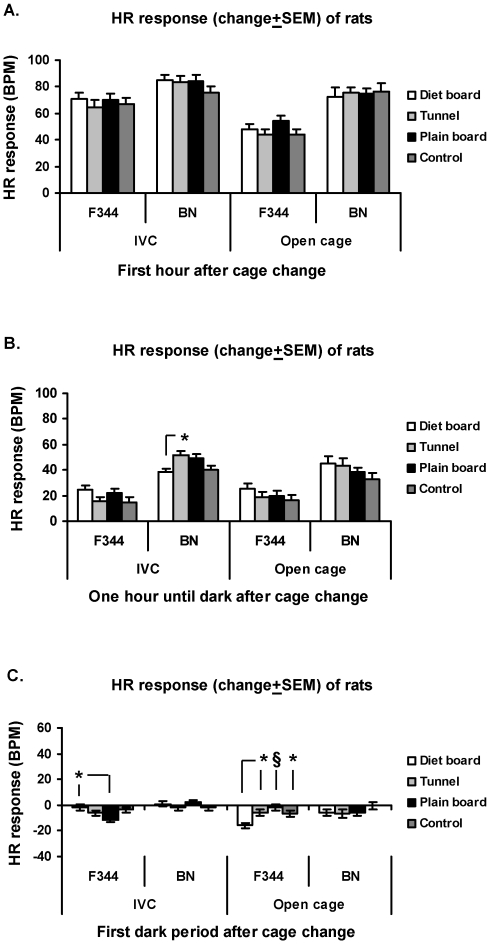
In both cage types of F344 rats, no differences HR response were seen before the dark period (Figure 5 A, B). The HR response of IVC F344 rats during the first dark phase was significantly (P < 0.05) lower in those exposed to the plain board as compared with the diet board. In open-top cages, the HR response was significantly decreased in F344 rats with the diet board in comparison with control rats (P < 0.05) and the plain board (P < 0.001) and tunnel (P < 0.05) groups (Figure 5 C).
Figure 5.
HR response (change from baseline ± SEM) of rats to cage change in IVCs and open-top cages. (A) First hour after cage change. (B) From 1 h after cage change to start of dark period. (C) First dark period after cage change. The y axis scale is the same in all figures. Overall there are 16 experimental units, 4 animals in 4 rounds. *, P < 0.05; #, P < 0.01; §, P < 0.001.
BN rats and HR response.
Regardless of cage type, no differences in HR response between groups of BN rats were detected during the first hour or the first dark period after the cage change (Figure 5 A, C). During the period after the first hour until the start of the dark period in IVCs, there was a significantly (P < 0.05) higher HR response in BN rats with the tunnel compared with the diet board group (Figure 5B).
IG-gavage
Locomotor activity response to gavage did not differ between groups of both strains in both cage types until the first dark period, when the F344 rats in IVCs with plain boards were significantly more active than control rats (P < 0.01) and those with diet boards (P < 0.05; Figure 6 A). The locomotor activity response in both strains and cage types diminished (P < 0.001) after the first hour (Figure 6). There were no significant differences in the durations of the MAP and HR responses.
Figure 6.
Activity (mean ± SEM) of rats after gavage in IVCs and open-top cages. (A) First hour after cage change. (B) From 1 h after cage change to start of dark period. (C) First dark period after cage change. Overall there are 16 experimental units, 4 animals in 4 rounds. *, P < 0.05; #, P < 0.01.
F344 rats and MAP response.
The F344 rats living in open-top cages and with access to the tunnels had a significantly smaller MAP response during the first hour after gavage than did control (P < 0.01) and plain board (P < 0.001) groups, whereas from that point until dark, the plain board group exhibited a higher (P < 0.001) MAP response compared with those of all other groups, including the controls (Figure 7 A, B). During the subsequent dark phase, F344 rats housed in IVCs with diet boards or plain boards showed smaller (P < 0.001 for both groups) MAP responses than that of the control group, but in open-top cages, the F344 rats in the tunnel group had a lower (P < 0.05) response than did control and plain board rats (Figure 7 C).
Figure 7.
