Abstract
The thymus has long been known as the generative organ for the T cell arm of the immune system. To perform this role, the thymus was thought to require protection from antigenic and cellular insult from the “outside world”, with the notable exception of the continual influx of progenitor cells required to initiate the complicated process of T cell differentiation. Overwhelming evidence that mature T cells can recirculate and persist in the thymus has required us to revamp this earlier view of the thymus as detached from outside influence. In this review, we consider the evidence for T cell recirculation into the thymus, discuss the likely means and location of mature T cell entry, and speculate on the potential consequences of such close apposition between differentiating thymocytes and mature recirculating lymphocytes.
Keywords: thymus, T cell recirculation, negative selection, positive selection
INTRODUCTION
Prior to focusing on the potential impact on the thymus of immigrating peripheral T cells, a discussion of the highly regulated development of T cells within the thymic stromal cell maze is in order. T cell differentiation in the thymus is a complex and tightly controlled process that begins with the immigration of bone marrow-derived progenitor cells, and ends with the generation of self-tolerant, lineage committed T cells capable of performing an array of immune functions upon recognition of antigen in the context of molecules encoded by self major histocompatibility (MHC) genes (reviewed in1). The T cell precursors that first enter the thymus do not express the antigen recognition machinery, lacking both the coreceptors (CD4 and CD8) that focus T cell attention on MHC molecules and the T cell receptors for antigen recognition (TCRαβ and TCRγδ). The genes encoding these highly diverse TCRs undergo a carefully programmed series of DNA rearrangements triggered within the thymus in a stepwise fashion, beginning in CD4−CD8− (double negative or DN) thymocytes (reviewed in2). For conventional TCRαβ T cells that recognize peptide antigens presented by classical MHC molecules, commitment to the T cell lineage is sealed by rearrangement of the TCRβ gene. The process of β selection tests the accuracy of this rearrangement event, and drives the proliferation and CD4 and CD8 coreceptor expression by those cells expressing a functional TCRβ chain (defined as one that pairs with the product of the unrearranged pre-Tα gene)3. The result is a large population of CD4+CD8+ (double positive or DP) thymocytes that initiate TCRα rearrangement. Accurate TCRα rearrangement along with successful TCRαβ chain pairing and surface expression are required for positive selection, the second critical checkpoint for maturing T cells. Positive selection is driven by the successful, low affinity interaction between the expressed TCRαβ receptor on a DP thymocyte and self-peptide in the context of self-MHC3,4. Positive selection rescues DP thymocytes from the alternative destiny of programmed cell “death by neglect”, and drives the accurate alignment between coreceptor expression and lineage commitment1,3,4. This process results in a population of CD4+CD8− (single positive or SP) thymocytes that can differentiate into helper T cells upon further recognition of peptide presented by MHC class II molecules, and CD4−CD8+ SP thymocytes that can differentiate into killer T cells upon encounter with antigen presenting cells whose MHC class I molecules carry the appropriate peptides. At the DP or SP stages, thymocytes are subjected to negative selection, the third checkpoint that regulates T cell development. During this process, central to the establishment of self-tolerance among developing T cells, TCRαβ+ thymocytes that react with inappropriately high avidity to self peptide/MHC complexes are deleted5–7. The 1% of thymocytes that successfully transit β selection, positive selection, and negative selection undergo additional maturation that promotes their regulated exit from the thymus. After further maturation in the lymphoid periphery, these recent thymic emigrants join the pool of mature peripheral T cells. The induction of self-tolerance among any remaining self-reactive T cells continues in the lymphoid periphery by several means, including through the activity of regulatory T cells (Tregs, reviewed in8). The population of mature peripheral T cells recirculates from blood to the lymph and back again, patrolling the spleen and lymph nodes for foreign invaders. Lymphocyte recirculation is a carefully controlled, multistep process, regulated by the expression on lymphocytes of homing receptors (including integrins and chemokine receptors) and by the expression or elaboration by stromal elements of addressins, the ligands for lymphocyte homing receptors (reviewed in9–11).
