Abstract
Positive reinforcement training (PRT) has successfully been used to train diverse species to execute behaviors helpful in the everyday care and wellbeing of the animals. Because little information is available about training sooty mangabeys (Cercocebus atys atys), we analyzed PRT with a group of 30 adult males as they were trained to shift from 1 side of their enclosure to the other. Over a 4-mo period we conducted 57 training sessions totaling 26.5 h of training and recorded compliance information. During training, compliance increased from 76% of the animals during the first 5 training sessions to 86% of the animals shifting during the last 5 sessions. This result indicated progress but fell short of our goal of 90% compliance. After 25 training sessions, problem-solving techniques were applied to help the consistently noncompliant animals become more proficient. The techniques included reducing social stress by shifting animals so that noncompliant monkeys could shift into an unoccupied space, using more highly preferred foods, and ‘jackpot’-sized reinforcement. To determine whether social rank affected training success, animals were categorized into high, medium, and low dominance groups, based on 7 h of behavioral observations. A Kruskal–Wallis test result indicated a significant difference in compliance according to the category of dominance. Although training a group this large proved challenging, the mangabeys cooperated more than 90% of the time during follow-up sessions. The training program improved efficiency in caring for the mangabeys.
Abbreviation: PRT, positive reinforcement training
Positive reinforcement training (PRT) has successfully been used to train diverse primate species to execute a variety of behaviors helpful in daily husbandry procedures, facilitating research, and promoting the wellbeing of the animals. Over the last 2 decades increasing emphasis has been placed on the use of PRT techniques, as nonhuman primates living in zoos and laboratories have been trained to cooperate with a variety of husbandry, research, and veterinary procedures. For example, PRT has been used to facilitate daily colony management such as moving animals within or between their enclosures3 and into transport cages. Positive reinforcement training facilitates veterinary and research procedures by training animals to cooperate with intramuscular7,21,23,28 and subcutaneous injections18,23 and to present for voluntary blood,4,8,12 semen17,23 and urine 12,27 collection. Training has also been used to improve social dynamics in primate groups by reducing competition and aggression over food2 and increasing affiliative behaviors between conspecifics.22
At its most basic level, PRT is a form of operant conditioning in which the animal receives a primary reinforcer or reward, such as food, for exhibiting a particular behavior. Receiving the reward increases the likelihood that the animal will exhibit the behavior again in the future. The reward typically is paired with a clicking sound (called a conditioned reinforcer or ‘bridge’), which serves to pinpoint the exact moment the desired behavior occurs. After multiple presentations of the conditioned reinforcer with the primary reinforcer, the clicking sound develops a meaning of “good job”20 to the animal. Positive reinforcement training contrasts with more traditional training techniques such as negative reinforcement. In negative reinforcement, the animal may be coerced into complying by applying a stimulus that an animal wants to avoid and removing that same stimulus when the animal complies.20 For example, directing water from a hose at an animal might be used to get the animal to move from inside to outside or the pressure of a squeeze mechanism in a cage is used to get the animal to come forward and hold a position. Both PRT and negative reinforcement training can be effective, but PRT is believed to have additional benefits such as reducing distress, increasing choice and control for the animal, and therefore improving wellbeing.7,10,11 The general principles of PRT are species-independent, and the training techniques are used successfully across taxa. Many species of nonhuman primates such as chimpanzees,2,3,7,8,12,16-18,21,27,28 macaques,4,6,19 and tamarins25 have been trained, yet no information has been published on the training of mangabeys (Cercocebus spp.), even though they live in both zoo and laboratory colonies.
Several publications have reported training monkeys to move between portions of their enclosures and to enter smaller transport cages or chute systems.5,6,13,19,24,29 These articles describe the use of a combination of PRT and negative reinforcement strategies to coerce animals into complying with the requested behavior and then rewarding them. Several of these publications6,19 describe animal care staff entering the monkeys’ enclosures as a part of the training process. None of these articles relied solely on PRT techniques to achieve their goals.
For this study, we wanted to train a group of mangabeys to move between different portions of their enclosure, on cue, to allow for daily cleaning and cage maintenance. To protect personnel, we wanted to conduct the training without having people enter the mangabey enclosure, and we chose to use only PRT procedures for the presumed benefits to the wellbeing of the mangabeys. This approach was similar to that of to an earlier study with chimpanzees,3 in which the animals were taught to shift between enclosures by using only PRT. We recorded the training time and number of sessions needed to train the mangabeys to move throughout their enclosure on cue. During the training process, we suspected that the dominance ranking of individual animals was affecting their success rate and therefore assessed this factor's contribution. In addition, we found that a few animals were not cooperating with the request to shift even after considerable training. We therefore used a problem-solving method to develop hypotheses about the lack of compliance and to test possible solutions to improve the performance of this subset of our test population.
