Abstract
Langerhans cells (LC) are epidermal dendritic cells capable, in several experimental systems, of Ag-presentation for stimulation of cell-mediated immunity. LC have been considered to play a key role in initiation of cutaneous immune responses. Additionally, administration of donor T cells to bone marrow chimeric mice with persistent host LC, but not mice whose LC have been replaced by donor cells, exhibit marked skin graft-versus-host disease, demonstrating that LC can trigger graft-versus-host disease. However, experiments with transgenic mice in which regulatory elements from human langerin were used to drive expression of diphtheria toxin, resulting in absence of LC, suggest that LC may serve to downregulate cutaneous immunity. LC are associated with nerves containing the neuropeptide calcitonin gene-related peptide (CGRP) and CGRP inhibits LC Ag-presentation in several models including presentation to a Th1 clone. We now report that CGRP enhances LC function for stimulation of Th2 responses. CGRP exposure enhanced LC Ag-presentation to a Th2 clone. Upon presentation of chicken ovalbumin (cOVA) by LC to T cells from DO11.10 cOVA T cell receptor transgenic mice,pretreatment with CGRP resulted in increased IL-4 production and decreased IFN-γ production. CGRP also inhibited stimulated production of the Th1 chemokines CXCL9 and CXCL10 but induced production of the Th2 chemokines CCL17 and CCL22 by a dendritic cell line and by freshly-obtained LC. Changes in production of these chemokines correlated with the effect of CGRP on mRNA levels for these factors. Exposure of LC to nerve-derived CGRP in situ may polarize them towards favoring Th2-type immunity.
This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI,holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
Keywords: rodent, chemokines, antigen presentation/processing, skin, dendritic cells
Introduction
Langerhans cells (LC)3 are dendritic cells that reside in the epidermis. LC are capable, in several experimental systems, of Ag-presentation for stimulation of cell-mediated immunity (1,2) and have been believed to play a key role in initiation of cutaneous immune responses. In support of their putative role in cutaneous Ag presentation, administration of donor T cells to bone marrow chimeric mice with persistent host LC, but not mice whose LC have been replaced by donor cells, exhibit marked skin graft-versus-host disease, demonstrating that LC can trigger graft-versus-host disease (3). Recently, however, data was presented that challenged this paradigm. Transgenic mice in which regulatory elements from human langerin were used to drive expression of diphtheria toxin, resulting in absence of LC, exhibited enhanced contact hypersensitivity responses, suggesting that LC may serve to downregulate cutaneous immunity (4).
In this regard, the finding that LC can produce both Th1-type and Th2-type chemokines suggests they participate in determining the character of immunity that is engendered in the skin or in draining lymph nodes after trafficking of LC to those sites. As LC in situ in the skin are frequently anatomically associated with calcitonin gene-related peptide (CGRP)-containing nerves (5), the possibility that CGRP influences the ability of LC to support Th1- or Th2-type immunity must be considered. Based on our earlier work showing that CGRP inhibits LC Ag-presentation in several models including presentation to a Th1 clone (5,6,7), we hypothesized that CGRP may act by biasing LC towards inducing Th2-type immunity.