MAP response (change from baseline ± SEM) of rats to gavage in IVCs and open-top cages. (A) First hour after cage change. (B) From 1 h after cage change to start of dark period. (C) First dark period after cage change. The y axis scale is the same in all figures. Overall there are 16 experimental units, 4 animals in 4 rounds. *, P < 0.05; #, P < 0.01; §, P < 0.001.
BN rats and MAP response.
BN rats in IVCs experienced a lower (P < 0.01) MAP response when the plain board was in the cage during the first hour after gavage, compared with controls. During the same period, the BN rats living in open-top cages with tunnels had a significantly lower MAP response than did control rats (P < 0.05) or those with diet boards (P < 0.01; Figure 7 A). During the subsequent light period (before dark), the MAP response in the IVC plain board group was decreased compared with those of control (P < 0.05), diet board (P < 0.01) and tunnel (P < 0.001) groups of BN rats. BN rats in open-top cages with plain boards displayed significantly (P < 0.01) greater MAP responses than did those with tunnels (Figure 7 B). During the dark in IVCs, the response differences disappeared, but in open-top cages, the MAP responses of the plain board group of BN rats were still significantly greater than those in control (P < 0.05), tunnel (P = 0.01), and diet board (P < 0.001) groups (Figure 7 C).
F344 rats and HR response.
Significant response differences during the first hour after gavage were seen only in rats in IVCs; that is, HR response was significantly (P < 0.01) lower in the tunnel group compared with controls (Figure 8 A). During the remainder of the light period in open-top cages, the plain board group displayed a significantly (P < 0.001 for all comparisons) larger HR response compared with that of controls, rats with diet boards, and with a rats with tunnels (Figure 8 B). During the subsequent dark phase, groups exposed to the diet board (P < 0.01) and plain board (P < 0.001) in IVCs had decreased HR responses when compared with those of controls, and in open-top cages, the plain board group of F344 rats showed a significantly higher HR response than did the diet board (P < 0.05) and tunnel (P < 0.01) groups (Figure 8 C).
Figure 8.
HR response (change from baseline ± SEM) of rats to gavage in IVCs and open-top cages. (A) First hour after cage change. (B) From 1 h after cage change to start of dark period. (C) First dark period after cage change. The y axis scale is the same in all figures. Overall there are 16 experimental units, 4 animals in 4 rounds. *, P < 0.05; #, P < 0.01; §, P < 0.001.
BN rats and HR response.
During the first hour after gavage, the BN rats in IVCs with tunnels or plain boards had smaller (P < 0.01 for both comparisons) HR response than did controls, whereas in open-top cages, the diet board group had higher HR responses than did controls (P < 0.05) and the tunnel group (P < 0.01; Figure 8 A). During the remaining light period, the plain board group of BN rats in IVCs displayed a significantly (P < 0.05) lower HR response than did controls, whereas in open-top cages, the BN rats in the tunnel group exhibited a significantly (P < 0.01 to 0.05) lower response compared with those of the rats with the 2 types of board (Figure 8 B). During the subsequent dark phase, the rats in the plain board group exhibited a significantly (P < 0.05) higher HR response compared with the diet board group in the IVCs and those provided with a tunnel and living in open-top cages (Figure 8 C).
Discussion
Virtually nothing is known about whether cage objects alter the responses of rats to routine care and research procedures (for example, cage change and gavage). Most published studies on cage change or gavage7,8,13,27,29,31-33,40 either lacked cage items or details regarding the objects provided or provided the same item for all groups. Only 1 study34 has addressed the effect of a multifaceted enrichment program on procedures in rats. The present study evaluates the effect of 2 different objects (a tunnel and dividing boards), 1 of which was used with and without restricted feeding. To achieve better representation of the rat as a species, 2 inbred strains of rats (F344 and BN) were used.16
The current study demonstrates that the cardiovascular response to both cage change and gavage in F344 and BN rats is modified by the cage item added and the strain of rat evaluated. These intraspecies differences in cardiovascular responses are not surprising, given that these rat strains differ extensively in their baseline systolic and diastolic blood pressures and HR,37 plasma corticosterone,28 and brain and pituitary mineralocorticoid receptor levels.23 These strains also differ in their motor activity level, diurnal rhythm21,37 and behavior.25,26,39 Therefore, the effect of cage objects on the procedures appears to include a genetic component. A previous study34 also showed a difference between SHR and SD rats in responses to procedures, and the authors concluded that making generalized recommendations to the animal care community regarding rat enrichment programs is difficult.