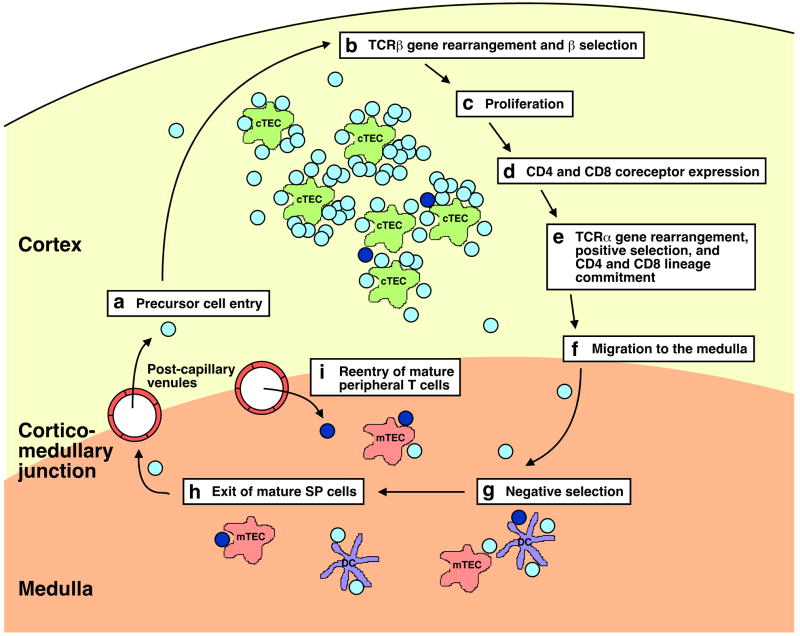
As with most developmental systems, the close association between differentiating thymocytes and the stromal elements of the thymus itself is key to the regulation of these maturation events. The thymus can be loosely categorized into densely packed cortical (outer) and more sparsely populated medullary (inner) regions (Figure 1)12. It is where these regions meet, the cortico-medullary junction, that the postcapillary venules lie13–16. There is a very close association between the thymic epithelium and these postcapillary venules17, which are the only vessels in the thymus large enough to allow cellular extravasation18, and are surrounded by a well-defined peri-vascular space. This space appears to serve as a transit pathway for both progenitor cell entry and emigration of mature thymocytes16. In addition, the thymus likely contains lymphatic vessels, and these may also serve as entry and exit routes for lymphocytes14,15. The nonthymocyte elements of the thymus include cortical and medullary thymic epithelial cells, dendritic cells, macrophages, and a small number of B cells. Cortical thymic epithelial cells are the primary mediators of positive selection3,4, although hematopoietic cells are known to positively select some populations of developing thymocytes19. Medullary thymic epithelial cells that are specialized to express tissue-specific antigens and dendritic cells mediate negative selection5,6. The importance of thymic architecture to T cell maturation is underscored by the recent observation that developing T cells traverse distinct thymic zones during the course of their differentiation20,21. Progenitor cells enter the thymus via the vessels at the cortico-medullary junction, and the immature DN thymocytes migrate to the outer cortex, over the course of approximately 2 weeks, differentiating along their journey. Their DP progeny then retrace this migration pattern, more rapidly and in retrograde fashion, traveling back to the inner cortex, and from there, into the medulla, where most SP thymocytes reside21. Thus, the various stages of T cell differentiation, including TCRβ gene rearrangement, β selection and cell proliferation, dual coreceptor expression, TCRα gene rearrangement, positive selection, lineage commitment, downregulation of inappropriate coreceptor expression, and negative selection, all take place in separate thymic microenvironments, defined by distinct stromal elements (Figure 1). It appears that most mature thymocytes leave for the periphery from the cortico-medullary junction, although when this pathway is blocked, exit from the cortex may also be possible22.
Figure 1. T cell development proceeds through the checkpoints of β selection, positive selection, and negative selection as developing thymocytes migrate through distinct thymic microenvironments. Reentering mature T cells may intersect this pathway, possibly impacting both positive and negative selection.
(a) and (i) Progenitor cells and mature peripheral T cells both likely enter the thymus through post-capillary venules at the cortico-medullary junction. Developing thymocytes (depicted as light blue cells) follow the migration patterns detailed below, while reentering mature peripheral T cells (depicted as dark blue cells) persist mainly, although not exclusively, in the medulla. (b) Developing T cells migrate to the outer cortex of the thymus and recombine TCRβ chain genes. Successful generation of a TCRβ chain drives cells to (c) proliferate and (d) co-express CD4 and CD8 coreceptors, and migrate deeper into the cortex. (e) DP thymocytes rearrange TCRα gene segments. Surface expression of the resulting TCRαβ molecules is required for positive selection, driven most efficiently by low-affinity interaction with self-MHC complexes on cortical thymic epithelial cells (cTECS). Positively selected cells will commit to the appropriate CD8 or CD4 T cell lineage. (f) The resulting SP thymocytes migrate into the medulla. In the medulla (g), expression of tissue-specific antigens by medullary thymic epithelial cells (mTECs) and presentation of self-antigens by dendritic cells (DC) enforce central tolerance by the clonal deletion of self-reactive SP thymocytes. (h) Mature CD4SP and CD8SP thymocytes likely exit via the post-capillary venules at the cortico-medullary junction.
MATURE LYMPHOCYTES CAN REENTER THE THYMUS
Recirculation of mature lymphocytes to the thymus was documented shortly after general acceptance of the notion that the thymus, despite the signs of intrathymic cellular death, was at least partly responsible for exporting immune cells to the lymphoid periphery. Cells labeled in vivo with 3H-thymidine were isolated from the lymph nodes draining an allogeneic skin graft, injected into syngeneic recipients, and recovered, in small numbers, 1–3 days post transfer from the host thymus23. Additional reports followed24,25, although the failure to consistently document the ability of lymphocytes to home to and persist in the thymus proved confusing26,27. The ability to track cells by their immune functions enabled the more consistent detection of often rare recirculating cells in the thymus. Mature lymphocyte immigration into the thymus has now been well documented in the mouse23,28–43, the rat25,44–48, and the pig49.
Which mature recirculating cell types are allowed access to the thymus? In most systems, B cells do not appear to enter the healthy adult thymus or persist there39,46, but there are reports to the contrary, in both healthy30,47 and autoimmune individuals32,50. Bone marrow-derived dendritic cells and other antigen presenting cells can travel to the thymus of allogeneic and syngeneic recipients under some conditions38,47,48,51,52, but not others39,48,53.
