Abstract
Assessment of fecal glucocorticoid metabolites has become a widely used method for monitoring stress responses. Because most small rodents are social animals whose physiologic parameters are affected by social stimuli, individual housing may compromise these data. Nevertheless, housing rodents in families or social groups may be an important limitation to the experimental design. The challenge is to collect samples from individual rodents while avoiding stress-associated effects from the sampling method itself. Here we present an apparatus and protocol allowing routine repeated collection of an individual rodent's fresh fecal samples without noticeable disturbance of any of the study animals; continuous maintenance of studied animals in a familiar environment; group housing; and uninterrupted visual and olfactory communication among group members during sampling. The apparatus consists of 1 central and 4 lateral compartments. The experimental animal was allowed to enter a lateral compartment voluntarily, where it remained for the short (4 h) period necessary for sample collection before rejoining the rest of the group. Evaluations involved Egyptian spiny mice, a social rodent increasingly studied in laboratories. The results confirmed the repeatability of the assessment of baseline levels of glucocorticoid metabolites. Moreover, keeping the animals in our experimental apparatus did not induce any increase in the levels of glucocorticoid metabolites, even when isolation in the compartment was relatively prolonged. We interpret these results as confirmation that our sampling procedure allows repeated individual sampling within a nearly undisturbed social unit.
Abbreviations: EIA, enzyme immunoassay; FCM, fecal cortisol metabolites
Glucocorticoid levels are used widely as a marker of stress in vertebrates.3,9,32,37 Traditionally, endocrine status is assessed from blood samples. This approach is limited, however, particularly in the case of glucocorticoids.32 Their concentrations not only exhibit considerable circadian and episodic fluctuations11,13,24,51-53 but also may be increased markedly by the sampling procedure itself. These problems are especially pronounced in small rodents. Any capture, handling, or bleeding technique almost inevitably causes an increase in glucocorticoid levels within a few minutes.7,11,43 To avoid this problem, noninvasive sampling methods are being introduced. An easy solution is to measure glucocorticoid metabolites in fecal samples. Usually, glucocorticoid metabolites extracted from feces and assessed by an enzyme immunoassay or radioimmunoassay accurately reflect glucocorticoid levels in circulating blood after a specified time lag.32,37,51
Even with this approach, the collection of the fecal samples may itself affect levels of measured metabolites. When repeated or even continuous sampling is necessary, any disturbance of the studied animal may significantly alter the levels of glucocorticoid metabolites in subsequent samples. A wire-mesh grid bottom is an appropriate solution for the collection of repeat samples from individually housed rodents.52 Nevertheless, many rodent species, including the most common laboratory animals such as mice and rats, are not solitary (except, for example, golden hamsters). They live in monogamous units (Mongolian gerbil1), polygynous or promiscuous families (house mouse8), and even larger societies (Norway rat5). Access to social contact with conspecifics promotes the welfare of these animals.35,54 Consequently, social isolation of the experimental subject, a common practice among studies assessing glucocorticoid levels, may act as a stressor directly14,49,56 or as a result of unavoidable manipulation of the animal. Consequently, even brief isolation may interfere with the experimental design.16,46 Furthermore, in laboratory animals such as guinea pigs, differences in glucocorticoid levels between group-housed and individually housed animals have been reported.25 In addition to the direct effect on glucocorticoid levels, prolonged social isolation of mice is known to enhance their aggression.4,44
In addition to isolation, various social factors can be stressors under both natural and laboratory conditions. Social stress plays a key role in the intrinsic regulation of rodent populations through mortality and reduction of reproductive activity.5,6 Social stress clearly accompanies almost all conflicts of interests between family or group members (for example, dwarf mongoose (Helogale parvula)7). Therefore, any social interaction is potentially stressful, and glucocorticoid levels should be monitored in most behavioral studies dealing with social animals.
Egyptian spiny mice (Acomys cahirinus Desmarest, 1819) are nocturnal,57 desert-dwelling rodents36,59 found in a wide range of habitats across North Africa. Despite their earlier systematic placement, spiny mice are more related to gerbils than to true murids, which belong to the subfamily Murinae and are represented by rats and house mice.29,30,50 Spiny mice have an extremely long gestation period (38 to 39 d) and produce small litters (mean, 2.3) consisting of well-developed precocial offspring. They exhibit no behavioral signs of stress under standard laboratory conditions and breed well. Therefore, this species has become widely used as an experimental model in both physiologic12,33,47 and behavioral38 studies. Cortisol is the main glucocorticoid in the blood of Egyptian spiny mice.23
We chose to study Egyptian spiny mice instead of more common laboratory rodents because spiny mice have a complex social organization involving individual and kin recognition,41,42 communal nesting40 and paternal care.26 Our experience with captive breeding suggests that they are visibly sensitive to social stress. Further, in nature, these rock-dwelling animals climb and live on sharp substrates. Moreover, spiny mice do not build nests. Therefore, a grid floor may be a less unnatural substrate for spiny mice than for mice and rats.
