Abstract
The short-term effects on rates and durations of self-injurious behavior and self-directed stereotypies associated with various doses of fluoxetine (FLX) and venlafaxine (VEN) were examined in rhesus macaques. Adult male macaques (Macaca mulatta; n = 17; age, 7 to 15 y) with at least 1 episode of severe SIB within the past 5 y were randomized to treatment with FLX (n = 6), VEN (n = 6), or placebo (PLC, n = 5), administered by voluntary consumption of medication provided in fruit-flavored tablets. After 4-wk baseline and 4-wk placebo lead-in phases, doses were increased monthly for 4 mo (FLX: 0.5, 2.0, 4.0, and 8.0 mg/kg; VEN: 2.0, 4.0, 8.0, and 16.0 mg/kg). Animals in the PLC condition received similar nonmedicated fruit-flavored tablets. Focal behavioral observations, plasma drug levels, and neurochemical data were obtained. Results indicated that rates and percentage time spent self-biting declined at all doses of FLX, with the greatest effect seen at 2.0 mg/kg. For VEN, percentage time spent self-biting was significantly lower only at the 4.0 mg/kg dose. Treatment-induced reductions in platelet serotonin and cerebrospinal fluid 5-hydroxyindoleacetic acid (CSF 5HIAA) concentrations were substantially greater in the FLX-treated condition than in the VEN-treated condition. Plasma FLX and norfluoxetine levels increased with FLX dose; plasma levels of VEN were low and not dose-related. Fluoxetine at a dose of 2.0 mg/kg daily was most efficacious in reducing SIB, and the observed reductions in platelet serotonin and CSF 5HIAA levels indicated substantial bioeffect at this dose. Treatment with VEN was marked by noncompliance, low bioeffect, and low efficacy.
Abbreviations: bpm, beats per minute; CSF 5HIAA, cerebrospinal fluid 5-hydroxyindoleacetic acid; FLX, fluoxetine; PLC, placebo; SIB, self-injurious behavior; SNRI, serotonin–norepinephrine reuptake inhibitor; VEN, venlafaxine
Self-injurious behavior (SIB) is a considerable problem in adult rhesus macaques socially deprived in infancy or individually housed in captivity.4,14,20,33-35,41,42,50,51 In 1 report,30 the incidence of self-injury was estimated to be as high as 14% in captive populations of rhesus monkeys, the vast majority of which are male, with self-biting being the most prevalent form of injury. In a survey of 362 individually housed rhesus monkeys (age, 2 to 21 y) that had been housed individually for 4.6 ± 0.18 y (mean ± SE),34 33.7% exhibited self-directed stereotypic behavior and 25% exhibited self-biting behavior. Self-directed stereotypic behavior was positively correlated with age. Animals with severe SIB (mean, 9.8 y) were significantly older than those without a history of wounding (mean, 7.0 y). Although self-wounding can be an infrequent event, self-biting in this severe SIB population occurs frequently throughout the day in approximately 78% of animals.40 Severe cases of self-biting require prolonged veterinary care and often result in the removal of animals from research protocols.
Current research has focused on identifying the etiology of SIB with the ultimate goal of prevention. Some monkeys have an increased vulnerability that is associated with stressful social experiences in the first 2 y of life, such as early weaning. In susceptible adult animals, the behavior may be triggered by separation from sexual partners or social groups,8,17 contact with fear-provoking personnel,45 or disruption of daily routines.30 In 1 study,19 outdoor housing resulted in decreases in SIB and self-directed stereotypic behavior among animals previously housed indoors. However, among indoor-housed animals, manipulation of the environment by increasing cage space29,31 or providing toys,39 puzzle-feeders,41 or forage boards32 appears to have little effect on SIB. The limited utility of environmentally based interventions suggests that pharmacologic treatment of SIB should be examined carefully.
Previous studies suggest that serotonergic compounds are useful for short-term treatment of SIB and self-directed stereotypic behavior in macaques18 and for treatment of compulsive behaviors in companion animal species.27 Selective serotonin reuptake inhibitors, such as fluoxetine (FLX) and sertraline, have efficacy in the treatment of acral lick dermatitis in dogs.44,52 The tricyclic antidepressant clomipramine has been used effectively to treat compulsive behavior in cats49 and dogs.10,22 To date, no studies have examined the effects of combined serotonin–norepinephrine reuptake inhibitors on anxiety or compulsive behaviors in companion animals. However, the serotonin–norepinephrine reuptake inhibitor (SNRI) venlafaxine (VEN) appears to offer a profile similar to clomipramine with decreased side effects and has efficacy in the treatment of depression, panic attacks, and obsessive–compulsive behavior in humans.11-13,28,38
We performed a dose-finding study to establish optimal doses of subchronic FLX and VEN for the treatment of SIB and stereotypic behavior in adult male rhesus macaques. Rates and durations of SIB and self-directed stereotypies and plasma drug levels associated with escalating of doses of FLX and VEN were quantified. In addition, the effects of FLX and VEN on platelet serotonin (5-hydroxytryptamine) and cerebrospinal fluid levels of the serotonin metabolite 5-hydroxyindoleacetic acid (5HIAA) were measured to assess the bioeffect of the agents.
Materials and Methods
Animals.
The study included 17 male Indian-origin rhesus macaques (Macaca mulatta; age, 7 to 15 y). All animals were screened for evidence of self-wounding and characterization of self-directed stereotypic behavior. To be included in the study, an animal had to be singly housed and have had at least 1 episode of severe SIB requiring veterinary intervention within the past 5 y. In addition, subjects were not treated with any medication specifically for SIB for at least 1 y prior to this study (Table 1).
Table 1.
Subject history: age, incidence of severe SIB, and weight
| Animal | Treatment | Age (y)a | No. months single-housed | No. of incidents of SIB during last 5 yb | No. months since last SIB incidentc | Weight (kg) |
| A | FLX | 7 | 61 | 5 | 7 | 10.70 |
| B | FLX | 7 | 84 | 3 | 13 | 12.60 |
| C | FLX | 8 | 73 | 3 | 11 | 11.25 |
| D | FLX | 9 | 64 | 3 | 44 | 10.80 |
| E | FLX | 9 | 83 | 9 | 31 | 11.90 |
| F | PLC | 10 | 102 | 4 | 27 | 10.60 |
| G | PLC | 7 | 69 | 2 | 6 | 9.60 |
| H | PLC | 9 | 73 | 5 | 15 | 13.75 |
| I | PLC | 9 | 64 | 2 | 13 | 14.35 |
| J | PLC | 9 | 94 | 2 | 40 | 14.10 |
| K | PLC | 10 | 89 | 7 | 5 | 11.10 |
| L | VEN | 9 | 85 | 4 | 48 | 11.40 |
| M | VEN | 9 | 67 | 8 | 15 | 11.90 |
| N | VEN | 10 | 107 | 1 | 4 | 12.00 |
| O | VEN | 12 | 67 | 5 | 34 | 15.05 |
| P | VEN | 13 | 65 | 1 | 8 | 12.00 |
| Q | VEN | 14 | 98 | 2 | 9 | 9.80 |
Age (mean ± SE): FLX, 8 ± 0.5 y; PLC , 9 ± 0.5 y; and VEN, 11 ± 0.9 y
Median no. of incidents of severe SIB: FLX, 3.5; PLC, 2.0; and VEN, 3.0
No. of months (mean ± SE) since last incident of severe SIB: FLX, 22.2 ± 5.8; PLC, 15.8 ± 6.4; and VEN, 19.7 ± 7.1
Animals were inspected twice daily, in the morning and afternoon, for the presence of wounds. Incidents of new injuries (that is, punctures and lacerations) were recorded daily. An incident was classified as the presence of any new punctures or lacerations that day.