Chemokines are chemotactic cytokine signals involved in leukocyte migration. CD4+ Th1 and Th17 cells express the CXCR3 while Th2 cells predominantly express CCR4 (8,9). It has been recently found that IFN-γ, LPS and polyinosinic-polycytidylic acid induce production of the Th1-type chemokines CXCL9 (monokine induced by IFN-γ, MIG) and CXCL10 (interferon-inducible protein 10, IP-10) by murine LC (10). These chemokines bind to CXCR3 and promote the migration of Th1 cells. The Th2-type chemokines CCL17 (thymus and activation-regulated chemokine, TARC) and CCL22 (macrophage-derived chemokine, MDC) promote the migration of Th2 cells via binding to CCR4. CXCR3 is also expressed by natural killer cells and Th1-type cytokines are involved in their migration (11). CXCR3 signaling plays a role in experimental autoimmune encephalitis and murine models of herpes simplex encephalitis, corneal and skin disease and multiple sclerosis, amongst others (12,13). CCR4 signaling is also involved in various other disorders including asthma and, interestingly, CCR4 signaling is believed to play a significant role in some types of malignancies (14,15). For example, in a subset of patients with CCR4+ T cell leukemia or lymphoma, the tumor cells themselves function as regulatory T cells, contributing to tumor survival in the face of host anti-tumor immunity (15). In other examples, CCL17 and CCL22 are sometimes produced by tumor cells and attract CCR4+ T regulatory cells to the tumor where they create an environment permissive for tumor escape from host immune responses (15). Overall, CCR3 and CCR4 signaling play crucial roles in determining the balance of Th1 versus Th2 immunity in many situations.
The experiments discussed in this paper evaluated the possibility that exposure of LC to CGRP may differentially modulate the ability of these cells to present Ag to Th1 and Th2 cells. The ability of CGRP to regulate the production of Th1 and Th2 chemokines, and thereby regulate the nature of a cutaneous immune response, was also evaluated. Some experiments were initially performed with XS106 cells [a dendritic cell line derived from neonatal BALB/c epidermis (16,17)] as a surrogate for LC. All experiments other than Northern blotting were also performed with fresh, primary LC. The results discussed below strongly suggest that CGRP biases LC towards favoring Th2-type immune responses.
Materials and Methods
Mice
Six- to 12 week old female BALB/c (H-2d), A/J (H-2k) and DO11.10 chicken ovalbumin T cell receptor transgenic mice on a BALB/c background [C.Cg-Tg(DO11.10)10Dlo/J] mice were purchased from The Jackson Laboratory and were kept in the animal facility of Weill Medical College of Cornell University on a 12-hr light/dark cycle. All experiments were approved by the Institutional Animal Care and Use Committee of the Weill Cornell Medical College.
Reagents
α-CGRP and α-CGRP8–37 were purchased from Bachem. Conalbumin and keyhole limpet hemocyanin (KLH) were purchased from Sigma-Aldrich. A fragment of chicken ovalbumin (cOVA) was obtained from Peptides International.
Media and cell lines
Complete medium (CM) consisted of RPMI 1640 (Mediatech), 10% FCS (Gemini Biotech), 100 U/ml penicillin, 100 μg/ml streptomycin, 0.1 mM nonessential amino acids, 0.1 mM essential amino acids, 2 mM L-glutamine, 1 mM sodium pyruvate, and 10 mM HEPES buffer (all from Mediatech).
The XS106 cell line is a dendritic cell line derived from neonatal A/J epidermis. It is capable of Ag presentation, and has many of the phenotypic characteristics of LC (16,17). XS106 cells were grown in CM with the addition of 2 ng/ml murine rGM-CSF (Chemicon), 10% NS cell supernatant [supernatant conditioned by a fibroblast-like cell line, known to support the growth of epidermal APC-derived cell lines (18)] and 5 × 10−5 M 2-ME (Sigma-Aldrich).
The HDK-1 cell line, a keyhole limpet hemocyanin (KLH)-specific, I-Ad-restricted Th1 clone (19), was maintained in CM supplemented with 5 × 10−5 M 2-ME, 10 ng/ml murine IL-2 (Chemicon) and 10% FCS (Gemini Biotech). The D10.G4.1 cell line (ATCC) is a conalbumin-specific, I-Ak-restricted Th2 clone (20). It was maintained in modified RPMI, supplemented with 0.05 mM 2-ME, 10 pg/ml mouse IL-1α (R&D Systems), 10% FCS and 10% T-STIM with Con A (rat IL-2 culture supplement, BD Biosciences Biosciences).