Cardiovascular telemetry allows continuous recording. The items themselves resulted in a variable baseline (Table 1), but this variability was accommodated through adding item-specific individual baseline values as covariates for both day and night responses. Consequently, the statistically significant differences obtained can be considered as true differences between groups. Overall, even small differences may be important because these procedures are done in many animals.
The current study assessed responses to cage change during 3 subsequent time windows. The response during the first hour after the procedure is considered to result from the combined effects of lifting and transferring the rat to a new cage and its exposure to the new environment. The period between the first hour after a procedure until the start of the dark phase is considered to reflect the rat's reaction to the new environment during the inactive (that is, lights-on) period. Finally, the subsequent 12-h active period should reveal any long-lasting consequences of the cage items.
The immediate MAP and HR responses to gavage appear to be smaller in magnitude than those associated with cage change (Figure 4 A and 7 A). In 1 study,40 the immediate responses in blood pressure and HR to cage change and gavage in an outbred Wistar stock were essentially the same as ours. Another study30 found a larger increase in the corticosterone level when rats were moved to a novel environment compared with that associated with short-term handling. Gavage is a short-term procedure that is usually considered more invasive than handling. A feature common to both gavage and handling is that the rats are returned back to the familiar home cage. In the cage change procedure, the animals are relocated to a new environment with new odors; therefore the more intense response to cage change is not surprising.
The locomotor activity of the rats increased immediately after placement into the clean cages (Figure 3). This finding agrees with several studies10,27,29 in which cage change has been shown to increase the motor activity of rats. Clean cages also change the behaviors displayed by rats: grooming, eating, drinking, resting, rearing, and bedding manipulation all decrease, whereas the walking and skirmishing increase immediately after cage change.10
Skirmishing or fighting of rats after cage changing may be attributable to the establishment of the dominance hierarchies within the group and is related to territory4 (for example, the new cage). Rats investigate their surroundings by ambulating and rearing.18 As a result of the increased exploratory behavior, cardiovascular parameters and activity increase after cage changes. Cage objects can provide an odor cue that makes the new cage more familiar to rats.
The cage change procedure increased MAP, HR, and locomotor activity in all groups, but the values returned to near baseline within 1 h (Figures 3 to 5). These findings are consistent with other studies,13,31-33 that also found increased blood pressure and HR after cage changes. However, in the cited studies, both parameters returned to baseline within 60 to 180 min.
Seemingly minor differences in the cage changing procedure can have cardiovascular effects in rats. The HR of the rats is increased even by moving the cage to a different location in the cage rack.17 In another study,29 if cage changes took place in the morning, during the resting period, the systolic and diastolic blood pressures and HR responses were larger than those when cage changes occurred during the active period in the evening.29 Another study1 reported that if rats experienced a cage change during the light period, they slept less and had more chromodacryorrhea, reduced thymus weight, increased aggression, and less object-directed behavior. Consequently, the authors suggested that performing husbandry procedures during the dark period rather than the light period might improve the wellbeing of rats. This timing may be impractical, however. In our current study, cages were changed at about noon—clearly within working hours.
We used strain-specific reference values (that is, night–day differences in MAP and HR calculated for the control group) to assess whether statistically significant differences detected in the current study have biologic or welfare relevance. Based on this comparison, the statistically significant MAP responses to both cage change and gavage for F344 rats in IVCs were not greater than the night–day MAP difference for this strain (that is, 6.4 mm Hg) or the corresponding HR value [that is, 55.5 beats per minute (bpm)]. Therefore, a biologically valid effect attributable to cage objects is not apparent in the current study.
The presence of a plain board in IVC-housing BN rats influenced the MAP response to gavage until dark (Figure 7 A and B). This significant difference exceeded the BN-specific MAP night–day difference (1.8 mm Hg). However, the corresponding HR differences (Figure 8 A through C) were less than the HR night–day difference (31.4 bpm).