The animals were housed individually in 0.56 m2 cages in a 1-tiered system and fed a commercially available primate chow twice daily. Water was provided ad libitum. Additional fresh fruit and foraging devices were provided at least 5 d each week for enrichment. All animals were provided with manipulanda, including commercially available toys and wood, in accordance with the University of Louisiana at Lafayette–New Iberia Research Center plan for Environmental Enhancement and Behavioral Management. Husbandry was performed daily between 07:30 to 08:30.
The animals were serologically negative for simian retrovirus, simian T-cell leukemia virus, simian immunodeficiency virus, and B virus (Cercopithecine herpesvirus 1). All experimental procedures were approved by the Institutional Animal Care and Use Committee.
Dose selection.
Doses were selected based on allometric scaling from therapeutic dosages in humans (average weight, 70 kg):46
Significant protein binding, capacity-limited biotransformation, and genetic polymorphisms of cytochrome P450 isoenzymes all may influence the application of this equation. However, extrapolation from therapeutic ranges in humans provides the best initial estimate for dosages in macaques.46 The dose (mg/kg) ranges used for the present study largely overlap the ranges prescribed for humans (that is, FLX: 20 to 60 mg/day or approximately 0.3 to 1 mg/kg daily; VEN: 75 to 450 mg/day or approximately 1 to 7 mg/kg). The midrange FLX dose of 2.0 mg/kg dose was used in our previous study18 and gives trough drug concentrations consistent with levels seen in humans.23
Randomization and dosing schedule.
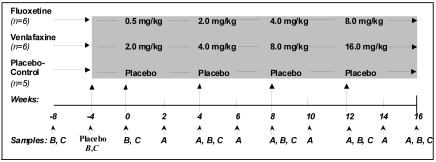
Animals were randomly assigned to 1 of 3 treatment conditions: FLX (n = 6), VEN (n = 6), and placebo (PLC; n = 5). After a 4-wk baseline period (B), a 4-wk lead-in placebo period (PP) was initiated. The animals then received, at 4-wk intervals, increasing doses of FLX (0.5, 2.0, 4.0, and 8.0 mg/kg daily) or VEN (2.0, 4.0, 8.0, and 16.0 mg/kg daily) (Figure 1). All medication was administered orally in a commercially prepared fruit-flavored scored tablet (Bioserv, Frenchtown, NJ). Tablets were available in 20 mg or 40 mg formulations. Animals in the PLC condition received nonmedicated fruit-flavored tablets. Animals were dosed daily between 10:00 to 12:30. Animals that did not consume the medicated tablets were offered medication in food or fruit treats. Not all animals consumed 100% of the medication daily; therefore, percentage consumption was recorded as the amount of the medicated food treat or tablet consumed.
Figure 1.
Study design. A, steady-state trough plasma drug samples; B, blood samples for biochemical analyses; C, cerebrospinal fluid samples for neurochemical analyses.
Plasma drug and metabolite concentrations.
Trough plasma levels of FLX, norfluoxetine, and VEN were determined from early-morning blood samples obtained before the daily dose of drug (Figure 1). Fluoxetine and norfluoxetine levels in plasma were assayed by using a method that we have previously used to measure the compounds in human plasma16 and were determined with within-assay coefficients of variation of 4% and 6%, respectively, and assay-to-assay coefficients of variation of 8 and 11%, respectively. Plasma VEN concentration was measured by using a similar HPLC–UV absorbance method with within-assay and assay-to-assay coefficients of variation of 4% to 5%.
The 4-wk dose interval allowed sufficient time to reach steady-state drug levels, which usually are attained after 4 to 5 half-lives. In humans, the half-life for each compound is: FLX, 2 to 4 d; norfluoxetine, 7 to 15 d; and VEN, 11 h.6,25,43,47
Behavioral observations.
Throughout the experiment, 10-min focal behavioral samples were collected from each animal twice weekly, including 1 morning and 1 afternoon sample. Animals were observed by trained technicians, who were blind to experimental condition, from a remote location by using a closed-circuit videocamera to eliminate interaction with observers. Behavioral data were collected by using handheld computers with The Observer software (Noldus Information Technology, Leesburg, VA). The Cohen kappa value for interobserver reliability was greater than 0.75.
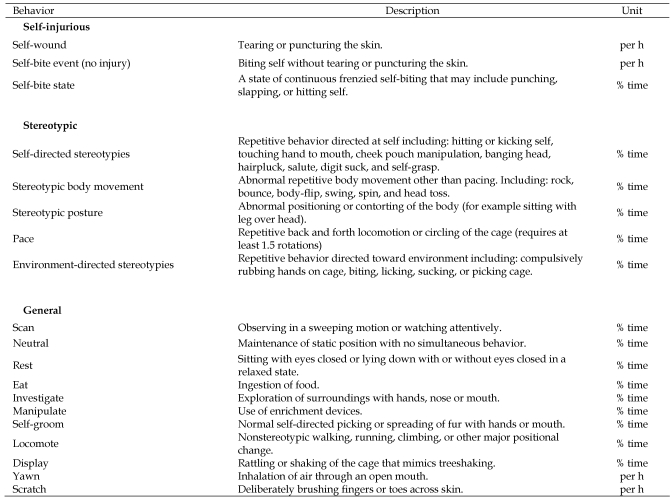
Behaviors that occurred nearly instantaneously (lasting less than 5 s) were categorized as events and calculated as rates (that is, no. events/h). Behaviors that occurred over a period of time were categorized as states and calculated as rates and percentage of total observation time. All behavior was averaged over 2-wk time periods. We distinguished self-biting behavior that does not cause injury from self-wounding. Stereotypic behavior was categorized based on our previous work19 and modified from another previous study.40 General behaviors were those associated with normal activity (Figure 2)
Figure 2.
Ethogram for adult male rhesus macaques.
Serum chemistries and physiologic measures.
Hepatic and renal functions were monitored throughout the study by assessing hepatic enzymes (alanine aminotransferase, aspartate aminotransferase, gamma glutamyltransferase), triglycerides, albumin, creatinine, and blood urea nitrogen concentrations. These samples were collected at 4-wk intervals (Figure 1). Normal reference ranges for rhesus are well-established.3 Serum chemistries were processed by the Center's clinical laboratory. At 4-wk intervals, animals were given a physical examination prior to CSF collection and anesthetized with ketamine HCl (15 mg/kg IM). Heart rate, blood pressure, body temperature, and body weight were obtained.
Neurochemical analyses.
Cerebrospinal fluid was collected from the cisterna magna by using sterile technique. Animals were fasted for 12 h before sample collection. Samples were obtained within 20 min of anesthesia by using a 1.5-in. 25-gauge needle. Approximately 1.0 mL CSF was obtained from each animal and frozen at –70 °C until assayed for neurochemical concentrations. Blood samples were obtained at 4-week intervals for determination of platelet serotonin concentration (Figure 1).