Preparation of epidermal cells (EC), LC-enriched EC (eEC), and purified LC (pLC)
EC were prepared using a modification of standard protocol (21). Briefly, the truncal skins of mice were shaved and chemically depilated. The subcuaneous fat and carnosus panniculus were removed by blunt dissection. The skins were then floated dermis side down for 45 min in Ca2+/Mg2+-free PBS containing 0.5 U of dispase/ml (BD Biosciences) and 0.38% trypsin (BD Biosciences). Epidermal sheets were collected by gentle scraping, washed and dissociated by continuous mild agitation for 20 min in HBSS (Mediatech) supplemented with 2% FCS. The EC were then filtered through a 40-μm cell strainer (BD Biosciences) to yield EC containing ~2–3% LC.
To prepare eEC, EC were incubated in a 1/2000 dilution of anti-Thy-1.2 monoclonal antibodies (Sigma) for 30 min at 4°C. Low-toxicity rabbit complement (Cederlane Laboratories) was added at a 1/40 dilution for another 30 min at 37°C. Dead cells were digested by treatment with 0.05% trypsin and 80 μg/ml DNase I (Sigma-Aldrich) for 4 min at room temperature. Finally, the cells were washed in CM. This procedure enriches LC content by selectively removing epidermal T cells and some keratinocytes. FACS analysis has shown that the resulting population consists of ~12% LC.
To prepare pLC, eEC were incubated with anti I-Ad monoclonal antibodies (BD Biosciences Biosciences) at a 1/50 dilution for 30 min at 4°C. They were then incubated with goat anti-mouse IgG conjugated to magnetic microspheres (Dynabeads M-450; Dynal Biotech) for 10 min with continuous, gentle agitation. The cells were then washed repeatedly (up to five times) to enrich LC, which adhered to the beads. By FACS analysis (using anti-I-Ad monoclonal antibodies), this procedure yields a cell population of ~95% LC.
CGRP receptor mRNA detection by RT-PCR
EC from BALB/c mice were obtained as described above and incubated with phycoerythrin-conjugated anti-mouse I-Ad (BD Biosciences). Then LC were sorted by FACS on a Beckman-Coulter flow cytometer (BD Biosciences). Total RNA was extracted from these freshly-obtained LC as well as from XS106 cells using a total RNA extraction kit (Qiagen). RNase free DNase (Qiagen) was used to eliminate any contamination with genomic DNA. Two hundred ng (LC) or 100 ng (XS106 cells) of RNA was reverse-transcribed into complementary DNA using 200 units of Superscript™ II reverse-transcriptase (Invitrogen) following the instructions of the manufacturer (Invitrogen). One-tenth of the synthesized cDNA was amplified by PCR using gene-specific primers made from published sequences for receptor activity modifying protein (RAMP)1, RAMP2 and RAMP3 (22). Primers sequences for calcitonin receptor-like receptor (CRLR) and calcitonin gene-related peptide-receptor component protein (CRCP) were designed from GenBank sequences for the mRNA of mouse CRLR and CRCP: CRLR, 5′-CTACTATTTTCTGC TTCTTT-3′ forward and 5′-TTTGTGCTTATTTTCTTTCC -3′ reversed; CRCP, 5′-TGGCGGAATAGGAGATAAGA-3′ forward and 5′-AGACAGAAGGGACCGCAT AA-3′ reversed. The PCR products were electrophoresed in 1% agarose gel, stained with ethidium bromide and visualized with UV radiation.