The aim of this study was to design an apparatus that accommodated 1) routine repeated collection of an individual animal's fresh fecal samples without disturbing other study animals; 2) continuous maintenance of study animals in a familiar environment; 3) group housing of animals; and 4) uninterrupted visual and olfactory communication between group members during sampling. We used Egyptian spiny mice to verify the usefulness of the apparatus and test the repeatability of the results.
Materials and Methods
Studied animals.
Our spiny mice are descendants of a dozen founders captured in the vicinity of the Abu Simbel archaeological site in southern Egypt. The laboratory colony has been maintained outbred in numbers exceeding 50 pairs for about 10 generations. The animals were kept in glass breeding cages (600 × 500 × 400 mm) with a sliding front door and wire-mesh ventilation in the upper part of the back side. Wood shavings were used as bedding material, a flowerpot with lateral opening served as a shelter, and some branches for climbing and gnawing were provided as environmental enrichment. The light schedule in the animal housing room corresponded to the outdoor light cycle. The room was maintained under standard laboratory conditions (temperature, 22 ± 1 °C; relative humidity, 37% ± 5%). Food (standard diet for rats and mice ST1, Velaz, Prague, Czech Republic) supplemented with a mixture of grains, bread, mealworms, apples, and fresh seasonal herb leaves and water were available ad libitum. During the experiment (and 2 wk before), the diet was standardized (solely ST1 diet). Spiny mice were kept in family groups consisting of 1 male, 2 females (sisters), and their offspring. After gradual exposure to experimenters who minimized their intrusiveness, the animals became habituated to their presence.
Harm to experimental animals was avoided, and only noninvasive methods for sample collection were used. The experiments were performed in accordance with Czech law implementing all corresponding European Union regulations and were approved by the institutional animal care and use committee.
The apparatus.
The front and top views of the experimental cage are depicted in Figures 1 and 2, respectively. The size of the apparatus was the same as that of the breeding cage. The experimental cage was subdivided into 5 compartments: a single central area (200 × 500 × 400 mm) and 4 lateral regions (200 × 250 × 400 mm), 1 in each corner of the cage. Each compartment had a separate sliding glass door to allow access while caring for the animals. Above this door was a 50-mm wire mesh screen for ventilation, with water bottle holders.
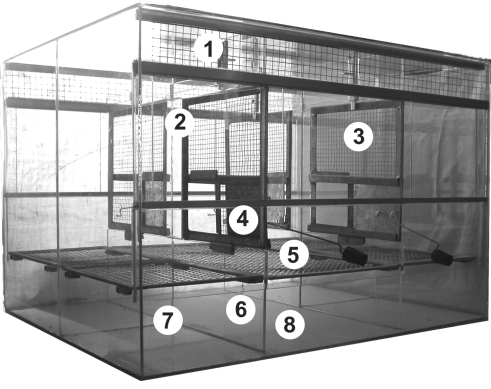
Figure 1.
Front view of the experimental apparatus. 1, wire-mesh screen; 2, glass sliding door; 3, wire-mesh partition; 4, metal sliding door operated from the outside; 5, grid bottom; 6, glass partition separating compartments; 7, open front and back space for removing samples; 8, bottom space for clean filter paper, allowing collection of fecal samples.
Figure 2.
Top view of the experimental apparatus. 9, central compartment; 10, lateral compartments; 11, fixed glass partition.
The central compartment was separated from the lateral ones by wire-mesh partitions fixed to low glass bases (125 mm). A metal sliding door (100 × 85 mm) operated from the outside is placed in each wire-mesh partition. The adjacent lateral compartments were separated by fixed glass partitions.
A 7 × 7-mm wire-mesh grid floor was positioned in each compartment at 90 mm above the glass bottom. The space below the grid was freely accessible from the open front and back. The glass bottom of each compartment was covered with clean filter paper, allowing the collection of fecal samples. As in the breeding cage, each compartment of the experimental cage was supplied with a shelter, a branch, food, and water.
Collection of fecal samples.