Levels of platelet serotonin were determined by using reversed-phase HPLC and fluorometric or electrochemical detection as previously described.2,15 Compounds were determined with within-day and day-to-day coefficients of variation of 4.0% to 14%.
Statistical analyses.
Because of poor compliance among animals treated with the highest dose level (DL4) of VEN, analyses of behavior, serum chemistry, and neurochemical data for animals treated with FLX, VEN, and PLC first were performed after excluding the highest dose level (DL4). Analyses of dose effects on behavior, serum chemistry, and neurochemical variables then were done for all dose levels in the FLX-treated and PLC animals. Analyses were done using Statistica (StatSoft, Inc, Tulsa, OK).
Total injuries for each animal were calculated for each treatment phase and analyzed by using repeated-measures ANOVA for animals in the VEN-treated condition through DL3 and for animals in the FLX-treated and PLC conditions through DL4.
Behavioral data were averaged over 2-wk time points to yield 2 baseline time points (B1, B2), 2 placebo time points (PP1, PP2), and 2 time points for each dose level (DL1-1, DL1-2, DL 2-1, DL2-2, DL 3-1, DL 3-2). Mixed-model ANOVA (1 between- and 2 within-subjects factors) was used to assess the effects of the 3 dose levels of each drug on SIB, stereotypic and general behavior, serum chemistry, and neurochemical data. The between-subjects factor was drug condition (FLX-treated, VEN-treated, PLC), and the within-subjects factors were phase (B, PP, DL1, DL2, DL3) and time points (weeks 1 to 2, weeks 3 to 4) within in each phase. All reported P values for effects involving within-subjects factors are Huynh–Feldt adjusted P values to correct for any possible violations of sphericity.26
Repeated-measures ANOVA was done to assess the effects of drug level on plasma drug (FLX and norfluoxetine) concentrations with phase (DL1 to DL4) as a within-subjects factor. Subsequent main effects and interactions were analyzed by using univariate contrasts. Correlations between CSF 5HIAA and platelet serotonin were computed separately for each subject in the FLX-treated and PLC conditions over the 6 phases (B through DL4) and for animals in the VEN-treated condition over 5 phases (B through DL3). These correlations (FLX-treated, PLC: n = 6; VEN-treated: n = 5) were first transformed to Fisher Z scores to meet normality assumptions and analyzed separately for each drug condition by using t tests. Mean Fisher Z scores were converted back to correlations for reporting. For animals in the FLX-treated condition, a similar analysis was used to assess the relationship between plasma drug concentrations, platelet serotonin, and CSF 5HIAA.
Results
Drug compliance (Table 2), as measured by the percentage of the offered drug that was consumed, was influenced by dose level and drug condition, as indicated by a phase × condition (FLX, VEN, or PLC) interaction (F3, 42 = 9.02; P < 0.001), a condition main effect (F2, 14 = 8.59; P < 0.01), and a phase main effect (F3, 42= 22.16; P < 0.001). Among animals in the FLX-treated condition, compliance decreased significantly during DL3 as compared with DL1 (F1, 14 = 13.20; P < 0.01) and DL2 (F1, 14 = 15.23; P < 0.01). Compliance among animals in the FLX-treated condition increased significantly for DL4 compared with DL3 (F 1, 14 = 7.19; P < 0.05) but remained significantly lower than DL1 (F1, 14 = 6.24, P < 0.05) and DL2 (F 1, 14 = 4.96; P < 0.05). Among animals in the VEN-treated condition compliance declined significantly for the 2 highest dose levels. Compliance during DL3 was significantly lower than DL1 (F1, 14 = 18.85; P < 0.001) and DL2 (F1, 14 = 21.23; P < 0.001). Compliance in DL4 was significantly lower than for DL1 (F1, 14 = 70.47; P < 0.001), DL2 (F1, 14 = 74.98; P < 0.001), and DL3 (F1, 14 = 6.15; P < 0.05). Compliance among animals in the PLC condition was 100% throughout the study.
Table 2.
Mean compliance (± 1 SE) among animals in the FLX and VEN conditions
| FLX |
VEN |
|||
| Phase | Dose (mg/kg/24 h) | % compliance | Dose (mg/kg/24 h) | % compliance |
| DL1 | 0.5 | 100 (0) | 2.0 | 96.8 (1.3) |
| DL2 | 2.0 | 96.1 (2.6) | 4.0 | 91.6 (5.3) |
| DL3 | 4.0 | 61.3 (12.6) | 8.0 | 50.5 (13.6) |
| DL4 | 8.0 | 80.8 (6.6) | 16.0 | 32.4 (11.8) |
Among animals in the drug-treated conditions, compliance decreased significantly over time within dose level as dose increased, as indicated by phase × time point × condition (F6, 42 = 4.25; P < 0.01) and phase × time point (F3, 42 = 6.67; P < 0.001) interactions and a time point main effect (F1, 14 = 9.59; P < 0.01). Animals in the FLX-treated condition were significantly less compliant during weeks 3 to 4 of DL3 (F1, 14 = 5.61; P < 0.05) compared with weeks 1 to 2 of DL3. For the 2 highest dose levels of the VEN-treated condition, compliance was significantly lower during weeks 3 to 4 compared with weeks 1 to 2 (DL3: F1, 14 = 15.42; P < 0.01; DL4: F1, 14 = 11.56; P < 0.01).
Due to the significant reduction in compliance among animals in the VEN-treated condition, further analysis of 3-way comparisons between FLX-treated, VEN-treated, and PLC conditions excluded DL4.
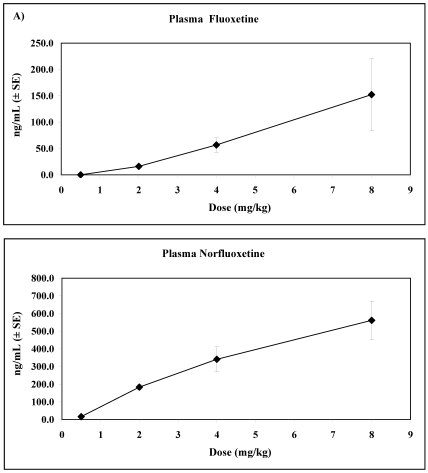
For animals in the FLX-treated condition, plasma drug concentrations increased as dose level increased. As shown in Figure 3 A, B, a significant phase main effect was found for plasma FLX (F3, 15 = 7.86; P < 0.05) and plasma norfluoxetine (F3, 15 = 24.94; P < 0.001). The plasma concentrations of FLX and norfluoxetine both increased with dose over the 4 phases (F1, 5= 9.73, P < 0.05; F1, 5 = 46.82, P < 0.001, respectively). The orthogonal polynomial contrast coefficients for the unequally spaced dose levels were examined, but the analysis indicated a nonlinearity trend for FLX (F1, 5 = 2.74, P = 0.16) and was not significant for norfluoxetine (F1, 5 = 0.37, P = 0.57). Plasma levels of VEN were only occasionally above the detection limit of the assay (4 ng/mL), precluding statistical analyses.
Figure 3.