Much evidence suggests that the thymus lobes of healthy individuals are perhaps uniquely accessible to recirculating T cells. The T cell identity of at least some recirculating lymphocytes recovered from the thymus became clear from experiments in which T cell lines, induced in vivo44 or in vitro45, homed (albeit inefficiently) to the thymus. In the latter case, these thymic immigrants were recovered up to several months later as immunocompetent T cells. A few years after this report, Thy-1 allotype marked lymph node cells were transferred to congenic hosts that were then primed to minor histocompatibility antigens to induce a killer T cell response. Thy-1 typing of the resulting cytolytic effector cells revealed that 5–60% of the resulting cytolytic activity recovered from the host thymus was mediated by donor cells28. The rule dictating the unidirectional traffic of mature T cells out of (but not into) the thymus clearly required rewriting.
Further technological advances have improved our ability to detect and quantify the contribution of recirculating T cells to the thymocyte pool. Whole body immunohistochemistry of adult recipients of allotype marked TCRαβ transgenic T cells primed to their cognate antigen in the host environment revealed the entry into the host thymus of donor T cells37. While the number of immigrant mature T cells peaked at approximately 106 per thymus, their numbers stabilized at 105/thymus for up to 60 days post transfer and priming. Entry of mature cells into the thymus is not an indirect result of associated in vitro manipulations of the donor cells, as delivery of donor cells through parabiosis has also demonstrated immigration of T cells from one partner into the thymic lobes of the second parabiont31,41. T cells do not reenter the thymus purely as a result of surgical perturbations or from the intravenous injection of a bolus of mature T cells. Recirculation of mature T cells to the thymus has also been demonstrated in unmanipulated mice in a genetically engineered model system. In mice transgenic for green fluorescent protein (GFP) under the control of the RAG2 promoter, developing thymocytes after the late DN stage fluoresce, as do mature SP thymocytes54. Analysis of GFP− mature T cells within the thymic lobes of such RAG2p-GFP transgenic mice has permitted the quantification of recirculating, nonfluorescent T cells in unmanipulated mice from 2 days to nearly 2 years of age40,42,54,55. Work by Hale and colleagues revealed that the proportion of the SP compartment that is comprised of GFP−, recirculating, mature (CD24lowQa2hi) cells increases with age (particularly within the first five months), although in the steadily shrinking thymic lobes, the numbers of immigrants remain somewhat more constant (Figure 2).
Figure 2. Mature peripheral T cells recirculate to the thymus of unmanipulated mice.
Thymocytes from RAG2p-GFP mice were analyzed by flow cytometry for cell surface markers and GFP. (a) Representative dot plots from an approximately 70 week old mouse, showing that populations gated as CD4SP (left) and CD8SP (right) contain GFP+ SP thymocytes and GFP− mature T cells that have reentered the thymus. (b) Cells gated as CD4SP (left) and CD8SP (right) were counterstained with the indicated antibodies to reveal the cell surface phenotype of GFP− (open histograms) and GFP+ cells (filled histograms). The GFP− populations consist of predominantly mature (CD24lowQa2hi) and previously activated (CD44hi) cells. (c) The percent of the total SP thymic compartment comprised of reentering peripheral T cells increases with age. (d) The number of GFP− mature T cells found in the thymus initially increases and then remains relatively stable with age.
WHAT TYPES OF MATURE T CELLS REENTER THE THYMUS?
Both CD4 and CD8 T cells appear to reenter the thymus29,33,40,42,54,56 in approximately the proportions they represent in secondary lymphoid organs and blood. Much has been written on the relative homing into the thymus of activated versus naive recirculating T cells. It is clear from experiments directly comparing the immigration of activated and naive T cells that prior exposure to antigen or mitogen enhances the homing of mature T cells to the thymus. Injection of alloantigen-activated blasts or naive T cells into Thy-1 allotypic hosts revealed an estimated 50-fold preference for thymic homing of the activated T cell population33. T cells activated by antigen, either in vivo37,38,48 or in vitro45, showed a similar pattern of thymic homing. Comparable results were obtained following injection of T cells sorted by phenotypic marker expression into naive and previously activated populations46,47, although the fold preference of homing by previously activated cells in these experiments was a more modest 4–6 fold. Analysis of recirculating mature T cells in the thymic lobes of unmanipulated mice (Figure 2) also demonstrated that most cells are CD44hi, and therefore previously activated40. Reentry into the thymus may be particularly robust by T cells that have entered the S phase of the cell cycle33, although it is clear that resting, previously activated cells also enter the thymus more efficiently than do naive cells46,47.