Except during the brief sampling period, all 4 metal doors were left open to allow free movement of the animals throughout the compartments. At the beginning of the sampling, when animal voluntarily entered a lateral compartment without conspecifics, the experimenter closed the sliding door from the outside using separate wire rods, shutting the animal inside. Because spiny mice are active animals, moving frequently from compartment to compartment, the procedure of sequestering individual subjects for sampling usually takes just a few minutes. The 4 lateral compartments in each cage allowed sampling of 4 individual animals simultaneously. The paper covering the glass bottom of the compartment was replaced with a new sheet, which was removed (with feces) 4 h later. The fecal samples were placed in 1.5-mL microfuge tubes and immediately frozen at –20 °C for subsequent analysis. The metal door was then opened, allowing the animal to rejoin the group.
Experimental procedures.
At the beginning of each experimental series, a family group was transferred from the breeding cage to the experimental cage. First we tested whether the introduction of spiny mice into the experimental apparatus itself increased fecal cortisol metabolites (FCM) levels. For this purpose, we moved 5 family groups (11 male and 5 female mice) into experimental cages and repeatedly collected fecal samples of each studied animal from 2 to 6 h after the beginning of the light period (that is, 1000 to 1400 h) to reflect circulating glucocorticoid levels at the end of the dark period. The sampling periods were designed to detect: 1) circulating glucocorticoid levels in undisturbed animals (these samples were collected immediately after transfer and could provide control or baseline values because of the delay between elevations in circulating glucocorticoid concentrations and the resultant increase in fecal concentrations of metabolites); 2) the putative adaptation period (days 7 and 14) after transfer; and 3) putative baseline on days 23 through 25 after transfer.
Second, we tested 5 family groups (16 male and 21 female mice). After a 7-d adaptation to the experimental cages, individual animals each were sampled on 3 consecutive days in the morning from 0800 to 1200 (that is, starting 1 h after the beginning of the light period to reflect circulating glucocorticoid levels at the dark period) and in the evening from 1800 to 2200 (that is, starting 1 h before the beginning of the dark period to reflect values of circulating glucocorticoid during the light period).
In addition, we tested the effect of prolonged sequestration of the experimental animal on FCM levels. Each of 4 male and 4 female spiny mice was confined to a lateral compartment for 3 d (76 h). Samples were collected daily from 1400 to 1800 (that is, starting 7 h after the beginning of the light period and reflecting circulating glucocorticoid during the light period). The first sampling period started immediately after the animals were confined to obtain samples reflecting baseline values.
Assessment of FCM.
Each sample was well homogenized with a mortar and pestle, a 0.05-g aliquot was placed in a1.5-mL tube, and 1 mL 80% methanol was added as described.53 Samples were vortexed for 15 min and centrifuged (11,500 × g; model, 5415 C microcentrifuge Eppendorf, Germany) for 2 min. The supernatant (800 µL) was transferred into new tubes. Aliquots of the supernatant were diluted 1:10 with assay buffer (Tris/HCl 20 mM, pH 7.5), transferred to new titer tubes and frozen at –20 °C until analysis. For determination of the amount of FCM, we used 2 established enzyme immunoassays (EIA). The first assay was designed to evaluate 5α-pregnane-3β,11β,21-triol-20-one (EIA1) and recognizes FCM with a 5α-3β,11β-diol structure (developed for laboratory mice52,53); sample supernatants were diluted 1:100 before being evaluated by this test. The second assay (EIA2) was developed for measuring 11-oxoetiocholanolone and reacted with FCM with a 5β-3α-ol-11-one structure (first developed for ruminants31). The intra- and interassay coefficients of variation were 9.1% and 14.0%, respectively, for EIA1 and 9.7% and 12.5%, respectively, for EIA2. These EIA procedures were validated for use in spiny mice by using an adrenocorticotropic hormone challenge test.34 The results of EIA1 and EIA2 were correlated (r = 0.53), but both were used, because each of the assays detects different FCM.
Statistical analysis.
Results were analyzed with Statistica 6.0 (StatSoft, Tulsa, OK) and a P value of 0.05 was selected as the threshold for statistical significance. We performed general linear model procedures in which the log-transformed concentration of FCM was used as a dependent variable. Animal identity was taken as a random factor to avoid pseudoreplications and sampling order as a continuous (or categorical in the case of the first series) fixed factor. The model evaluating samples collected both in the morning and evening also included time of sample collection as a fixed factor. Because FCM did not differ between male (mean: EIA1, 2738 ng/g; EIA2, 411 ng/g) and female (mean: EIA1, 2670 ng/g; EIA2, 447 ng/g34) spiny mice, sex was not included as a factor in the model.