Effects of dose level on concentrations (mean ± SE) of (A) plasma FLX and (B) plasma norfluoxetine among animals in the FLX-treated condition.
Daily visual inspection.
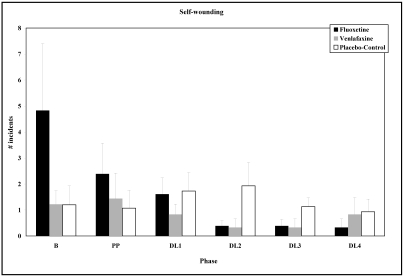
Among animals in the FLX- and VEN-treated conditions, self-wounding decreased as dose level increased. This effect approached significance in the FLX-treated condition only (Figure 4; F5, 25 = 2.86; P = 0.06, 1-tailed test).
Figure 4.
Effects of dose level on incidents of self-wounding (mean ± SE) among animals in the FLX-treated, VEN-treated, and PLC conditions.
Incidents of self-wounding recorded by daily visual examination did not differ significantly among animals in the VEN-treated and PLC conditions.
Effects of fluoxetine, venlafaxine, and placebo: excluding DL4.
SIB and stereotypic behavior.
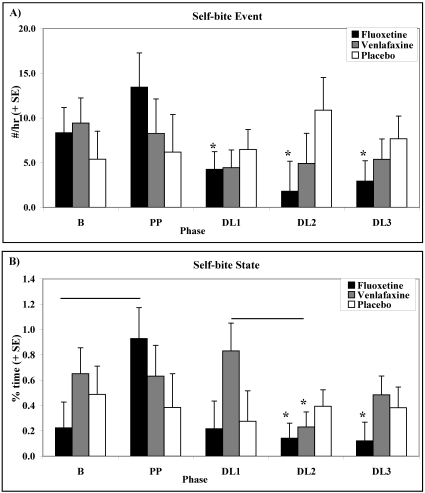
As depicted in Figure 5 A, rates of self-biting were influenced by dose level, drug condition, and time point as indicated by a phase × time point × condition interaction (F8, 56 = 3.50; P < 0.01), a phase × time interaction (F4, 56 = 4.37; P < 0.01), and a nearly significant phase × condition interaction (F8, 56 = 2.13; P = 0.066). For animals in the FLX-treated conditions, rates of self-biting increased significantly during weeks 1 to 2 of PP compared with B (F1, 14 = 11.83; P < 0.01) then declined significantly during weeks 3 to 4 of PP. In addition, among animals in the FLX-treated condition, rates of self-biting were significantly lower during DL1 (F1, 14 = 6.80; P < 0.05), DL2 (F1, 14 = 5.51; P < 0.05), and DL3 (F1, 14 = 6.67; P < 0.05) than they were in the averaged B and PP phases. Among animals in the FLX-treated condition, rates of self-biting did not differ significantly among DL1, DL2, and DL3. Rates of self-biting among animals in the FLX-treated condition during DL2 only were lower, approaching significance, compared with animals in the concomitant PLC condition (F1, 14 = 3.29; P = 0.09).
Figure 5.
Effects of dose level on (A) rates (mean ± SE) of self-biting and (B) percentage (mean ± SE) time spent self-biting among animals in the FLX-treated, VEN-treated, and PLC conditions. Horizontal bars represent P < 0.05 and * represent significant (P < 0.05) differences compared with averaged B and PP.
Self-biting did not decrease significantly among animals in the VEN-treated or PLC conditions. No incidence of self-wounding was observed directly during the study.
Self-biting state behavior, measured in percentage time, was affected by dose level and drug condition (Figure 5 B; phase × condition interaction: F8, 56 = 2.19, P < 0.05; phase main effect: F4, 56 = 2.74, P < 0.05). In the FLX-treated condition, percentage time self-biting increased significantly during PP compared with B (F1, 14 = 8.62; P < 0.01). Fluoxetine-treated animals spent significantly less percentage time self-biting during DL2 (F1, 14 = 6.71; P < 0.05) and DL3 (F1, 14 = 6.47; P < 0.05) compared with the averaged B and PP phases. Animals in the VEN-treated condition spent significantly less percentage time self-biting during DL2 compared with the averaged B and PP (F1, 14 = 6.00; P < 0.05) phases and DL1 (F1, 14 = 7.93; P < 0.01). There were no significant differences in percentage time self-biting among animals in the PLC condition.
There were no significant differences in phase or condition for percentage time spent performing self-directed stereotypies, stereotypic body movement, stereotypic posture, pacing, or environment-directed stereotypies.
General behavior.
A significant phase main effect (F4, 56 = 6.93; P < 0.001) was found for percentage time locomoting. Animals in all conditions spent significantly less % time locomoting in DL1 (mean ± SE, 1.65% ± 0.34%; F1, 14 = 5.41; P < 0.05) and DL2 (1.44% ± 0.34%; F1, 14 = 16.21; P < 0.001) compared with the averaged B and PP phases. Percentage time spent locomoting in DL3 (1.09% ± 0.18%) was significantly lower than the averaged B and PP phases (2.17% ± 0.47%; F1, 14 = 19.04; P < 0.001), DL1 (F1, 14 = 5.76; P < 0.05), and DL2 (F1, 14 = 5.12; P < 0.05).
A significant phase main effect (F4, 56 = 3.70; P < 0.05) was found for percentage time spent performing aggressive displays. Animals in all conditions spent significantly less time performing aggressive displays during DL2 (mean ± SE, 0.15% ± 0.05%; F1, 14 = 4.62; P < 0.05) and DL3 (0.07% ± 0.03%; F1, 14 = 10.77; P < 0.01) compared with the averaged B and PP phases (0.23% ± 0.07%).
In addition, a significant phase main effect (F4, 56 = 3.99; P < 0.01) was found for rates of yawning. Animals in all conditions yawned significantly less in DL1 (mean ± SE, 0.18% ± 0.02%; F1, 14 = 5.41; P < 0.05) and DL2 (0.18% ± 0.02%; F1, 14 = 16.21; P < 0.001) and DL3 (0.15% ± 0.02%; F1, 14 = 5.41; P < 0.05) compared with the averaged B and PP phases (0.25% ± 0.04%).
No significant main effects or interactions in percentage time scanning, resting, eating, investigating, or grooming nor in rates of scratching were found.
Serum chemistries and physiologic measures.
No significant effects of drug condition or dose level on concentrations of hepatic enzymes (alanine aminotransferase, aspartate aminotransferase, gamma glutamyltransferase), triglycerides, albumin, creatinine, or blood urea nitrogen concentration were found.
A significant phase × condition interaction (F8, 56 = 2.61; P < 0.05) was found for heart rate. In the FLX-treated condition, heart rate decreased during DL3 [111 ± 5 beats per minute (bpm); F1, 14 = 7.62; P < 0.05] compared with the averaged B and PP phases (127 ± 5 bpm). During DL3, heart rate among animals in the FLX-treated condition was significantly lower than in animals in the VEN-treated (130 ± 6 bpm; F1, 14 = 5.37; P < 0.05) and PLC (138 ± 7 bpm; F1, 14 = 9.74; P < 0.01) conditions. No significant effects of drug condition or dose levels on heart rate were found among animals in the VEN-treated and PLC conditions.