The MAP response to cage change of BN rats in the tunnel group, as compared with both control and diet board groups, was greater during the second sampling window (Figure 4 B), and the magnitude of the difference exceeded the corresponding night–day difference. This effect appeared to be related to the light phase, because during the subsequent dark period, the presence of the plain dividing board did not change the MAP as compared with the controls (Figure 4 C). The between-groups HR differences were not close to the corresponding night-day difference. BN rats generally rested on top of rather than inside of the tunnel and tend to be aggressive toward their cagemates. A single tunnel may not have provided sufficient numbers of compartments to function as safe havens compared with those created by inclusion of the plain board. This explanation could account for the MAP changes that occurred when a tunnel was present in the cage.
In open-top cages, the F344 night–day difference of the control group was 7.9 mm Hg for MAP and 68.0 bpm for HR. Although many statistically significant differences were detected in the responses to both procedures (Figures 4, 5, 7, and 8), none of them were large enough to achieve biologic significance.
Compared with the F344 strain, BN rats in open-top cages showed lower night–day differences than they had shown 8 wk earlier when they were in IVCs (Table 1). In BN rats, the presence of the tunnel changed responses to gavage during the first hour in terms of the MAP response, as compared with the diet board and control groups (Figure 7 A), although with respect to HR, the tunnel-associated difference was significant only in comparison to the diet board (Figure 8 A). Thereafter the presence of the plain board lessened the response, especially in MAP during the subsequent dark period (Figure 7 B, C). A similar trend occurred for HR, but a biologically meaningful effect occurred only for comparison of the plain board and tunnel during the 5 h before the dark period (Figure 8 B).
After the cage change, the BN rats showed no significant differences in HR. The change in MAP in BN rats supplied with a diet board, as compared with the controls, reduced the response during the period from 1 h after cage change until dark (Figure 4 B). Perhaps the new diet board allowed easier access to food than did the old, worn-out board, thereby leading to the changes in MAP.
When living in open-top cages, rats can smell other rats in the room. This situation may influence postprocedural responses. Comparison of the cage types used in the present study is difficult due to the 8-wk age difference in the rats in the 2 cage types and the different physical environments.20
Overall, cardiovascular telemetry allowed us to assess the impact of cage objects on responses to procedures. However, the importance of statistically significant effects must be interpreted with regard to biologic significance. In this regard, we detected no meaningful effect of cage objects on the responses of F344 rats to either cage change or gavage. In BN rats, the presence of the plain board in the IVC modified the responses to both procedures. The only effect observed in open-top cages during the first 60 min was associated with gavage and the presence of the tunnel in BN rats. In conclusion, the response of rats to various husbandry procedures appears to be specific to strain and cage objects, and perhaps also to age and cage type, complicating the establishment of valid general recommendations.
Acknowledgments
This study was supported by the Academy of Finland, the Finnish Ministry of Education, ECLAM and ESLAV Foundation, the North-Savo Cultural Foundation, the Finnish Research School for Animal Welfare, the Oskar Öflund Foundation, and the University of Kuopio. We acknowledge Mr Heikki Pekonen for help with the surgical procedures and Ms Virpi Nousiainen for carrying out the gavage procedure.
References