Materials and Methods
Subjects and housing.
The subjects for this study were a group of 30 adult (age, 8 to 21 y) male sooty mangabeys (Cercocebus atys atys). The group was formed in 2001 and recently was moved into new housing at the Yerkes National Primate Research Center of Emory University. The group was housed in 12 adjoining runs measuring 5.18 × 2.44× 2.44 m each. There were 6 connected runs on each side of a corridor that were linked by a single tunnel (3.05 × 0.91 × 0.91 m). Each run had 2 guillotine doors leading to the adjacent run. The guillotine doors were accessed by inner and outer corridors. The goal of this study was to train the mangabeys, using only PRT, to move (shift) on cue from 1 side of their enclosure through a tunnel to the other side. The subjects needed to shift twice daily so that both sides of their enclosure could be cleaned in a safe and efficient manner. The mangabeys were fed a standard primate chow (5037, LabDiet, Richmond, IN) twice daily and given produce and other enrichment daily (destructible items, foraging devices, and food enrichment); water was provided ad libitum.
The mangabeys were maintained in accordance with federal regulations and the recommendations from the Guide for the Care and Use of Laboratory Animals15 at an AAALAC-accredited facility. All research conducted with these mangabeys was approved by the Institutional Animal Care and Use Committee of Emory University.
Training method.
Our training plan used only PRT. Trainers gave the subjects a verbal cue (“over”) along with a visual cue (moving the hand from one side of the body to the other). Initially 2 trainers were present to close the doors on the inner and outer corridors. As the animals shifted thorough the runs, the guillotine doors were closed behind them. When they moved through the tunnel, they were given verbal praise of “good over,” the tunnel door was closed, and a preferred food was given as a primary reinforcer. If a subject did not go through the tunnel, trainers worked with the subject on an individual basis, using the same verbal and visual cues, and the behavior was reinforced after the animal moved through the guillotine door toward the tunnel. If the subject chose not to shift after several attempts, the doors were closed, and the training session ended with no consequence to those mangabeys that did not comply. Animals that did not comply were locked in the run, and the runs on both sides of them were cleaned. Once the runs were cleaned, the doors were opened and the cues to shift were given. Most of the time, the animals moved over. If the animal did not move, the doors were left open until they moved into a clean run, and then the remaining run was cleaned. Subjects that did not comply with the cue to shift were recorded as noncompliant for that training session. Noncompliance was used as a dependent variable throughout the study.
Training sessions were between 15 and 40 min (mean, 27.8 min) in duration for the group. The subjects were trained 3 to 5 times each week during a 4-mo period. The group was considered to be reliably trained when 90% of the group shifted on cue 5 times in a row.
Behavioral observations.
Two observers recorded 7 h of behavioral observation to measure the general dominance status among subjects. Affilative, aggressive, and submissive behaviors and the identities of the initiator and recipient were recorded (Figure 1). A modification of an all-occurrence method1 was used. Because the observer could not see all of the animals at the same time, the observer walked through the enclosure and recorded all behaviors that were observed. The index of concordance for interobserver reliability was 0.913.
Figure 1.
The ethogram26 used during behavioral observations.
Dominance hierarchy.
Once the less-compliant animals were identified, we were interested in determining whether there was a relationship between dominance rank and compliance with shifting. The behavioral observations were used to create a dominance hierarchy. The behavioral observations were compiled by recording the number of interactions between all pairs of subjects and placing the collected data into a matrix. The matrix was organized so that animals that always showed dominant behavior or were never supplanted were put on the top, whereas animals that were always supplanted were placed on the bottom of the hierarchy. Other subjects then were arranged in the hierarchy such that the number of interactions in which they were supplanted increased.14 Because there were not sufficient interactions between all the animals to create a complete dominance hierarchy, the animals were ranked into 3 categories—high, medium, and low rank—based on these data.
Statistics.
The relationship between dominance category and compliance with the shifting request was tested by a Kruskal–Wallis statistical test (SPSS version 13.0, Chicago, IL). This test was chosen because of the lack of homogeneity of variance across groups and the modest sample size.