Neither drug condition nor dose levels affected blood pressure, body temperature, or body weight among animals in the FLX-treated, VEN-treated, or PLC conditions.
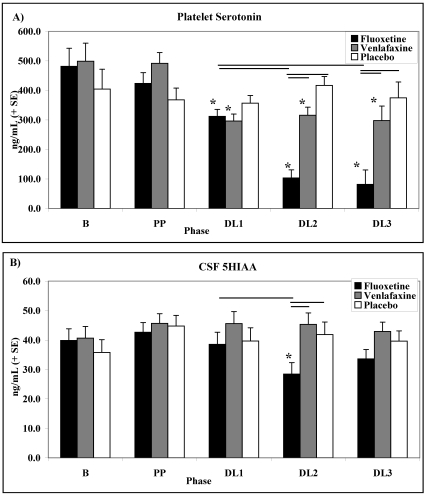
Platelet serotonin.
Among animals in the FLX-treated and VEN-treated conditions, platelet serotonin concentrations decreased as dose levels increased (Figure 6 A; phase × condition interaction: F8, 56 = 6.88; P < 0.01; condition main effect: F2, 14 = 4.59; P < 0.05; phase main effect: F4, 56 = 18.51; P < 0.001). Among animals in the FLX-treated condition, platelet serotonin concentrations decreased significantly during DL1 (F1, 14 = 22.40; P < 0.01), DL2 (F1, 14 = 60.84; P < 0.001), and DL3 (F1, 14 = 31.21; P < 0.001) compared with the averaged B and PP phases. In addition, among animals in the FLX-treated condition, platelet serotonin concentrations decreased significantly during DL2 (F1, 14 = 56.72; P < 0.001) and DL3 (F1, 14 = 20.79; P < 0.001) compared with DL1.
Figure 6.
Effects of dose level on (A) platelet serotonin concentrations (mean ± SE) and (B) cerebrospinal fluid 5HIAA concentrations (mean ± SE) among animals in the FLX-treated, VEN-treated, and PLC conditions. Horizontal bars represent P < 0.05 and * represent significant (P < 0.05) differences compared with averaged B and PP.
Among animals in the VEN-treated condition, platelet serotonin concentrations decreased significantly during DL1 (F1, 14 = 44.91; P < 0.001), DL2 (F1, 14 = 16.10; P < 0.001), and DL3 (F1, 14 = 8.82; P < 0.001) compared with the averaged B and PP phases.
During DL2 and DL3, platelet serotonin concentrations were significantly lower in the FLX-treated condition compared with the VEN-treated (DL2: F1, 14 = 29.89; P < 0.001; DL3: F1, 14 = 9.76; P < 0.01) and PLC (DL2: F1, 14 = 59.18; P < 0.001; DL3: F1, 14 = 16.32; P < 0.001) conditions.
No significant main effects or interactions in platelet serotonin concentrations between phases were found among animals in the PLC condition.
Cerebrospinal fluid monoamines.
Cerebrospinal fluid 5HIAA concentrations were influenced by dose level and drug condition, as indicated by a significant phase × condition interaction (Figure 6 B; F8, 56 = 2.90, P < 0.01) and a phase main effect (F4, 56 = 3.72, P < 0.01). For animals in the FLX-treated condition, CSF 5HIAA was significantly lower during DL2 compared with the averaged B and PP phases (F1, 14 = 61.33; P < 0.001) and DL1 (F1, 14 = 14.14; P < 0.01).
During DL2, CSF 5HIAA concentrations among animals in the FLX-treated conditions were significantly lower compared with animals in the VEN-treated (F1, 14 = 14.31; P < 0.01) and PLC (F1, 14 = 8.22; P < 0.01) conditions.
Relationship between platelet serotonin and CSF metabolite concentrations.
Platelet serotonin correlated significantly with CSF 5HIAA only in the FLX-treated condition (r = 0.85, t5 = 5.85, P < 0.01).
Effects of fluoxetine and placebo: DL4 comparisons.
SIB and stereotypic behavior.
In addition to the findings described previously, animals in the FLX-treated condition continued to exhibit decreased rates of self-biting and lower percentage time self-biting during DL4 (8 mg/kg) compared with the averaged B and PP phases (Table 3; F1, 9 = 6.38, P < 0.05 and F1, 9 = 10.68, P < 0.01, respectively). Rates of self-biting and percentage time self-biting in the PLC condition did not differ significantly between DL4 and the other phases. In addition, there were no significant differences for DL4 in self-directed stereotypies, stereotypic body movement, stereotypic posture, pacing, and environment-directed stereotypies.
Table 3.
Mean (1 SE) rates and % time self-biting, platelet serotonin, and CSF 5HIAA concentrations among animals in the FLX and PLC conditions
| Phase | Self-bite event (no./h) | Self-bite state (% time) | Platelet serotonin (ng/mL) | CSF 5HIAA (ng/mL) |
| FLX (n = 6) | ||||
| B | 8.28 (2.40) | 0.22 (0.22) | 481.45 (49.67) | 39.86 (2.43) |
| PP | 13.44 (4.56) | 0.93 (0.25) | 423.68 (35.88) | 42.66 (4.34) |
| DL1 | 4.26 (2.34) | 0.22 (0.11) | 312.13 (24.74) | 38.56 (2.63) |
| DL2 | 1.80 (3.90) | 0.14 (0.13) | 103.42 (18.77) | 28.46 (3.09) |
| DL3 | 2.94 (2.34) | 0.12 (0.14) | 81.57 (34.26) | 33.61 (3.59) |
| DL4 | 2.22 (1.80) | 0.15 (0.12) | 31.92 (23.50) | 30.24 (3.10) |
| PLC (n = 5) | ||||
| B | 5.40 (2.64) | 0.49 (0.24) | 404.46 (54.42) | 35.79 (2.67) |
| PP | 6.18 (4.98) | 0.39 (0.27) | 367.96 (39.03) | 44.78 (4.76) |
| DL1 | 6.48 (2.58) | 0.28 (0.12) | 256.92 (27.10) | 39.69 (2.88) |
| DL2 | 10.80 (4.26) | 0.39 (0.14) | 416.78 (20.56) | 41.89 (3.39) |
| DL3 | 7.38 (2.58) | 0.38 (0.15) | 374.92 (37.53) | 39.66 (3.93) |
| DL4 | 5.94 (1.98) | 0.36 (0.14) | 380.98 (25.74) | 42.25 (3.40) |
General behavior.
Percentage time spent locomoting remained significantly lower during DL4 (1.38% ± 0.32%; F1, 9 = 13.25; P < 0.001) compared with the averaged B and PP phases (2.26% ± 0.52%).
No significant main effects or interactions in percentage time scanning, resting, eating, investigating, grooming or performing aggressive displays were found during DL4, nor were any significant differences found for rates of yawning and scratching.
Heart rate, blood pressure, temperature, and body weight.