- 1.Abou-Ismail UA, Burman OHP, Nicol CJ, Mendl M. 2008. Let sleeping rats lie: does the timing of husbandry procedures affect laboratory rat behavior, physiology, and welfare? Appl Anim Behav Sci 111:329–341 [Google Scholar]
- 2.Alban L, Dahl PJ, Hansen AK, Hejgaard KC, Jensen AL, Kragh M, Thomsen P, Steensgaard P. 2001. The welfare impact of increased gavaging doses in rats. Anim Welf 10:303–314 [Google Scholar]
- 3.Azar TA, Sharp JL, Lawson DM. 2008. Effect of housing rats in dim light or long nights on heart rate. J Am Assoc Lab Anim Sci 47:25–34 [PMC free article] [PubMed] [Google Scholar]
- 4.Barnett SA. 1958. An analysis of social behaviour in wild rats. Proc Zool Soc Lond 130:107–152 [Google Scholar]
- 5.Batūraitė Z, Voipio HM, Rukšenas O, Luodonpää M, Leskinen H, Apanavičiene N, Nevalainen T. 2005. Comparison of and habituation to four common methods of handling and lifting of rats with cardiovascular telemetry. Scand J Lab Anim Sci 32:137–148 [Google Scholar]
- 6.Björk E, Nevalainen T, Hakumäki M, Voipio HM. 2000. R-weighting provides better estimation for rat hearing sensitivity. Lab Anim 34:136–144 [DOI] [PubMed] [Google Scholar]
- 7.Bonnichsen M, Dragsted N, Hansen AK. 2005. The welfare impact of gavaging laboratory rats. Anim Welf 14:223–227 [Google Scholar]
- 8.Brown AP, Dinger N, Levine BS. 2000. Stress produced by gavage administration in the rat. Contemp Top Lab Anim Sci 39:17–21 [PubMed] [Google Scholar]
- 9.Burn CC, Peters A, Day MJ, Mason GJ. 2006. Long-term effects of cage-cleaning frequency and bedding type on laboratory rat health, welfare, and handleability: a cross laboratory study. Lab Anim 40:353–370 [DOI] [PubMed] [Google Scholar]
- 10.Burn CC, Peters A, Mason GJ. 2006. Acute effects of cage cleaning at different frequencies on laboratory rat behavior and welfare. Anim Welf 15:161–171 [Google Scholar]
- 11.Chamove AS. 1989. Environmental enrichment: a review. Anim Technol 40:155–178 [Google Scholar]
- 12.Council of Europe 2007. [Internet] Appendix A of the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (ETS No. 123). Guidelines for accommodation and care of animals. Available at http://conventions.coe.int/Treaty/en/Treaties/Html/123.htm.
- 13.Duke JL, Zammit TG, Lawson DM. 2001. The effects of routine cage-changing on cardiovascular and behavioural parameters in male Sprague–Dawley rats. Contemp Top Lab Anim Sci 40:17–20 [PubMed] [Google Scholar]
- 14.Eskola S, Lauhikari M, Voipio HM, Nevalainen T. 1999. The use of aspen blocks and tubes to enrich the cage environment of laboratory rats. Scand J Lab Anim Sci 26:1–10 [Google Scholar]
- 15.European Union 2007. [Internet] Commission recommendation on guidelines for the accommodation and care of animals used for experimental and other scientific purposes (2007/526/EC). Available at: http://eur-lex.europa.eu/JOHtml.do?uri=OJ:L:2007:197:SOM:EN:HTML
- 16.Festing M, Overend P, Gaines Das R, Borja MC, Berdoy M. 2004. The design of animal experiments. Laboratory animal handbooks, no. 14. London: The Royal Society of Medicine Press [Google Scholar]
- 17.Gärtner K, Büttner D, Döhler K, Friedel R, Lindena J, Trautschold I. 1980. Stress response of rats to handling and experimental procedures. Lab Anim 14:267–274 [DOI] [PubMed] [Google Scholar]
- 18.Hughes RN. 1968. Behaviour of male and female rats with free choice of two environments differing in novelty. Anim Behav 16:92–96 [DOI] [PubMed] [Google Scholar]
- 19.Jegstrup IM, Vestergaard R, Vach W, Ritskes-Hoitinga M. 2005. Nest-building behaviour in male rats from three inbred strains: BN/HsdCpb, BDIX/OrlIco and LEW/Mol. Anim Welf 14:149–156 [Google Scholar]
- 20.Kemppinen N, Meller A, Björk E, Kohila T, Nevalainen T. 2008. Exposure in the shoebox; comparison of physical environment of IVC- and open rat cages. Scand J Lab Anim Sci 35:97–103 [Google Scholar]
- 21.Kemppinen N, Meller A, Mauranen K, Kohila T, Nevalainen T. 2008. Work for food – a solution for restricted feeding in group housed rats? Scand J Lab Anim Sci 35:81–90 [Google Scholar]