While there is general agreement regarding the preferential thymic reentry of activated relative to naive T cells, it is likely this preference is a quantitative one. Thus, many reports have demonstrated that small numbers of naive, nonactivated T cells can also gain access to the thymus24,25,29,36,40,46,47. In RAG2p-GFP transgenic mice, reentering GFP− mature T cells are predominantly, but not exclusively, CD44hi. Thus, 5–15% of GFP− thymocytes are CD44low, and therefore naive (Figure 2 and data not shown). Hale and coworkers found that approximately 600 recirculating T cells seeded the thymus of young adult recipients 4 days following injection of 6×106 naive, allotype marked T cells40. Given the estimated 10% take of intravenously injected spleen and lymph node cells57 (a number that agrees well with the number of donor cells recovered from the spleens of these recipients), an estimated 0.1% of naive T cells reenter the thymus and persist there40. In contrast, injection of 107 alloantigen-activated T cell blasts resulted in 5×104 cells donor cells in the host thymus by day 6. Assuming a 10% take and no proliferation (an assumption that is very likely wrong), this translates to 5% of activated T cells that reenter the thymus33.
WHERE AND HOW DO MATURE T CELLS REENTER THE THYMUS?
There appears to be general agreement that recirculating T cells enter the thymus through the postcapillary venules at the cortico-medullary junction13–16 and persist in the medulla29–31,33,37,47,56. There are reports of recirculating cells in the cortex23, although in these earlier experiments, a clear distinction between mature T cells and progenitor cells (known to take up residence in the cortex) could not be established. In more recent experiments, unambiguously mature T cells made a rare appearance in the cortex, at a frequency of 5-fold56 or 20–60 fold lower than in the medulla, in which 0.2 to 0.3% of the cells were calculated to be of donor recirculating T cell origin29.
A few groups have investigated the molecules that promote mature T cell immigration into the thymus. Migration into the thymus of previously activated peripheral T cells in the rat was found to be α4 integrin dependent. Entry into the thymus was blocked by antibody against VCAM-1, implicating the interaction between α4 integrin expressed on the T cell surface (probably paired with β1) and VCAM-1 expressed on the thymic vasculature in T cell migration across the thymic endothelium and into the medullary compartment of the thymus46. In the hyperplastic mouse thymus, expression on the T cell of L-selectin and α4β7 integrin mediates the respective interaction between PNAd and MAdCAM-1 on the thymic vasculature or stromal cells58, thereby promoting T cell immigration59,60. Whether T cell recirculation to the normal thymus is similarly controlled is unclear, although L-selectin expression by immigrant donor T cells in the thymus has been noted previously in adults30,42, but not in neonates35. It is clear that much more needs to be learned about the control of T cell immigration into the thymus. In particular, it would be interesting to understand whether expression of CCR7, known to be important for medullary access by developing thymocytes21,22, is also required for efficient reentry of mature peripheral T cells into the thymic medulla.
WHAT PROMOTES LYMPHOCYTE IMMIGRATION INTO THE THYMUS?
Under what conditions is thymic immigration of mature T cells enhanced? The neonatal thymus is very permeable to both naive and previously activated CD4 and CD8 T cells26,35. Within a day after injection of 5–10×106 naive adult T cells, 2–5% of the mature (CD24low) SP compartment of the host thymus was comprised of transferred cells in week old neonates35. Thymus lobes from aged mice are also much more leaky to naive T cells than are those from young adults40. While an average of 600 cells lodged in the young adult thymus after intravenous injection of 6–8×106 naive CD8 T cells, approximately 6,500 cells homed to the thymus of 70–76 week old recipients40. This permeability is unlikely to be due solely to the sparse DP compartment found in the aged thymus. Thus, irradiation and cortisone injection, known to deplete the DP compartment, failed to promote the thymic immigration of naive T cells in young adults, although irradiation did increase the frequency of previously activated cells reentering the thymus33.
Certain disease states have also been shown to promote thymic immigration by recirculating T cells. Mature resting T cells readily enter the atrophic thymus of T cell deficient SCID mice, and persist there for months34. Lymphopenia induced by deletion of the pre-Tα gene or by the generation of radiation bone marrow chimeras engineered such that thymic output is approximately 1% of normal levels both increase migration to the thymus of mature T cells41. At the opposite end of the spectrum, the hyperplastic thymus of AKR mice is similarly permissive to immigrating mature T cells. In this case, an increase in the number of medullary blood vessels expressing appropriate addressins has been identified as the likely driving force behind this influx59,60. In another model, intravenously injected T cells localize to the thymus lobes of old (but not young) (NZB x SJL) F1 mice, correlating with the onset of disease in this autoimmune-prone strain50.
Setting aside the findings in hyperplastic AKR thymuses and aged (NZB x SJL) F1 mice, which analyze the immigration of mature T cells that may themselves display aberrant characteristics, T cell lymphopenia characterizes each of the conditions shown to promote enhanced donor T cell migration into the thymus. Why this might be has not been experimentally addressed.
DO RECIRCULATING LYMPHOCYTES SERVE A FUNCTION IN THE THYMUS?
Why are the thymic lobes of individuals across many species permeable to mature T cells? The preferential migration of activated relative to naive T cells into the thymus and their long term persistence within this organ have led to the notion that the thymus may serve as a repository of T cell memory28,45. A parallel function for the B cell generative compartment, the bone marrow, has been supported by considerable experimental evidence61–64. What would be the advantage of selective recruitment of memory T cells into the thymus and bone marrow? This sheltered environment may protect the memory pool from cytokine deprivation, environmental insult, or triggers of homeostatic proliferation. It could also help rid the generative organs of infected cells that could negatively select antigen-reactive thymocytes, most needed in the periphery during an infection. Thus, retaining some memory T cells within the thymus could help keep this generative organ free of foreign antigens and ensure the development of new antigen-specific T cells during times of need.