Heart rate (mean ± SE) remained lower among animals in the FLX-treated condition during DL4 (116 ± 7 bpm) compared with the averaged B and PP phases (127 ± 5 bpm; F1, 9 = 6.06, P < 0.05). Animals in the PLC condition exhibited an increase in heart rate during DL4 (154 ± 6 bpm) compared with averaged B and PP phases (133 ± 7 bpm; F1, 9 = 14.39, P < 0.01), DL2 (132 ± 7 bpm; F1, 9 = 13.60, P < 0.01), and DL3 (138 ± 7 bpm; F1, 9 = 9.00, P < 0.01). Heart rate was lower during DL4 among animals in the FLX-treated condition (116 ± 7 bpm) compared with animals in the PLC condition (154 ± 7 bpm; F1, 9 = 15.78, P < 0.01).
Platelet serotonin.
In addition to the reductions described previously, animals in the FLX-treated condition continued to exhibit decreased levels of platelet serotonin during DL4 compared with the averaged B and PP phases (F1, 9 = 105.6, P < 0.001), DL1 (F1, 9 = 102.1, P < 0.001), and DL2 (F1, 9 = 23.4, P < 0.001; Table 3). Among animals in the PLC condition, platelet serotonin concentrations during DL4 did not differ from the previous phases. Platelet serotonin concentrations during DL4 were lower among animals in the FLX-treated condition compared with animals in the PLC condition (F1, 9 = 100.3, P < 0.001).
Cerebrospinal fluid monoamines.
Among animals in the FLX-treated condition, CSF 5HIAA concentrations during DL4 were significantly lower than those for the averaged B and PP phases (Table 3; F1, 9 = 20.6, P < 0.001) and DL1 (F1, 9 = 16.5, P < 0.01). In addition, CSF 5HIAA concentrations were significantly lower during DL4 among animals in the FLX-treated condition compared with animals in the PLC condition (F1, 9 = 6.8, P < 0.05). No significant differences in CSF 5HIAA were found among animals in the PLC condition.
Relationships among plasma drug concentrations and neurochemical variables.
Plasma FLX significantly positively correlated with plasma norfluoxetine (r = 0.98, t5 = 18.7, P < 0.001) and negatively correlated with platelet serotonin (r = –0.75, t5 = –7.85, P < 0.001) and CSF 5HIAA (r = –0.52, t5 = –2.63, P < 0.05). Plasma norfluoxetine concentrations were also significantly negatively correlated with platelet serotonin (r = –0.82, t5 = –6.64, P < 0.001) and CSF 5HIAA (r = –0.53, t5 = –3.34, P < 0.05).
Discussion
Fluoxetine treatment at doses of 0.5, 2.0, 4.0, and 8.0 mg/kg daily was efficacious in reducing rates of self-biting. A trend in reductions in new incidents of self-wounding was noted among animals treated with FLX. Percentage time spent self-biting was reduced in animals treated at doses of 2.0, 4.0, and 8.0 mg/kg daily. Compared with those in animals receiving placebo, rates of self-biting showed the greatest reduction at a dose of 2.0 mg/kg FLX daily. Drug treatment produced no significant effects on general behavior. Therefore, reductions in SIB seen in animals treated with FLX and VEN were not likely to be due to sedation. These results are consistent with previous research that demonstrated a significant reduction in rates of self-biting at 1 to 4 wk of treatment with 2.0 mg/kg FLX.18 However, the current study did not find significant effects of FLX on durations of self-directed stereotypic behavior. In the previous study,18 the effects on self-directed stereotypic behavior did not occur until 5 wk of treatment with 2.0 mg/kg daily. In addition, baseline levels of percentage time spent displaying self-directed stereotypic behavior in the current study were substantially lower than observed previously.18
Compliance in the FLX-treated condition was generally high (100.0% to 96.1%) at the 0.5 and 2.0 mg/kg doses and decreased to 61.3% at 4.0 mg/kg and was 80.8% for the 8.0 mg/kg dose. Plasma concentrations of FLX and the active metabolite norfluoxetine increased with dose overall despite the reduced compliance at higher doses. Plasma FLX levels in animals receiving doses of 4.0 and 8.0 mg/kg daily approached those seen in human patients receiving a fixed dose of 20 mg daily for 8 wk and who were classified as responsive to treatment.1 However, plasma norfluoxetine levels in rhesus monkeys were at least 3-fold higher than typical human levels, as was the norfluoxetine:FLX ratio. Similar to the patient population, animals exhibited a large individual variability in plasma levels of FLX and norfluoxetine at all dose levels and showed no significant correlation between plasma drug or metabolite levels and reductions in self-biting.5
Species differences in cytochrome P450 enzyme isoforms (for example, 2C, 2D, 3A) may contribute to variable metabolic profiles.36 As depicted in Figure 3, plasma FLX level showed an apparent nonlinear increase with dose, with increased dose leading to greater than proportional increases in levels. Although only trend-level significant (1-tailed P = 0.08) when fit to a polynomial, this effect of FLX has been reported for humans and appears to result from FLX ‘s inhibition of cytochrome P450 enzymes.21
Venlafaxine also reduced the percentage time spent self-biting at a dose of 4.0 mg/kg daily, with an average compliance of 91.6%. Percentage time spent self-biting at higher doses of VEN were not significantly different from averaged B and PP levels; however, compliance was significantly lower at the 2 highest dose levels (that is, 50.5% at 8.0 mg/kg daily and 32.4% at 16.0 mg/kg daily). Plasma VEN levels were not detectable (that is, less than 4 ng/mL) at all dose levels. VEN apparently is not only highly unpalatable to rhesus macaques but also is metabolized extremely rapidly.
Reductions in platelet serotonin, which result from inhibition of the platelet serotonin transporter, are thought to be closely related to the degree of peripheral and central blockade.15,37 In FLX-treated animals, plasma FLX and norfluoxetine levels correlated negatively with platelet serotonin and CSF 5HIAA. Platelet serotonin levels decreased significantly among animals in both the FLX-treated and VEN-treated conditions at all dose levels examined. However, platelet serotonin levels were significantly lower among animals in the FLX-treated condition compared with animals in the VEN-treated and PLC conditions. This result suggests that FLX treatment produced a greater blockade of serotonin transporter than did VEN.
Concentrations of 5HIAA in CSF were significantly decreased in animals treated with FLX at 2.0 and 8.0 mg/kg daily. Problems with compliance among animals treated with 4.0 mg/kg daily of FLX may have influenced the apparent lack of response during this phase. Previous studies reported a 42% to 50% reduction in CSF 5HIAA after chronic SSRI administration to monkeys9,18 and is consistent with the 40% to 60% declines typically seen in human lumbar CSF after sustained clinical SSRI treatment.48 The SSRI-induced reduction in human CSF 5HIAA is well established and has been presumed to be due to uptake blockade leading to increased extracellular serotonin, resulting in a compensatory terminal autoreceptor-mediated decrease in serotonin turnover.2 As expected given the proposed mechanisms for the reductions seen in platelet serotonin and CSF 5HIAA, the 2 measures were highly correlated in the FLX-treated animals.
Given the absence of effects on CSF 5HIAA and the minimal effects on platelet serotonin, VEN appeared to have little effect on peripheral or central serotonin transport at the doses examined. Therefore, other mechanisms, such as noradrenergic reuptake inhibition, may be responsible for the decreases in percentage time spent self-biting found among animals treated with 4 mg/kg VEN (DL2). Venlafaxine has shown efficacy for treatment of SIB and repetitive behavior in patients with developmental disorders.7,24 Further studies will be required to fully address the potential efficacy of VEN or other SNRIs on self-injurious behavior in macaques.