Results
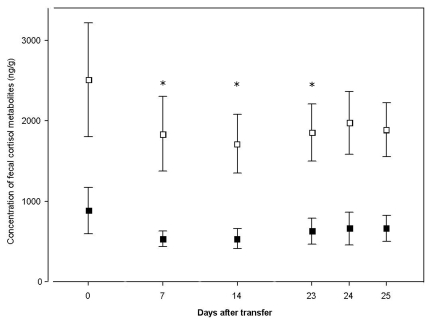
The FCM of male and female spiny mice varied significantly among subjects (EIA1: F15,75 = 14.76, P < 0.0001; EIA2: F15,75 = 3.15, P = 0.0005). The effect of the sampling order was significant only for EIA1 (EIA1: F5,75 = 3.53, P = 0.0064). In both EIAs, the highest mean FCM levels were found in control samples (Figure 3). The differences between the controls and subsequent values from days 7, 14, and 23 were significant according to the Dunnett test in the case of EIA1 (P = 0.0053, 0.0014, and 0.0360, respectively).
Figure 3.
Effect of transfer of spiny mice (n = 16) from the breeding cage to the experimental apparatus. Samples were collected between 1000 and 1400 on various days after transfer (day 0, samples collected immediately after transfer; days 7 and 14, adaptation period; days 23 through 25, putative baseline levels). Means (95% confidence intervals) of FCM levels (ng/g) measured by enzyme immunoassays 1 (open squares) and 2 (solid squares). Details of the immunoassays are given in the Material and Methods section. *, P < 0.05 compared with day 0 (control) value.
We found no effect of repeated sampling on FCM levels (EIA1: F1,173 = 1.96, P = 0.16; EIA2: F1,173 = 1.87, P = 0.17). Nevertheless, the results revealed clear differences in FCM levels among subjects (EIA1: F36,173 = 5.09, P < 0.0001; EIA2: F36,173 = 5.52, P < 0.0001). Mean log values ranged from 7.16 to 8.80 (that is, 1288 to 6609 ng/g feces) for EIA1 and from 5.45 to 6.71 (that is, 233 to 818 ng/g feces) for EIA2. In the case of EIA1, a significant difference was present between samples collected in the morning and evening (F1,173 = 8.27, P = 0.005). Evening values measured by EIA1 were somewhat higher (mean = 3125 ng/g) than the morning ones (mean, 2300 ng/g). No such difference was revealed by EIA2 (means of 431 and 433 ng/g for morning and evening, respectively). To illustrate repeatability of the results, we calculated correlations between mean values of the morning and the evening triads of samples: 0.62 (P = 0.0001) and 0.74 (P < 0.0001) for EIA1 and EIA2, respectively.
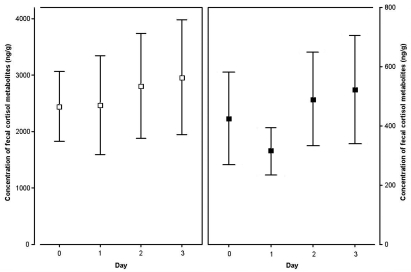
Prolonged confinement of an animal in 1 of the lateral compartments had no significant effect on FCM levels (EIA1: F1,23 = 3.17, P = 0.0882; EIA2: F1,23 = 3.31, P = 0.0820; Figure 4).
Figure 4.
Effect of prolonged confinement of a spiny mouse (n = 8) from the rest of the family group by using a wire-mesh partition to allow olfactory, acoustic, and visual communication. Samples were collected between 1400 and 1800 on successive days after separation (day 0, samples collected immediately after confinement). Means (95% confidence intervals) of FCM levels (ng/g) measured by enzyme immunoassays EIA1 (open squares; left panel) and EIA2 (solid squares; right panel). Details of the enzyme immunoassays are given in the Materials and Methods section.
Discussion
These results can be interpreted as a test of our apparatus. FCM levels were not elevated after the introduction of family groups of spiny mice into the experimental apparatus. Surprisingly, in the case of EIA1, initial FCM levels corresponding to the previous environment of a standard breeding cage were even slightly higher than the subsequent ones reflecting experimental conditions. Thus, subdivision of the cage into the compartments with separate shelters, multiple food and water sources, and vertical wire-mesh surfaces available for climbing can be viewed as a specific form of enrichment.
Moreover, repeated sampling of the same mice yielded consistent results, which are a prerequisite for the applicability of the procedure. Because repeated sampling did not affect FCM values, we conclude that the sampling procedure itself had no effect on subsequent values. Of course, closing an experimental animal in the sample-collection compartment could itself potentially affect glucocorticoid levels. Nevertheless, the length of our sampling bouts was designed to be shorter than the time lag between elevation of glucocorticoid in the blood and the appearance of the corresponding metabolites in feces. Accordingly, the intervals between subsequent sampling bouts were much longer than the lag period.