Problem solving.
We also used a standardized problem-solving process9 to attempt to increase compliance with shifting for the mangabeys. The problem-solving process included a group discussion of everyone working with the mangabeys on the training strategy, social dynamics of the group, enrichment strategies, and animal management procedures9 to identify any potential factors of compliance with training. After the problem-solving session, we hypothesized that social dynamics (that is, dominance relationships) might be having a negative effect on the compliance of some subjects and that the food rewards being used might be ineffective for some animals. We implemented different strategies based on these 2 hypotheses to try to increase compliance. To reduce the likelihood of a more-dominant animal intimidating less-dominant animals from moving into a certain portion of the enclosure, we shifted all of the subjects who would readily move and then emptied the run across from the tunnel so that the less-compliant animals could enter an area without any other animals present. In addition, we used more desirable reinforcers (such as yogurt, banana, grapes) and ‘jackpots’ (a large volume of a reinforcer; such as an entire ear of corn, a whole banana) when rapid progress was made in training.
Results
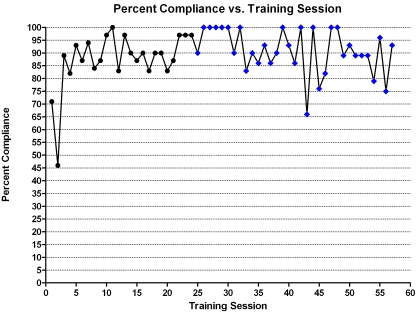
Over a 4-mo period, we conducted 57 training sessions totaling 26.5 h of training time. In the first 5 training sessions, 76% of the monkeys cooperated with the request to move, indicating that the monkeys were already cooperating to some extent even though no explicit training had been done. During the last 5 of the 57 sessions, 86% of the monkeys cooperated, indicating progress but falling short of the reliable performance we intended to obtain. Inspection of the data revealed that 5 of the 30 subjects complied less than 75% of the time, over all of the training sessions.
The Kruskal–Wallis test result indicated a significant [χ2 (df = 2) = 6.256, P = 0.044] difference in compliance relative to the category of dominance. Inspection of the data revealed that low-ranking subjects complied less often than did those in the other dominance categories (high-dominance group, 97% compliant; medium, 89% compliant; low, 85% compliant).
After 25 training sessions, problem-solving techniques were applied to help the 5 consistently noncompliant animals become more proficient. The changes included emptying of other monkeys from 1 portion of the enclosure that we were asking a noncompliant monkey to move into, using highly preferred foods and jackpot reinforcement, and providing additional enrichment after training sessions. All of these changes were implemented simultaneously. The 5 training sessions immediately following implementation of these changes had 100% success, but this rate of compliance was not maintained over time (Figure 2).
Figure 2.
Percentage compliance of the group of mangabeys during the initial 57 training sessions. The blue diamonds indicate the implementation of problem solving techniques (‘jackpot’ reinforcement, providing additional enrichment, and emptying 1 portion of the enclosure of other monkeys).
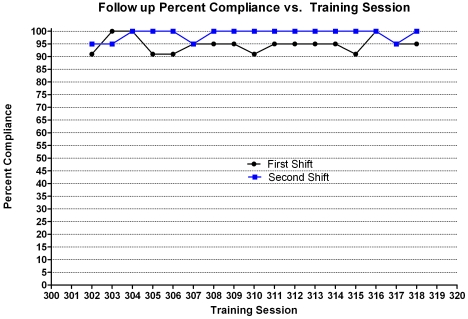
Data collection was halted after 57 sessions, due to constraints on personnel availability, but the training procedures were still conducted. Approximately 34 wk later, we resumed data collection for 34 sessions. These data indicated improved performance: all 34 follow-up training sessions had greater than 90% compliance (Figure 3), thus reaching our predetermined definition of reliable performance. The average training time per session was 5 min. There were 23 incidents of noncompliance during the 34 training sessions; the same animal was responsible for 48% of the refusals.
Figure 3.
Follow-up percentage compliance for the group; data were collected approximately 34 wk after initial training was completed. There were 2 shifting sessions daily to move the animals from 1 side of the enclosure to the other (indicated as ‘first’ and ‘second’ on the graph). All sessions had greater than 90% compliance.