In conclusion, given the relatively high rate of compliance and significant decreases in rates and percentage time spent self-biting, FLX at a dose of 2.0 mg/kg daily demonstrated the most efficacy. The reductions in platelet serotonin and CSF 5HIAA levels at this dose reflect substantial decreases in serotonin reuptake (bioeffect). At this dosage, no adverse effects on hepatic or renal indices or on physiologic variables (that is, heart rate, blood pressure, and body weight) were noted. Long-term studies are required to provide a more comprehensive characterization of the utility and effectiveness of FLX in the prevention and treatment of SIB in macaques. Although the SNRI VEN appeared less promising, other, less-aversive SNRIs might prove to be useful. Such studies likely will improve the care of captive macaques and may lead to useful models of human self-injurious behavior.
Acknowledgments
We thank Dr Thomas J Rowell for administrative support. We appreciate the technical assistance of Amy Dupuy, Amber Wright, and Michelle Grisham. We thank Charlie Perioux and his staff from the Division of Animal Resources for their care of the animals. This research was supported in part from a 2005 grant from the ACLAM Foundation.
References
- 1.Amsterdam JD, Fawcett J, Quitkin FM, Reimherr FW, Rosenbaum JF, Michelson D, Hornig-Rohan M, Beasley CM. 1997. Fluoxetine and norfluoxetine plasma concentrations in major depression: a multicenter study. Am J Psychiatr 154:963–969 [DOI] [PubMed] [Google Scholar]
- 2.Anderson GM, Barr CS, Lindell S, Durham AC, Shifrovich I, Higley JD. 2005. Time course of the effects of the serotonin-selective reuptake inhibitor sertraline on central and peripheral serotonin neurochemistry in the rhesus monkey. Psychopharmacology 178:339–346 [DOI] [PubMed] [Google Scholar]
- 3.Bernacky BJ, Gibson SV, Keeling ME, Abee CR. 2002. Nonhuman primates, p 675–791. In: Fox JG, Anderson LC, Loew FM, Quimby FW. Laboratory animal medicine. New York (NY): Academic Press [Google Scholar]
- 4.Brocco M. 1980. The isolation syndrome in rhesus monkeys: behavioral, pharmacological, and electrophysiological analyses. Dissertation Abstracts International 41-12B of 4484. (University Microfilms No. AAl8111977). [Google Scholar]
- 5.Brunswick DJ, Amsterdam JD, Fawcett J, Quitkin FM, Reimherr FW, Rosenbaum JF, Beasley CM., Jr 2002. Fluoxetine and norfluoxetine plasma concentrations during relapse-prevention treatment. J Affect Disord 68:243–249 [DOI] [PubMed] [Google Scholar]
- 6.Carchman SH, Crowe JT, Jr, Wright GJ. 1987. The bioavailability and pharmacokinetics of guanfacine after oral and intravenous administration to healthy volunteers. J Clin Pharmacol 27:762–767 [DOI] [PubMed] [Google Scholar]
- 7.Carminati GG, Deriaz N, Bertschy G. 2006. Low-dose venlafaxine in three adolescents and young adults with autistic disorder improves self-injurious behavior and attention deficit–hyperactivity disorders (ADHA)-like symptoms. Prog Neuropsychopharmacol Biol Psychiatry 30:312–315 [DOI] [PubMed] [Google Scholar]
- 8.Chamove A, Anderson J, Nash V. 1984. Social and environmental influences on self aggression in monkeys. Primates 25:319–325 [Google Scholar]
- 9.Clarke AS, Ebert MH, Schmidt DE, McKinney WT, Kraemer GW. 1999. Biogenic amine activity in response to fluoxetine and desipramine in differentially reared rhesus monkeys. Biol Psychiatry 46:221–228 [DOI] [PubMed] [Google Scholar]
- 10.Crowell-Davis SL, Seibert LM, Sung W, Parthasarathy V, Curtis TM. 2003. Use of clomipramine, alprazolam, and behavior modification for treatment of storm phobia in dogs. J Am Vet Med Assoc 222:744–748 [DOI] [PubMed] [Google Scholar]
- 11.Cordioli AV, Basso de Sousa M, Bouchi D. 2003. Intravenous clomipramine in severe and refractory obsessive–compulsive disorder. J Clin Psychopharmacol 23:665–666 [DOI] [PubMed] [Google Scholar]
- 12.de Montigny C, Silverstone PH, Debonnel G, Blier P, Bakish D. 1999. Venlafaxine in treatment-resistant major depression: a Canadian multicenter, open-label trial. J Clin Psychopharmacol 19:401–406 [DOI] [PubMed] [Google Scholar]
- 13.den Boer JA. 1998. Pharmacotherapy of panic disorder: differential efficacy from a clinical viewpoint. J Clin Psychiatry 59Suppl 8:30–36; discussion 37–38 [PubMed] [Google Scholar]
- 14.Eaton GG, Worlein JM, Kelley ST, Vijayaraghavan S, Hess DL, Axhelm MK, Bethea CL. 1999. Self-injurious behavior is decreased by cyproterone acetate in adult male rhesus (Macaca mulatta). Horm Behav 35:195–203 [DOI] [PubMed] [Google Scholar]
- 15.Epperson N, Czarkowski KA, Ward-O'Brien D, Weiss E, Gueorguieva R, Jatlow P, Anderson GM. 2001. Maternal sertraline treatment and serotonin transport in breast-feeding mother–infant pairs. Am J Psychiatry 158:1631–1637 [DOI] [PubMed] [Google Scholar]
- 16.Epperson CN, Jatlow PI, Czarkowski K, Anderson GM. 2003. Maternal fluoxetine treatment in the postpartum period: effects on platelet serotonin and plasma drug levels in breastfeeding mother–infant pairs. Pediatrics 112:e425–e429 [DOI] [PubMed] [Google Scholar]
- 17.Erwin J, Mitchell G, Maple T. 1973. Strangers in a strange land: abnormal behavior in non-isolate-reared rhesus monkeys. Psychol Rep 33:515–523 [DOI] [PubMed] [Google Scholar]
- 18.Fontenot MB, Padgett EE 3rd, Dupuy AM, Lynch CR, DePetrillo PB, Higley JD. 2005. The effects of fluoxetine and buspirone on self-injurious and stereotypic behavior in adult male rhesus macaques. Comp Med 55:67–74 [PubMed] [Google Scholar]
- 19.Fontenot MB, Wilkes MN, Lynch CS. 2006. Effects of outdoor housing on self-injurious and stereotypic behavior in adult male rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 45:35–43 [PubMed] [Google Scholar]
- 20.Gluck JP, Sackett GP. 1974. Frustration and self-aggression in social isolate rhesus monkeys. J Abnorm Psychol 83:331–334 [DOI] [PubMed] [Google Scholar]
- 21.Goodnick PJ, Goldstein BJ. 1998. Selective serotonin reuptake inhibitors in affective disorders. I. Basic pharmacology. J Psychopharmacol 12Suppl B:S5–S20 [DOI] [PubMed] [Google Scholar]
- 22.Hewson CJ, Luescher UA, Parent JM, Conlon PD, Ball RO. 1998. Efficacy of clomipramine in the treatment of canine compulsive disorder. J Am Vet Med Assoc 213:1760–1766 [PubMed] [Google Scholar]
- 23.Higley J. 2004. Personal communication.