Interindividual differences in FCM of spiny mice were comparable to those of Meriones meridianus22 and M. unguiculatus56 (for review, see references 51 and 58). In our study, interindividual differences explained most of the observed variance in FCM levels. This finding does not necessarily indicate that some experimental animals suffer from heavy social stress. The distribution of individual means was unimodal and likely encompasses the normal range of basal values. The mean FCM levels in the present study (nontransformed values: EIA1, 2988 ng/g; EIA2, 473 ng/g) are one-third those obtained after administration of adrenocorticotropic hormone to simulate stressful conditions (EIA1, 7656 ng/g; EIA2, 1831 ng/g).34
Although the mean FCM levels obtained by using EIA1 were higher in the evening than in the morning, we interpret this finding with caution. EIA2, which assesses a different spectrum of glucocorticoid metabolites, revealed no such difference. The diurnal pattern of FCM in spiny mice therefore requires further research.
The assessment of glucocorticoid metabolites from fecal samples is an easy noninvasive method of evaluating adrenocortical activity and is widely used (for review, see references 32 and 51). The important advantage of this procedure is a relatively long lag time between a stressor and subsequent elevation of glucocorticoid metabolites in the feces. Assessment of FCM depends on gut transit time,37 which in small rodents has been described to take several h (laboratory rat, 10 to 12 h;24 Spermophilus beldingi, 18 h;28 Clethrionomys gapperi, 8 h; and Peromyscus maniculatus, 12 h).14 The shortest lag times are associated with small granivorous species, such as the laboratory mouse (4 to 10 h)53 and Peromyscus polionotus (4 h).11 Our sampling period (4 h) was therefore short enough to ensure that the samples reflected the circulating plasma concentration of cortisol before the animal was confined in the compartment.
Increasing evidence suggests that wild rodents locked in live traps exhibit elevated levels of glucocorticoid (Tamias amoenus,20,39 Spermophilus parryi,2 Peromyscus maniculatus, Clethrionomys gapperi,15 Microtus pennsylvanicus10). To avoid such a response, our samples were collected from animals that entered voluntarily a familiar compartment (perceived as a part of their home range). The locking procedure was gentle, and the experimental apparatus enabled uninterrupted olfactory and visual communication between the sampled animal and the rest of the group, which was in the central part of the apparatus. These circumstances may explain the fact that the FCM levels remained about the same even when the confinement period was substantially prolonged to 3 d. However, the sample size for this experiment was small, and the result should be interpreted with caution.
Several studies address glucocorticoid levels from individually housed animals.60 In comparison, the collection of samples from group-housed animals is difficult. Facing this problem, most researchers adopted protocols including either isolation of experimental animals from their social group for at least a short time18,46,48,56 or artificially induced defecation by handling.28 Both these procedures require manipulations that potentially cause stress in the studied animal and remaining group members. For example, isolation from the group in a new environment16,19,45 or even cage cleaning55 can change glucocorticoid levels. Although the time lag we described may reduce the impact on the actual measured values, an effect on subsequent sampling cannot be excluded.
Our approach allowed us to rule out several important confounding factors (manipulation, social isolation, new environment) that may affect the use of fecal samples for the detection of stress in social rodents. However, our mice were maintained on wire-grid floors, which are reported to adversely affect laboratory rats.17,21,27 Animals kept in our experimental cages exhibited neither physical harm nor any behavioral signs of discomfort. We suggest that spiny mice as rock dwellers are much more adapted to live and climb on surfaces of this kind than are more common laboratory rodents. Nevertheless, this factor warrants consideration before the described apparatus is used for laboratory mice and rats without modification.
To our knowledge, our experimental apparatus is the first to accommodate collection of fecal samples from individual small rodents housed in a social group without any disturbance of studied animals. Such arrangement allows measuring not only isolation stress but also social stress, which is a complex phenomenon requiring sophisticated experimental designs. The results here support the use of the apparatus and sampling procedure for measuring FCM in group-housed spiny mice. Our apparatus might be adapted and applied to other model species of small mammals living in families or more complex groups.
Acknowledgments
We thank Professor Ladislav Janský for advice and stimulating discussions, Věra Říčánková for initial encouragement, Edith Klobetz-Rassam for technical assistance in assessment of fecal glucocorticoid metabolites, Václav Hryz for the implementation of the experimental apparatus and valuable improvements of its construction, and other colleagues and students for comments. The project was supported by grants 206/05/2655 and 206/05/H012 (personal and travel costs of MN only) from the Czech Science Foundation.