Discussion
In this study, PRT was used as the sole training method to teach sooty mangabeys to move throughout their enclosure on cue. These methods were similar to others used in the past to train groups of chimpanzees3 to move around their enclosure. Positive reinforcement training improved the voluntary movement of the group-housed mangabeys. Although the group failed to reach 100% compliance, the animals ultimately met our training goal of 90% compliance (Figure 3). Fluctuating social dynamics in the group was a likely reason for the short-lived success of 100% compliance. Social and dominance issues will always be present when working with a group of animals. Awareness of these issues allows the development of plans to resolve them. Our plan included emptying the run across from the tunnel so that the less-compliant animals could enter an area without any other animals present. Over time, this practice helped less-compliant animals become more successful, and eventually we were able to stop emptying the run. Social rank did influence the responsiveness of the mangabeys to their training tasks: the high-ranked group was 97% compliant, the medium-ranked group was 89% compliant, and the low-ranked group was 85% compliant.
Although PRT requires an investment of time, the rewards are great. Training this group of mangabeys to shift between enclosures markedly decreased the amount of time that was required for daily husbandry procedures. The average training session was 28 min long during the 57 initial training sessions. During the later period of data collection, about 5 min was required to shift the animals, which saved 23 min per session. Because the animals are moved twice daily, this training saves 46 min per day of animal care time. The 1590 min of training time was essentially ‘paid back’ in fewer than 35 d (1590/46) of working with the mangabeys. In addition, PRT enabled the animal care staff to interact with their animals in a way that was personally rewarding.
Previous studies have shown that positive3,4,6,7,16-18,21,23 and negative19 reinforcement training both can be used to train nonhuman primates to do a large variety of tasks. Our study showed that although a PRT program can be effective in training nonhuman primates, other factors such as social structure need to be evaluated carefully to ensure the success of the training program.
Acknowledgments
We would like to thank Tracy Meeker and Katie Larkin for their help with behavioral observations. We also thank the animal care staff, especially Brittany Jordan, Hugh Denbow, and Cassandra Johnson, who worked with these animals on a daily basis.
This study was supported by the Base Grant to the Yerkes National Primate Research Center (NCRR/NIH P51RR 00165). The facility is fully accredited by the Association for the Assessment and Accreditation of Laboratory Care International (AAALAC International).
References
- 1.Altmann J. 1974. Observational study of behavior: sampling methods. Behaviour 49:227–267 [DOI] [PubMed] [Google Scholar]
- 2.Bloomsmith MA, Laule GE, Alford PL, Thurston RH. 1994. Using training to moderate chimpanzee aggression during feeding. Zoo Biol 13:557–566 [Google Scholar]
- 3.Bloomsmith MA, Stone AM, Laule GE. 1998. Positive reinforcement training to enhance the voluntary movement of group-housed chimpanzees within their enclosures. Zoo Biol 17:333–341 [Google Scholar]
- 4.Coleman K, Pranger L, Maier A, Lambeth SP, Perlman JE, Thiele E, Schapiro SJ. 2008. Training rhesus macaques for venipuncture using positive reinforcement techniques: a comparison with chimpanzees. J Am Assoc Lab Anim Sci 47:37–41 [PMC free article] [PubMed] [Google Scholar]
- 5.Dettmer EL, Phillips KA, Rager DR, Bernstein IS, Fragaszy DM. 1996. Behavioral and cortisol responses to repeated capture and venipuncture in Cebus apella. Am J Primatol 38:357–362 [DOI] [PubMed] [Google Scholar]
- 6.Knowles L, Fourrier M, Eisele S. 1995. Behavioral training of group-housed rhesus macaques (Macaca mulatta) for handling purposes. Laboratory Primate Newsletter 34:1–4 [Google Scholar]
- 7.Lambeth SP, Hau J, Perlman JE, Martino M, Schapiro SJ. 2006. Positive reinforcement training affects hematologic and serum chemistry values in captive chimpanzees (Pan troglodytes). Am J Primatol 68:245–256 [DOI] [PubMed] [Google Scholar]