A role in tolerance induction has also been ascribed to immigrating T cells within the thymus. The permeability of the neonatal thymus to naive T cells facilitates the intrathymic induction of tolerance to superantigens expressed by injected CD8 T cells, as measured by the deletion of reactive thymocytes65. A similar function has been ascribed to allogeneic T cells experimentally introduced into the adult39. Thus, induction of self-tolerance among developing thymocytes to antigens expressed primarily by mature T cells might benefit from the reentry of antigen-expressing mature T cells into the thymus. Conversely, migration into the thymus of self-activated T cells may promote the deletion of those cells upon encounter with their cognate antigens in the thymus. For example, T cells that develop in young individuals and are specific for antigens expressed only in the adult may be more easily deleted upon encounter with the relevant antigen while sequestered within the thymus38,48. It has also been proposed that T cells can acquire self-antigens that they recognize, and through their migration into the thymus, present these passively acquired antigens in tolerogenic form to developing T cells33,44.
It is apparent that there is considerable crosstalk between positively selected thymocytes and medullary thymic epithelial cells. Thus, formation of a medullary compartment requires the presence of mature thymocytes66. Reentering mature T cells may also alter the stromal environment within the thymus by helping regulate the size and integrity of the medullary thymic epithelial cell compartment34. The close apposition of the thymic vasculature and medullary epithelial cells is also of interest17, as it is through this area that mature T cells are likely to enter. Given the importance of the thymic medullary epithelial compartment for inducing self tolerance5–7, immigrating mature T cells may indirectly help regulate autoimmunity by influencing the thymic environment. In fact, recent evidence indicates that mature CD4 T cells (SP thymocytes in these experiments) help regulate the expansion of medullary thymic epithelial cells expressing tissue specific antigens67,68. It is these cells that have been implicated in negative selection. It is still unknown whether reentering CD4 T cells can serve this same function.
The recently recognized potential for mature Tregs to migrate into the thymus has renewed the debate on the potential intrathymic function for immigrating T cells. Parabiosis between wildtype mice and those lacking the gene encoding the preTα chain of the pre-TCR demonstrated that Tregs found in the thymus of the latter mice originated predominantly from the pool of recirculating Tregs derived from the wildtype partner41. The depletion of peripheral CD4+ Tregs in anti-CD4 antibody transgenic mice has revealed that at least 20% of thymic Tregs are likely derived from the pool of recirculating Tregs that reenter the thymus43. The use of RAG2p-GFP transgenic mice has also enabled the characterization of immigrating mature (GFP−) T cells within the thymus of unmanipulated individuals. Approximately 50% of thymic Tregs were found to be GFP− nonproliferating cells, and therefore represent reentering peripheral T cells42,55. It is not clear what intrathymic function can be ascribed to these returning Tregs, but a role in tolerance induction is attractive, given their ability to control self-reactivity among peripheral T cell populations8.
One final intrathymic function has been proposed for mature T cell immigrants. Recent work has suggested that reentering mature T cells expressing the requisite MHC class I molecules can positively select CD8+ T cells expressing a uniform TCR56. In these experiments, positive selection was defined as the appearance of mature CD8 SP thymocytes, while the appearance of functional T cells was not analyzed. Despite the somewhat inefficient appearance of these mature CD8 SP thymocytes, this work offers an exciting addition to the repertoire of functions performed by mature T cells that manage to reenter the thymus, and may help explain how functional T cell immunity can be achieved under experimental conditions in which hematopoietic cells differentiate in mismatched thymic epithelial compartments56. Table 1 summarizes each of the potential functions outlined above that returning peripheral T cells might serve as they masquerade as thymocytes.
Table 1.
Possible Roles for Mature T cells that Recirculate to the Thymus
| Function | References |
|---|---|
| Provide a repository for memory T cells | Naparstek, 1982; Fink, 1984; Gopinathan, 2001 |
| Eliminate foreign antigens from the thymus during an infection | Naparstek, 1982; Fink, 1984 |
| Induce negative selection to antigens they express | Webb, 1990; Surh, 1993; Tian, 2004 |
| Induce self tolerance in the returning, activated T cells themselves | Ben-Nun, 1982; Chau, 2002 |
| Induce negative selection to antigens they passively acquire through their TCRs | Agus, 1991; Gopinathan, 2001 |
| Maintain medullary thymic epithelial cells | Surh, 1992; Hikosaka, 2008; Irla, 2008 |
| Provide the thymus with Tregs that have been exposed to antigens in the periphery | Bosco, 2006; McCaughtry, 2007;Zhan, 2007; Weinreich, 2008 |
| Mediate positive selection | Kirberg, 2008 |
CONCLUDING COMMENTS
The well-documented immigration of mature T cells into the thymus has required us to alter our earlier views that this generative organ has evolved in part to provide a foreign antigen- and mature cell-free zone to promote thymocyte maturation without environmental insult. We now know that mature CD4 and CD8 T cells do return to the thymus, and that their reentry is facilitated by prior activation. The means by which these cells enter the thymus, and the reasons why their immigration is apparently promoted in adults under lymphopenic conditions and in neonates are currently unknown. Several non-mutually exclusive functions have been proposed for these migrants, including roles in providing a repository for memory T cells, eliminating foreign antigen in the thymus during times of infection, inducing negative selection to antigens they express or passively acquire, maintaining medullary thymic epithelial cells, providing a population of Tregs exposed to tissue-specific antigens in the lymphoid periphery, and mediating positive selection.