References
- 1.Agren G. 1984. Pair formation in the Mongolian gerbil. Anim Behav 32:528–535 [Google Scholar]
- 2.Boonstra R, Hubbs AH, Lacey EA, McColl CJ. 2001. Seasonal changes in glucocorticoid and testosterone concentrations in free-living arctic ground squirrels from the boreal forest of the Yukon. Can J Zool 79:49–58 [Google Scholar]
- 3.Boonstra R, McColl CJ. 2000. Contrasting stress response of male arctic ground squirrels and red squirrels. J Exp Zool 286:390–404 [PubMed] [Google Scholar]
- 4.Brain P. 1975. What does individual housing mean to a mouse? Life Sci 16:187–200 [DOI] [PubMed] [Google Scholar]
- 5.Calhoun JB. 1962. The ecology and sociology of the Norway rat. Public Health Service publication 1008. Bethesda (MD): US Department of Health, Education and Welfare [Google Scholar]
- 6.Chitty D. 1967. The natural selection of self-regulatory behaviour in animal populations. Proc Ecol Soc Aust 2:51–78 [Google Scholar]
- 7.Creel S. 2001. Social dominance and stress hormones. Trends Ecol Evol 16:491–497 [Google Scholar]
- 8.Crowcroft P, Rowe FP. 1963. Social organization and territorial behaviour in the wild house mouse (Mus musculus L.). Proc Zool Soc Lond 140:517–531 [Google Scholar]
- 9.De Villiers MS, van Jaarsveld AS, Meltzer DG, Richardson PR. 1997. Social dynamics and the cortisol response to immobilization stress of the African wild dog, Lycaon pictus. Horm Behav 31:3–14 [DOI] [PubMed] [Google Scholar]
- 10.Fletcher QE, Boonstra R. 2006. Impact of live trapping on the stress response of the meadow vole (Microtus pennsylvanicus). J Zool 270:473–478 [Google Scholar]
- 11.Good T, Khan MZ, Lynch JW. 2003. Biochemical and physiological validation of a corticosteroid radioimmunoassay for plasma and fecal samples in oldfield mice (Peromyscus polionotus). Physiol Behav 80:405–411 [DOI] [PubMed] [Google Scholar]
- 12.Haim A, Alma A, Neuman A. 2006. Body mass is a thermoregulatory adaptation of diurnal rodents to the desert environment. J Therm Biol 31:168–171 [Google Scholar]
- 13.Halberg F, Albrecht PG, Bittner JJ. 1959. Corticosterone rhythm of mouse adrenal in relation to serum corticosterone and sampling. Am J Physiol 197:1083–1085 [DOI] [PubMed] [Google Scholar]
- 14.Harper JM, Austad SN. 2000. Fecal glucocorticoids: a noninvasive method of measuring adrenal activity in wild and captive rodents. Physiol Biochem Zool 73:12–22 [DOI] [PubMed] [Google Scholar]
- 15.Harper JM, Austad SN. 2004. Fecal corticosteroid levels in free-living populations of deer mice (Peromyscus maniculatus) and southern red-backed voles (Clethrionomys gapperi). Am Midl Nat 152:400–409 [Google Scholar]
- 16.Hayssen V, Harper JM, DeFina R. 2002. Fecal corticosteroids in agouti and nonagouti deer mice (Peromyscus maniculatus). Comp Biochem Physiol A Mol Integr Physiol 132:439–446 [DOI] [PubMed] [Google Scholar]
- 17.Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, Feldon J, Moran MC, Nelson P. 2000. Behavioral, neurochemical, and endocrinological characterization of the early social isolation syndrome. Neuroscience 100:749–768 [DOI] [PubMed] [Google Scholar]
- 18.Hunt C, Hambly C. 2006. Faecal corticosterone concentrations indicate that separately housed male mice are not more stressed than group-housed males. Physiol Behav 87:519–526 [DOI] [PubMed] [Google Scholar]
- 19.Hurst JL, Barnard CJ, Nevison CM, West CD. 1998. Housing and welfare in laboratory rats: welfare implications of isolation and social contact among caged females. Anim Welf 7:121–136 [Google Scholar]
- 20.Kenagy GJ, Place NJ. 2000. Seasonal changes in plasma glucocorticosteroids of free-living female yellow-pine chipmunks: effects of reproduction and capture and handling. Gen Comp Endocrinol 117:189–199 [DOI] [PubMed] [Google Scholar]
- 21.Krohn TC, Hansen AK, Dragsted N. 2003. Telemetry as a method for measuring the impact of housing conditions on rats’ welfare. Anim Welf 12:53–62 [Google Scholar]