- 22.Krohn TC, Hansen AK, Dragsted N. 2003. Telemetry as a method for measuring the impact of housing conditions on rats’ welfare. Anim Welf 12:53–62 [Google Scholar]
- 23.Marissal–Arvy N, Mormède P, Sarrieau A. 1999. Strain differences in corticosteroid receptor efficiencies and regulation in Brown Norway and Fischer 344 rats. J Neuroendocrinol 11:267–273 [DOI] [PubMed] [Google Scholar]
- 24.Quignon P, Giraud M, Rimbault M, Lavigne P, Tacher S, Morin E, Retout E, Valin AS, Lindblad-Toh K, Nicolas J, Galibert F. 2005. The dog and rat olfactory receptor repertoires. Genome Biol 6:R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramos A, Berton O, Mormede P, Chalouff F. 1997. A multiple-test study of anxiety-related behaviours in six inbred rat strains. Behav Brain Res 85:57–69 [DOI] [PubMed] [Google Scholar]
- 26.Rex A, Sondern U, Voigt JP, Franck S, Fink H. 1996. Strain differences in fear-motivated behavior of rats. Pharmacol Biochem Behav 54:107–111 [DOI] [PubMed] [Google Scholar]
- 27.Saibaba P, Sales GD, Stodulski G, Hau J. 1996. Behaviour of rats in their home cages: daytime variations and effects on routine husbandry procedures analysed by time sampling techniques. Lab Anim 30:13–21 [DOI] [PubMed] [Google Scholar]
- 28.Sarrieau A, Mormède P. 1998. Hypothalamic–pituitary–adrenal axis activity in the inbred Brown Norway and Fischer 344 rat strains. Life Sci 62:1417–1425 [DOI] [PubMed] [Google Scholar]
- 29.Schnecko A, Witte K, Lemmer B. 1998. Effects of routine procedures on cardiovascular parameters of Sprague–Dawley rats in periods if activity and rest. J Exp Anim Sci 38:181–190 [Google Scholar]
- 30.Seggie JA, Brown GM. 1975. Stress response pattern of plasma corticosterone, prolactin, and growth hormone in rat, following handling or exposure to novel environment. Can J Physiol Pharmacol 53:629–637 [DOI] [PubMed] [Google Scholar]
- 31.Sharp J, Zammit T, Azar T, Lawson D. 2002. Stress-like responses to common procedures in male rats housed alone or with other rats. Contemp Top Lab Anim Sci 41:8–14 [PubMed] [Google Scholar]
- 32.Sharp JL, Zammit TG, Lawson DM. 2002. Stress-like responses to common procedures in rats: effect of the estrous cycle. Contemp Top Lab Anim Sci 41:15–22 [PubMed] [Google Scholar]
- 33.Sharp J, Zammit T, Azar T, Lawson D. 2003. Stress-like responses to common procedures in individually and group-housed female rats. Contemp Top Lab Anim Sci 42:9–18 [PubMed] [Google Scholar]
- 34.Sharp J, Azar T, Lawson D. 2005. Effects of a cage enrichment program on heart rate, blood pressure, and activity of male Sprague–Dawley and spontaneously hypertensive rats monitored by radiotelemetry. Contemp Top Lab Anim Sci 44:32–40 [PubMed] [Google Scholar]
- 35.Stauffacher M, Peters A, Jennings M, Hubrecht R, Holgate B, Francis R, Elliot H, Baumans V, Hansen AK. 2002. [Internet]. Future principles for housing and care of laboratory rodents and rabbits. Report for the revision of the Council of Europe Convention ETS 123 Appendix A for rodents and rabbits. Part B. Available at www.coe.int/t/e/legal_affairs/legal_co-operation/biological_safety%2C_use_of_animals/Laboratory_animals/
- 36.Townsend P. 1997. Use of in-cage shelters by laboratory rats. Anim Welf 6:95–103 [Google Scholar]
- 37.Van Den Brandt J, Kovács P, Klöting I. 1999. Blood pressure, heart rate, and motor activity in 6 inbred rat strains and wild rats (Rattus norvegicus): a comparative study. Exp Anim 48:235–240 [DOI] [PubMed] [Google Scholar]
- 38.van der Harst JE, Fermont PCJ, Bilstra AE, Spruijt BM. 2003. Access to enriched housing is rewarding to rats as reflected by their anticipatory behaviour. Anim Behav 66:493–504 [Google Scholar]
- 39.van der Staay FJ, Blokland A. 1996. Behavioral differences between outbred Wistar, inbred Fischer 344, Brown Norway, and hybrid Fischer 344 × Brown Norway rats. Physiol Behav 60:97–109 [DOI] [PubMed] [Google Scholar]
- 40.Okva K, Tamoseviciute E, Ciziute A, Pokk P, Ruksenas O, Nevalainen T. 2006. Refinements for intragastric gavage in rats. Scand J Lab Anim Sci 33:243–252 [Google Scholar]