Acknowledgments
The authors gratefully acknowledge the NIH for its support though R01 AI 064318 (to P.J.F.) and the Cancer Research Institute’s Predoctoral Emphasis Pathway in Tumor Immunology Program (to J.S.H.).
References
- 1.Singer AL, Adoro S, Park JH. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat Rev Immunol. 2008;8:788–801. doi: 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schatz DG. V(D)J recombination. Immunol Rev. 2004;200:5–11. doi: 10.1111/j.0105-2896.2004.00173.x. [DOI] [PubMed] [Google Scholar]
- 3.von Boehmer H. Selection of the T-cell repertoire: receptor-controlled checkpoints in T-cell development. Adv Immunol. 2004;84:201–238. doi: 10.1016/S0065-2776(04)84006-9. [DOI] [PubMed] [Google Scholar]
- 4.Jameson SC, Hogquist KA, Bevan MJ. Positive selection of thymocytes. Annu Rev Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- 5.Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- 6.Gallegos AM, Bevan MJ. Central tolerance: good but imperfect. Immunol Rev. 2006;209:290–296. doi: 10.1111/j.0105-2896.2006.00348.x. [DOI] [PubMed] [Google Scholar]
- 7.Mathis D, Benoist C. Back to central tolerance. Immunity. 2004;20:509–516. doi: 10.1016/s1074-7613(04)00111-6. [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 9.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 10.Cyster JG. Specifying the patterns of immune cell migration. Nov Found Symp. 2007;281:54–61. doi: 10.1002/9780470062128.ch6. [DOI] [PubMed] [Google Scholar]
- 11.Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells, and the programming of tissue-selective lymphocyte trafficking. Nat Immunol. 2008;9:981–987. doi: 10.1038/ni.f.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boehm T. Thymus development and function. Curr Opin Immunol. 2008;20:178–184. doi: 10.1016/j.coi.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Raviola E, Karnovsky MJ. Evidence for a blood-thymus barrier using electron-opaque tracers. J Exp Med. 1972;136:466–498. doi: 10.1084/jem.136.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ushiki T. A scanning electron-microscopic study of the rat thymus with special reference to cell types and migration of lymphocytes into the general circulation. Cell Tiss Res. 1986;244:285–298. doi: 10.1007/BF00219204. [DOI] [PubMed] [Google Scholar]
- 15.Kato S. Thymic microvascular system. Micros Res Tech. 1997;38:287–299. doi: 10.1002/(SICI)1097-0029(19970801)38:3<287::AID-JEMT9>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 16.Mori K, Itoi M, Tsukamoto N, Kubo H, Amagai T. The perivascular space as a path of hematopoietic progenitor cells and mature T cells between the blood circulation and the thymic parenchyma. Int Immunol. 2007;19:745–753. doi: 10.1093/intimm/dxm041. [DOI] [PubMed] [Google Scholar]
- 17.Anderson M, Anderson SK, Farr AG. Thymic vasculature: organizer of the medullary epithelial compartment? Int Immunol. 2000;12:1105–1110. doi: 10.1093/intimm/12.7.1105. [DOI] [PubMed] [Google Scholar]
- 18.Ernstrom U, Gyllensten L, Larsson B. Venous output of lymphocytes from the thymus. Nature. 1965;207:540–541. doi: 10.1038/207540b0. [DOI] [PubMed] [Google Scholar]
- 19.Bix M, Coles M, Raulet D. Positive selection of Vβ8+ CD4-8- thymocytes by class I molecules expressed by hematopoietic cells. J Exp Med. 1993;178:901–908. doi: 10.1084/jem.178.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lind EF, Prockop SE, Porritt HE, Petrie HT. Mapping precursor movement through the postnatal thymus reveals specific microenvironments supporting defined stages of early lymphoid development. J Exp Med. 2001;194:127–134. doi: 10.1084/jem.194.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrie HT, Zuniga-Pflucker JC. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu Rev Immunol. 2007;25:649–679. doi: 10.1146/annurev.immunol.23.021704.115715. [DOI] [PubMed] [Google Scholar]
- 22.Kurobe H, Liu C, Ueno T, Saito F, Ohigashi I, Seach N, et al. CCR7-dependent cortex-to-medulla migration of positively selected thymocytes is essential for establishing central tolerance. Immunity. 2006;24:165–177. doi: 10.1016/j.immuni.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Galton M, Reed PB. Entry of lymph node cells in the normal thymus. Transplantation. 1966;4:168–177. doi: 10.1097/00007890-196603000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Ford WL. Lymphocyte migration and immune responses. Prog Aller. 1975;19:1–59. doi: 10.1159/000313381. [DOI] [PubMed] [Google Scholar]
- 25.Rannie GH, Donald KJ. Estimation of the migration of thoracic duct lymphocytes to non-lymphoid tissues. A comparison of the distribution of radioactivity at intervals following i.v. transfuson of cells labelled with 3H, 14C, 75Se, 99mTc, and 51Cr in the rat. Cell Tiss Kinet. 1977;10:523–541. [PubMed] [Google Scholar]