- 24.Hollander E, Kaplan A, Cartwright C, Reichman D. 2000. Venlafaxine in children, adolescents, and young adults with autism spectrum disorders: an open retrospective clinical report. J Child Neurol 15:132–135 [DOI] [PubMed] [Google Scholar]
- 25.Howell SR, Hicks DR, Scatina JA, Sisenwine SF. 1994. Pharmacokinetics of venlafaxine and O-desmethylvenlafaxine in laboratory animals. Xenobiotica 24:315–327 [DOI] [PubMed] [Google Scholar]
- 26.Huynh H, Feldt LS. 1976. Estimation of the Box correction for degrees of freedom from sample data in the randomized block split plot designs. J Educ Stat 1:69–82 [Google Scholar]
- 27.International Veterinary Information Service [Internet] Evaluation and management of behavioral conditions [cited 18 Feb 2009]. Available at http://www.ivis.org/advances/Vite/overall/chapter_frm.asp?LA=1
- 28.Januel D, Poirier M, D'alche-Biree F, Dib M, Olie J. 2003. Multicenter double-blind randomized parallel-group clinical trial of efficacy of the combination clomipramine (150 mg/day) plus lithium carbonate (750 mg/day) versus clomipramine (150mg/day) plus placebo in the treatment of unipolar major depression. J Affect Disord 76:191–200 [DOI] [PubMed] [Google Scholar]
- 29.Jorgensen M, Kinsey J, Novak M. 1997. Effects of cage size on self-injurious behavior and abnormal behavior in macaques. Am J Primatol 42:120 [Google Scholar]
- 30.Jorgensen M, Kinsey J, Novak M. 1998. Risk factors self-injurious behavior in captive rhesus macaques (Macaca mulatta). Am J Primatol 45:187 [Google Scholar]
- 31.Kaufman B, Pouliot A, Tiefenbacher S, Novak M. 2004. Short- and long-term effects of a substantial change in cage size on individually housed, adult male rhesus monkeys (Macaca mulatta). Appl Anim Behav Sci 88:319–330 [Google Scholar]
- 32.Kinsey J, Jorgensen M, Novak M. 1997. The effects of grooming boards on abnormal behavior in rhesus monkeys (Macaca mulatta). Am J Primatol 42:122–123 [Google Scholar]
- 33.Kraemer GW, Clarke AS. 1990. The behavioral neurobiology of self-injurious behavior in rhesus monkeys. Prog Neuropsychopharmacol Biol Psychiatry 14:S141–S164 [DOI] [PubMed] [Google Scholar]
- 34.Lutz C, Well A, Novak M. 2003. Stereotypic and self-injurious behavior in rhesus macaques: a survey and retrospective analysis of environment and early experience. Am J Primatol 60:1–15 [DOI] [PubMed] [Google Scholar]
- 35.Mitchell GD. 1968. Persistent behavior pathology in rhesus monkeys following early social isolation. Folia Primatol (Basel) 8:132–147 [DOI] [PubMed] [Google Scholar]
- 36.Martignoni M, Groothuis GMM, de Kanter R. 2006. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol 2:875–894 [DOI] [PubMed] [Google Scholar]
- 37.Mulder EJ, Anderson GM, Kema IP, Minderaa RB. 2002. Reactivity of whole blood serotonin. Biol Psychiatry 51:266–268 [DOI] [PubMed] [Google Scholar]
- 38.Nelson JC, Mazure CM, Jatlow PI, Bowers MB, Jr, Price LH. 2004. Combining norepinephrine and serotonin reuptake inhibition mechanisms for treatment of depression: a double-blind, randomized study. Biol Psychiatr 55:296–300 [DOI] [PubMed] [Google Scholar]
- 39.Novak M. 1997. Enrichment of the environment—benefits to the animals: environmental enrichment for nonhuman primates, p 7–9. Performance standards and animal welfare: definition, application, and assessment. Part 1. In: Gonder JC, Krulische L. Greenbelt (MD): Scientists Center for Animal Welfare [Google Scholar]
- 40.Novak MA. 2003. Self-injurious behavior in rhesus monkeys: new insights into its etiology, physiology, and treatment. Am J Primatol 59:3–19 [DOI] [PubMed] [Google Scholar]
- 41.Novak MA, Kinsey JH, Jorgensen MJ, Hazen TJ. 1998. Effects of puzzle feeders on pathological behavior in individually housed rhesus monkeys. Am J Primatol 46:213–227 [DOI] [PubMed] [Google Scholar]
- 42.O'Neil M. 1982. Effects of an antidepressant drug given to isolated primates who display self-injurious behaviors: a comparative study. Psychology-General 43-07B of 2320. Dissertation Abstracts International [Google Scholar]
- 43.Preskorn SH. 1997. Clinically relevant pharmacology of selective serotonin reuptake inhibitors: an overview with emphasis on pharmacokinetics and effects on oxidative drug metabolism. Clin Pharmacokinet 32Suppl 1:1–21 [DOI] [PubMed] [Google Scholar]
- 44.Rapoport JL, Ryland DH, Kriete M. 1992. Drug treatment of canine acral lick. An animal model of obsessive–compulsive disorder. Arch Gen Psychiatr 49:517–521 [DOI] [PubMed] [Google Scholar]
- 45.Reinhardt V. 1999. Pair-housing overcomes self-biting behavior in macaques. Lab Primate Newsl 38:4 [Google Scholar]
- 46.Riviere JE. 1999. Comparative pharmacokinetics: principles, techniques, and applications. Ames (IA): Iowa State Press [Google Scholar]
- 47.Sanchez C, Hyttel J. 1999. Comparison of the effects of antidepressants and their metabolites on reuptake of biogenic amines and on receptor binding. Cell Mol Neurobiol 19:467–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheline Y, Bardgett ME, Csernansky JG. 1997. Correlated reductions in cerebrospinal fluid 5HIAA and MHPG concentrations after treatment with selective serotonin reuptake inhibitors. J Clin Psychopharmacol 17:11–14 [DOI] [PubMed] [Google Scholar]
- 49.Seksel K, Lindeman MJ. 1998. Use of clomipramine in the treatment of anxiety-related and obsessive–compulsive disorders in cats. Aust Vet J 76:317–321 [DOI] [PubMed] [Google Scholar]
- 50.Tiefenbacher S, Novak MA, Jorgenson MJ, Meyer JS. 2000. Physiological correlates of self-injurious behavior in captive, socially reared rhesus monkeys. Psychoneuroendocrinology 25:799–817 [DOI] [PubMed] [Google Scholar]
- 51.Weld KP, Mench JA, Woodward RA, Bolesta MS, Suomi SJ, Higley JD. 1998. Effect of tryptophan treatment on self-biting and central nervous system serotonin metabolism in rhesus monkeys. Neuropsychopharmacology 19:314–321 [DOI] [PubMed] [Google Scholar]
- 52.Wynchank D, Berk M. 1998. Fluoxetine treatment of acral lick dermatitis in dogs: a placebo-controlled randomized double-blind trial. Depress Anxiety 8:21–23 [PubMed] [Google Scholar]