- 8.Lambeth SP, Perlman JE, Thiele E, Schapiro SJ. 2005. Changes in hematology and blood chemistry parameters in captive chimpanzees (Pan troglodytes) as a function of blood sampling technique: trained versus anesthetized samples. Am J Primatol 66:182–183 [Google Scholar]
- 9.Laule G. 1994. Planning for the individual: situational problem solving. AZA Annual Conference, Zoo Atlanta, Atlanta, GA [Google Scholar]
- 10.Laule G, Whittaker M. 2007. Enhancing nonhuman primate care and welfare through the use of positive reinforcement training. J Appl Anim Welf Sci 10:31–38 [DOI] [PubMed] [Google Scholar]
- 11.Laule GE. 2003. Positive reinforcement training and environmental enrichment: enhancing animal wellbeing. J Am Vet Med Assoc 223:969–973 [DOI] [PubMed] [Google Scholar]
- 12.Laule GE, Thurston RH, Alford PL, Bloomsmith MA. 1996. Training to reliably obtain blood and urine samples from a diabetic chimpanzee (Pan troglodytes). Zoo Biol 15:587–591 [Google Scholar]
- 13.Luttrell L, Acker L, Urben M, Reinhardt V. 1994. Training a large troop of rhesus macaques to cooperate during catching: analysis of the time investment. Anim Welf 3:135–140 [Google Scholar]
- 14.Martin P, Bateson P. 1993. Measuring behaviour, an introductory guide. Cambridge (UK): Cambridge University Press [Google Scholar]
- 15.National Research Council 1996. Guide for the care and use of laboratory animals Washington (DC): National Academy Press [Google Scholar]
- 16.Perlman J, Guhad FA, Lambeth S, Fleming T, Lee D, Martino M, Schapiro S. 2001. Using positive reinforcement training techniques to facilitate the assessment of parasites in captive chimpanzees. Am J Primatol 54:56 [Google Scholar]
- 17.Perlman JE, Bowsher TR, Braccini SN, Kuehl TJ, Schapiro SJ. 2003. Using positive reinforcement training techniques to facilitate the collection of semen in chimpanzees (Pan troglodytes). Am J Primatol 60:77–7812874839 [Google Scholar]
- 18.Perlman JE, Thiele E, Whittaker MA, Lambeth SP, Schapiro SJ. 2004. Training chimpanzees to accept subcutaneous injections using positive reinforcement training techniques. Am J Primatol 62:96 [Google Scholar]
- 19.Phillippi-Falkenstein K, Clarke MR. 1992. Procedure for training corral-living rhesus monkeys for fecal and blood-sample collection. Lab Anim Sci 42:83–85 [PubMed] [Google Scholar]
- 20.Pryor K. 1999. Don't shoot the dog! The new art of teaching and training. New York (NY): Bantam Books [Google Scholar]
- 21.Russell JL, Taglialatela JP, Hopkins WD. 2006. The use of positive reinforcement training in chimpanzees (Pan troglodytes) for voluntary presentation for intramuscular injections. Am J Primatol 68:122 [Google Scholar]
- 22.Schapiro SJ, Bloomsmith MA, Laule GE. 2003. Positive reinforcement training as a technique to alter nonhuman primate behavior: quantitative assessments of effectiveness. J Appl Anim Welf Sci 6:175–187 [DOI] [PubMed] [Google Scholar]
- 23.Schapiro SJ, Perlman JE, Thiele E, Lambeth S. 2005. Training nonhuman primates to perform behaviors useful in biomedical research. Lab Anim 34:37–42 [DOI] [PubMed] [Google Scholar]
- 24.Smith EO. 1981. Device for capture and restraint of nonhuman primates. Lab Anim Sci 31:305–306 [PubMed] [Google Scholar]
- 25.Smith TE, McCallister JM, Gordon SJ, Whittikar M. 2004. Quantitative data on training New World primates to urinate. Am J Primatol 64:83–93 [DOI] [PubMed] [Google Scholar]
- 26.Stahl D. 1998. Food competition in captive sooty mangabeys (Cercocebus torquatus atys). Gottingen (Germany): Cuvillier Verlag; [DOI] [PubMed] [Google Scholar]
- 27.Stone AM, Bloomsmith MA, Laule GE, Alford PL. 1994. Documenting positive reinforcement training for chimpanzee urine collection. Am J Primatol 33:242 [Google Scholar]
- 28.Videan EN, Fritz J, Murphy J, Borman R, Smith HF, Howell S. 2005. Training captive chimpanzees to cooperate for an anesthetic injection. Lab Anim (NY) 34:43–48 [DOI] [PubMed] [Google Scholar]
- 29.Walker ML, Gordon TP, Wilson ME. 1982. Reproductive performance in capture-acclimated female rhesus monkeys (Macaca mulatta). J Med Primatol 11:291–302 [PubMed] [Google Scholar]