- 26.Gowans JL, Knight EL. The route of re-circulation of lymphocyes in the rat. Proc Roy Soc, B. 1964;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- 27.Weissman IL. Thymus cell maturation. Studies on the origin of cortisone-resistant thymic lymphocytes. J Exp Med. 1973;137:504–510. doi: 10.1084/jem.137.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fink PJ, Bevan MJ, Weissman IL. Thymic cytotoxic T lymphocytes are primed in vivo to minor histocompatibility antigens. J Exp Med. 1984;159:436–451. doi: 10.1084/jem.159.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michie S, Kirkpatrick EA, Rouse RV. Rare peripheral T cells migrate to and persist in normal mouse thymus. J Exp Med. 1988;168:1929–1934. doi: 10.1084/jem.168.5.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michie SA, Rouse RV. Traffic of mature lymphocytes into the mouse thymus. Thymus. 1989;13:141–148. [PubMed] [Google Scholar]
- 31.Hirokawa K, Utsuyama M, Sado T. Immunohistological analysis of immigration of thymocyte-precursors into the thymus: evidence for immigration of peripheral T cells into the thymic medulla. Cell Immunol. 1989;119:160–170. doi: 10.1016/0008-8749(89)90232-3. [DOI] [PubMed] [Google Scholar]
- 32.Hart M, Zan-Bar I. Modulation of B cell maturation and migration to the thymus of SJL mice. B cell migration to the thymus. Thymus. 1991;18:209–223. [PubMed] [Google Scholar]
- 33.Agus DB, Surh CD, Sprent J. Reentry of T cells to the adult thymus is restricted to activated T cells. J Exp Med. 1991;173:1039–1046. doi: 10.1084/jem.173.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Surh CD, Ernst B, Sprent J. Growth of epithelial cells in the thymic medulla is under the control of mature T cells. J Exp Med. 1992;176:611–616. doi: 10.1084/jem.176.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Surh CD, Sprent J, Webb SR. Exclusion of circulating T cells from the thymus does not apply in the neonatal period. J Exp Med. 1993;177:379–385. doi: 10.1084/jem.177.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardy CL, Godfrey DI, Scollay R. The effect of antigen stimulation on the migration of mature T cells from the peripheral lymphoid tissues to the thymus. Devel Immunol. 2001;8:123–131. doi: 10.1155/2001/20728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 38.Chau LA, Rohekar S, Wang JJ, Lian D, Chakrabarti S, Zhang L, et al. Thymic re-entry of mature activated T cells and increased negative selection in vascularized allograft recipients. Clin Exp Immunol. 2002;127:43–52. doi: 10.1046/j.1365-2249.2002.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian C, Bagley J, Forman D, Iacomini J. Induction of central tolerance by mature T cells. J Immunol. 2004;173:7217–7222. doi: 10.4049/jimmunol.173.12.7217. [DOI] [PubMed] [Google Scholar]
- 40.Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proc Natl Acad Sci USA. 2006;103:8447–8452. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bosco N, Agenes F, Rolink AG, Ceredig R. Peripheral T cell lymphopenia and concomitant enrichment in naturally arising regulatory T cells: the case of the pre-Tα gene-deleted mouse. J Immunol. 2006;177:5014–5023. doi: 10.4049/jimmunol.177.8.5014. [DOI] [PubMed] [Google Scholar]
- 42.McCaughtry TM, Wilden MS, Hogquist KA. Thymic emigration revisited. J Exp Med. 2007;204:2513–2520. doi: 10.1084/jem.20070601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhan Y, Bourges D, Dromey JA, Harrison LC, Lew AM. The origin of thymic CD4+CD25+ regulatory T cells and their co-stimulatory requirements are determined after elimination of recirculating peripheral CD4+ cells. Int Immunol. 2003;19:455–463. doi: 10.1093/intimm/dxm010. [DOI] [PubMed] [Google Scholar]
- 44.Ben-Nun A, Cohen IR. Spontaneous remission and acquired resistance to autoimmune encephalomyelitis (EAE) are associated with suppression of T cell reactivity: suppressed EAE effector T cells recovered as T cell lines. J Immunol. 1982;128:1450–1457. [PubMed] [Google Scholar]
- 45.Naparstek Y, Holoshitz J, Eisenstein S, Reshef T, Rappaport S, Chekei J, et al. Effector T lymphocyte line cells migrate to the thymus and persist there. Nature. 1982;300:262–264. doi: 10.1038/300262a0. [DOI] [PubMed] [Google Scholar]
- 46.Bell EB, Sparshott SM, Ager A. Migration pathways of CD4 T cell subsets in vivo: the CD45RC− subset enters the thymus via α4 integrin-VCAM-1 interaction. Int Immunol. 1995;7:1861–1871. doi: 10.1093/intimm/7.11.1861. [DOI] [PubMed] [Google Scholar]