- 22.Kuznetsov VA, Tchabovsky AV, Kolosova IE, Moshkin MP. 2004. Effect of habitat type and population density on the stress level of midday gerbils (Meriones meridianus pall.) in free-living populations. Biol Bull 31:628–632 [PubMed] [Google Scholar]
- 23.Lamers WH, Mooren PG, Griep H, Endert E, Degenhart HJ, Charles R. 1986. Hormones in perinatal rat and spiny mouse: relation to altricial and precocial timing of birth. Am J Physiol 251:E78–E85 [DOI] [PubMed] [Google Scholar]
- 24.Lepschy M, Touma C, Hruby R, Palme R. 2007. Noninvasive measurement of adrenocortical activity in male and female rats. Lab Anim 41:372–387 [DOI] [PubMed] [Google Scholar]
- 25.Machatschke IH, Wallner B, Schams D, Dittami J. 2004. Social environment affects peripheral oxytocin and cortisol during stress responses in guinea-pigs. Ethology 110:161–176 [Google Scholar]
- 26.Makin JW, Porter RH. 1984. Paternal behavior in the spiny mouse (Acomys cahirinus). Behav Neural Biol 41:135–151 [DOI] [PubMed] [Google Scholar]
- 27.Manser CE, Morris TH, Broom DM. 1995. An investigation into the effects of solid or grid cage flooring on the welfare of laboratory rats. Lab Anim 29:353–363 [DOI] [PubMed] [Google Scholar]
- 28.Mateo JM, Cavigelli SA. 2005. A validation of extraction methods for noninvasive sampling of glucocorticoids in free-living ground squirrels. Physiol Biochem Zool 78:1069–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michaux J, Catzeflis F. 2000. The bushlike radiation of muroid rodents is exemplified by the molecular phylogeny of the LCAT nuclear gene. Mol Phylogenet Evol 17:280–293 [DOI] [PubMed] [Google Scholar]
- 30.Michaux J, Reyes A, Catzeflis F. 2001. Evolutionary history of the most speciose mammals: molecular phylogeny of muroid rodents. Mol Biol Evol 18:2017–2031 [DOI] [PubMed] [Google Scholar]
- 31.Möstl E, Maggs JL, Schrotter G, Besenfelder U, Palme R. 2002. Measurement of cortisol metabolites in faeces of ruminants. Vet Res Commun 26:127–139 [DOI] [PubMed] [Google Scholar]
- 32.Möstl E, Palme R. 2002. Hormones as indicators of stress. Domest Anim Endocrinol 23:67–74 [DOI] [PubMed] [Google Scholar]
- 33.Nesher R, Cerasi E. 1994. The spiny mouse: in search of an animal model for the pathogenesis of type 2 diabetes mellitus. Isr J Zool 40:185–197 [Google Scholar]
- 34.Nováková M, Palme R, Kutalová H, Janský L, Frynta D. 2008. The effects of sex, age, and commensal way of life on levels of fecal glucocorticoid metabolites in spiny mice (Acomys cahirinus). Physiol Behav 95:187–193 [DOI] [PubMed] [Google Scholar]
- 35.Olsson IAS, Westlund K. 2007. More than numbers matter: the effect of social factors on behaviour and welfare of laboratory rodents and non-human primates. Appl Anim Behav Sci 103:229–254 [Google Scholar]
- 36.Osborn DJ, Helmy I. 1980. The contemporary land mammals of Egypt (including Sinai). Fieldiana Zool 5:l–579 [Google Scholar]
- 37.Palme R, Rettenbacher S, Touma C, El-Bahr SM, Möstl E. 2005. Stress hormones in mammals and birds: comparative aspects regarding metabolism, excretion and noninvasive measurement in fecal samples. Trends in comparative endocrinology and neurobiology. Ann N Y Acad Sci 1040:162–171 [DOI] [PubMed] [Google Scholar]
- 38.Pinter-Wollman N, Dayan T, Eilam D, Kronfeld-Schor N. 2006. Can aggression be the force driving temporal separation between competing common and golden spiny mice? J Mammal 87:48–53 [Google Scholar]
- 39.Place NJ, Kenagy GJ. 2000. Seasonal changes in plasma testosterone and glucocorticosteroids in free-living male yellow-pine chipmunks and the response to capture and handling. J Comp Physiol B Biochem Syst Environ Physiol 170:245–251 [DOI] [PubMed] [Google Scholar]
- 40.Porter RH, Cavallaro SA, Moore JD. 1980. Developmental parameters of mother–offspring interactions in Acomys cahirinus. Z Tierpsychol 53:153–170 [Google Scholar]