- 47.Westermann J, Smith T, Peters U, Tschernig T, Pabst R, Steinhoff G, et al. Both activated and nonactivated leukocytes from the periphery continuously enter the thymic medulla of adult rats: phenotypes, sources, and magnitude of traffic. Eur J Immunol. 1996;26:1866–1874. doi: 10.1002/eji.1830260830. [DOI] [PubMed] [Google Scholar]
- 48.Gopinathan R, DePaz HA, Oluwole OO, Ali AO, Garrovillo M, Engelstad K, et al. Role of reentry of in vivo alloMHC peptide-activated T cells into the adult thymus in acquired systemic tolerance. Transplantation. 2001;72:1533–1541. doi: 10.1097/00007890-200111150-00011. [DOI] [PubMed] [Google Scholar]
- 49.Binns RM, Licence ST, Whyte A, Wilby MN, Rothkotter HJ, Bacon M. Genetically determined CD45 variant of value in leucocyte tracing in vivo in the pig. Immunology. 1995;86:25–33. [PMC free article] [PubMed] [Google Scholar]
- 50.Dumont RJ, Barrois R, Jacobson EB. Migration of peripheral T and B cells into the thymus of aging (NZB x SJL)F1 female mice. Cell Immunol. 1984;83:292–301. doi: 10.1016/0008-8749(84)90308-3. [DOI] [PubMed] [Google Scholar]
- 51.Emmanouilidis N, Guo Z, Dong Y, Newton-West M, Adams AB, Lee ED, et al. Immunosuppressive and trafficking properties of donor splenic and bone marrow dendritic cells. Transplantation. 2006;81:455–462. doi: 10.1097/01.tp.0000195779.01491.4e. [DOI] [PubMed] [Google Scholar]
- 52.Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat Immunol. 2006;7:1092–1100. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- 53.Modigliani Y, Coutinho A, Pereira P, Le Douarin N, Thomas-Vaslin V, Burien-Defranoux O, et al. Establishment of tissue-specific tolerance is driven by regulatory T cells selected by thymic epithelium. Eur J Immunol. 1996;26:1807–1815. doi: 10.1002/eji.1830260822. [DOI] [PubMed] [Google Scholar]
- 54.Boursalian TE, Golub J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nat Immunol. 2004;5:418–425. doi: 10.1038/ni1049. [DOI] [PubMed] [Google Scholar]
- 55.Weinreich MA, Hogquist KA. Thymic emigration: when and how T cells leave home. J Immunol. 2008;181:2265–2270. doi: 10.4049/jimmunol.181.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kirberg J, Bosco N, Deloulme JC, Ceredig R, Agenes F. Peripheral T lymphocytes recirculating back into the thymus can mediate thymocyte positive selection. J Immunol. 2008;181:1207–1214. doi: 10.4049/jimmunol.181.2.1207. [DOI] [PubMed] [Google Scholar]
- 57.Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312:114–116. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- 58.Lepique AP, Palencia S, Irjala H, Petrie HT. Characterization of vascular adhesion molecules that may facilitate progenitor homing in the post-natal mouse thymus. Clin Devel Immunol. 2003;10:27–33. doi: 10.1080/10446670310001598492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Michie SA, Rouse RV. Traffic of peripheral B and T lymphocytes to hyperplastic, preneoplastic thymuses of AKR mice. Amer J Pathol. 1991;138:1015–1025. [PMC free article] [PubMed] [Google Scholar]
- 60.Michie SA, Streeter PR, Butcher EC, Rouse RV. L-selectin and alpha 4 beta 7 integrin homing receptor pathways mediate peripheral lymphocyte traffic to AKR mouse hyperplastic thymus. Amer J Pathol. 1995;147:412–421. [PMC free article] [PubMed] [Google Scholar]
- 61.Slifka MK, Whitmire JK, Ahmed R. Bone marrow contains virus-specific cytotoxic T lymphocytes. Blood. 1997;90:2103–2108. [PubMed] [Google Scholar]
- 62.Di Rosa F, Santoni A. Memory T-cell competition for bone marrow seeding. Immunology. 2003;108:296–304. doi: 10.1046/j.1365-2567.2003.01593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, Lefrancois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20:551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 64.Mazo IB, Honczarenko M, Leung H, Cavanagh LL, Bonasio R, Weninger W, et al. Bone marrow is a major reservoir and site of recruitment for central memory CD8 T cells. Immunity. 2005;22:259–270. doi: 10.1016/j.immuni.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 65.Webb SR, Sprent J. Induction of neonatal tolerance to Mlsa antigens by CD8+ T cells. Science. 1990;248:1643–1646. doi: 10.1126/science.1973003. [DOI] [PubMed] [Google Scholar]
- 66.van Ewijk W, Shores EW, Singer A. Crosstalk in the mouse thymus. Immunol Today. 1994;15:214–217. doi: 10.1016/0167-5699(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 67.Hikosaka Y, Nitta T, Ohigashi I, Yano K, Ishimaru N, Hayashi Y, et al. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity. 2008;29:438–450. doi: 10.1016/j.immuni.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 68.Irla M, Hugues S, Gill J, Nitta T, Hikosaka Y, Williams IR, et al. Autoantigen-specific interactions with CD4+ thymocytes control mature medullary thymic epithelial cell cellularity. Immunity. 2008;29:451–463. doi: 10.1016/j.immuni.2008.08.007. [DOI] [PubMed] [Google Scholar]