- 41.Porter RH, Matochik JA, Makin JW. 1983. Evidence for phenotype matching in spiny mice (Acomys cahirinus). Anim Behav 31:978–984 [Google Scholar]
- 42.Porter RH, Matochik JA, Makin JW. 1986. Discrimination between full-sibling spiny mice (Acomys cahirinus) by olfactory signatures. Anim Behav 34:1182–1188 [Google Scholar]
- 43.Romero LM, Reed JM. 2005. Collecting baseline corticosterone samples in the field: is under 3 min good enough? Comp Biochem Physiol A Mol Integr Physiol 140:73–79 [DOI] [PubMed] [Google Scholar]
- 44.Roubertoux PL, Guillot PV, Mortaud S, Pratte M, Jamon M, Cohen-Salmon C, Tordjman S. 2005. Attack behaviors in mice: from factorial structure to quantitative trait loci mapping. Eur J Pharmacol 526:172–185 [DOI] [PubMed] [Google Scholar]
- 45.Sachser N, Durschlag M, Hirzel D. 1998. Social relationships and the management of stress. Psychoneuroendocrinology 23:891–904 [DOI] [PubMed] [Google Scholar]
- 46.Scheibler E, Weinandy R, Gattermann R. 2004. Social categories in families of Mongolian gerbils. Physiol Behav 81:455–464 [DOI] [PubMed] [Google Scholar]
- 47.Shafrir E. 2000. Overnutrition in spiny mice (Acomys cahirinus): beta-cell expansion leading to rupture and overt diabetes on fat-rich diet and protective energy-wasting elevation in thyroid hormone on sucrose-rich diet. Diabetes Metab Res Rev 16:94–105 [DOI] [PubMed] [Google Scholar]
- 48.Shimozuru M, Kikusui T, Takeuchi Y, Mori Y. 2006. Scent-marking and sexual activity may reflect social hierarchy among group-living male Mongolian gerbils (Meriones unguiculatus). Physiol Behav 89:644–649 [DOI] [PubMed] [Google Scholar]
- 49.Späni D, Arras M, König B, Rülicke T. 2003. Higher heart rate of laboratory mice housed individually versus in pairs. Lab Anim 37:54–62 [DOI] [PubMed] [Google Scholar]
- 50.Steppan S, Adkins R, Anderson J. 2004. Phylogeny and divergence-date estimates of rapid radiations in muroid rodents based on multiple nuclear genes. Syst Biol 53:533–553 [DOI] [PubMed] [Google Scholar]
- 51.Touma C, Palme R. 2005. Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of validation. Ann N Y Acad Sci 1046:54–74 [DOI] [PubMed] [Google Scholar]
- 52.Touma C, Palme R, Sachser N. 2004. Analyzing corticosterone metabolites in fecal samples of mice: a noninvasive technique to monitor stress hormones. Horm Behav 45:10–22 [DOI] [PubMed] [Google Scholar]
- 53.Touma C, Sachser N, Möstl E, Palme R. 2003. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen Comp Endocrinol 130:267–278 [DOI] [PubMed] [Google Scholar]
- 54.Van Loo PLP, Van de Weerd HA, Van Zutphen LFM, Baumans V. 2004. Preference for social contact versus environmental enrichment in male laboratory mice. Lab Anim 38:178–188 [DOI] [PubMed] [Google Scholar]
- 55.Van Loo PLP, Van der Meer E, Kruitwagen CLJJ, Koolhaas JM, Van Zutphen LFM, Baumans V. 2004. Long-term effects of husbandry procedures on stress-related parameters in male mice of two strains. Lab Anim 38:169–177 [DOI] [PubMed] [Google Scholar]
- 56.Waiblinger E, Konig B. 2004. Refinement of gerbil housing and husbandry in the laboratory. Anim Welf 13:S229–S235 [DOI] [PubMed] [Google Scholar]
- 57.Weber ET, Hohn VM. 2005. Circadian activity in the spiny mouse, Acomys cahirinus. Physiol Behav 86:427–433 [DOI] [PubMed] [Google Scholar]
- 58.Williams TD. 2008. Individual variation in endocrine systems: moving beyond the ‘tyranny of the Golden Mean’. Philos Trans R Soc Lond B Biol Sci 363:1687–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson DE, Reeder DM. 2005. Mammal species of the world. A taxonomic and geographic reference, vol. 2. Bethesda (MD): The Johns Hopkins University Press [Google Scholar]
- 60.Yamaguchi H, Kikusui T, Takeuchia Y, Yoshimura H, Mori Y. 2005. Social stress decreases marking behavior independently of testosterone in Mongolian gerbils. Horm Behav 47:549–555 [DOI] [PubMed] [Google Scholar]