In vitro Ag presentation to Th1 and Th2 clones
To examine the effect of CGRP on LC Ag presentation to Th1 and Th2 clones in vitro, EC or pLC were prepared from A/J or BALB/c mice and plated in 96-well round bottom plates at 1 × 105 cells/well (EC) or 1 × 104 cells/well (pLC) in CM. They were then incubated with varying concentration of CGRP (0.1 nM, 1 nM, 10 nM and 100 nM) at 37°C. After 2.5 h, cells from A/J were exposed to conalbumin and cells from BALB/c were exposed to KLH at a final concentration of 100 μg/ml, still in the presence of CGRP. After an additional 2.5 h, cells were extensively washed with CM. Then, A/J cells were co-cultured with D10.G4.1 cells (Th2) (104 cells/well) and cells from BALB/c mice were co-cultured with HDK cells (Th1) (104 cells/well). Supernatants were collected after 24 h (Th2) or 72 h (Th1) respectively. IL-4 production by D10.G4.1 cells was measured by a sandwich ELISA kit (R&D Systems). IFN-γproduction by HDK cells was analyzed by a sandwich ELISA using purified rat anti-mouse IFN-γmonoclonal capture antibodies, biotinylated rat anti-mouse IFN-γmonoclonal detection antibodies, avidin-HRP (1/1000 dilution) and ABTS substrate, read at 405 nm (all reagents BD Biosciences Biosciences).
Ag presentation to DO11.10 transgenic T cells
Spleens were isolated from DO11.10 transgenic mice and mechanically disrupted to yield a single cell suspension. Erythrocytes were then lysed by brief exposure to hypotonic medium. Then, splenocytes were passed through a nylon-wool column to yield a suspension of cells enriched for T cell content (“T cells”). pLC from BALB/c mice were cultured for 3 hours in CM containing 10 nM CGRP or medium alone. Then, cells were washed 3 times and cocultured in 96 well round-bottom plates with DO11.10 T cells. Ten thousand CGRP treated or untreated pLC were cultured in each well with 1 × 105 T cells (for IFN-γ assay) or 2 × 105 T cells (for IL-4 assay) in 200 μl of CM along with varying concentrations of cOVA 323–339 in triplicate. Supernatants were harvested 48 hours later and content of IL-4 and IFN-γ was assessed by sandwich ELISA kits (R&D Systems).
Preparation of culture supernatants
XS106 cells and pLC were cultured at a concentration of 0.5 × 106 cells per well in 6-well plates, stimulated with or without 100 ng/ml IFN-γ in the presence or absence of varying concentration of CGRP. Supernatants were collected at different times and CXCL9, CXCL10, CCL17 and CCL22 content was measured by sandwich ELISA kits (R&D Systems) according to the manufacturer’s protocol.
RNA extraction and Northern blot analysis
XS106 cells were cultured at a concentration of 5 × 106 cells per 100 mm tissue culture dish and cultured with or without 100 ng/ml IFN-γ in the presence or absence of CGRP (10 nM) for 16 h. Cells were collected and total cellular RNA extracted by TRIzol (Invitrogen), according to the manufacturer’s protocol. Ten μg of total RNA was electrophoresed on 1.5% agarose-formadehyde gels, transferred in 20 × saline sodium citrate (SSC) onto a Hybond-N+ membrane (Amersham Biosciences) and cross-linked to the membrane using UV light.
Probes for murine chemokines and GAPDH were generated by RT-PCR using specific primers for CXCL9 (23), CXCL10 (24), CCL17 (25) and CCL22 (26). Oligonucleotides were end-labeled with 32P-dCTP (Perkin-Elmer) by using Klenow fragment of DNA polymerase I (Invitrogen). The RNA-containing membranes were prehybridized for 24 h at 42°C and hybridized for 24 h at 45°C with labeled probes [106 cpm] in Hybrisol®-1 buffer (Millipore). The membranes were then washed twice in 2X SSC containing 0.1% SDS (20 min; 25°C) and once with 0.1% SSC containing 0.1% SDS (10 min; 55°C). The membranes were then exposed to X-ray film (Kodak). The intensity of the transcript was digitized and quantified using a phosphor imaging system (Typhoon Trio+, GE Healthcare) and then normalized to the intensity of GAPDH mRNA with results expressed as fold-increase over the level obtained with medium alone.
Statistics
The significance of differences between groups was assessed by Student's two-tailed t-test for unpaired values.
Results
CGRP enhances LC Ag presentation to a Th2 clone while inhibiting Ag presentation to a Th1 clone
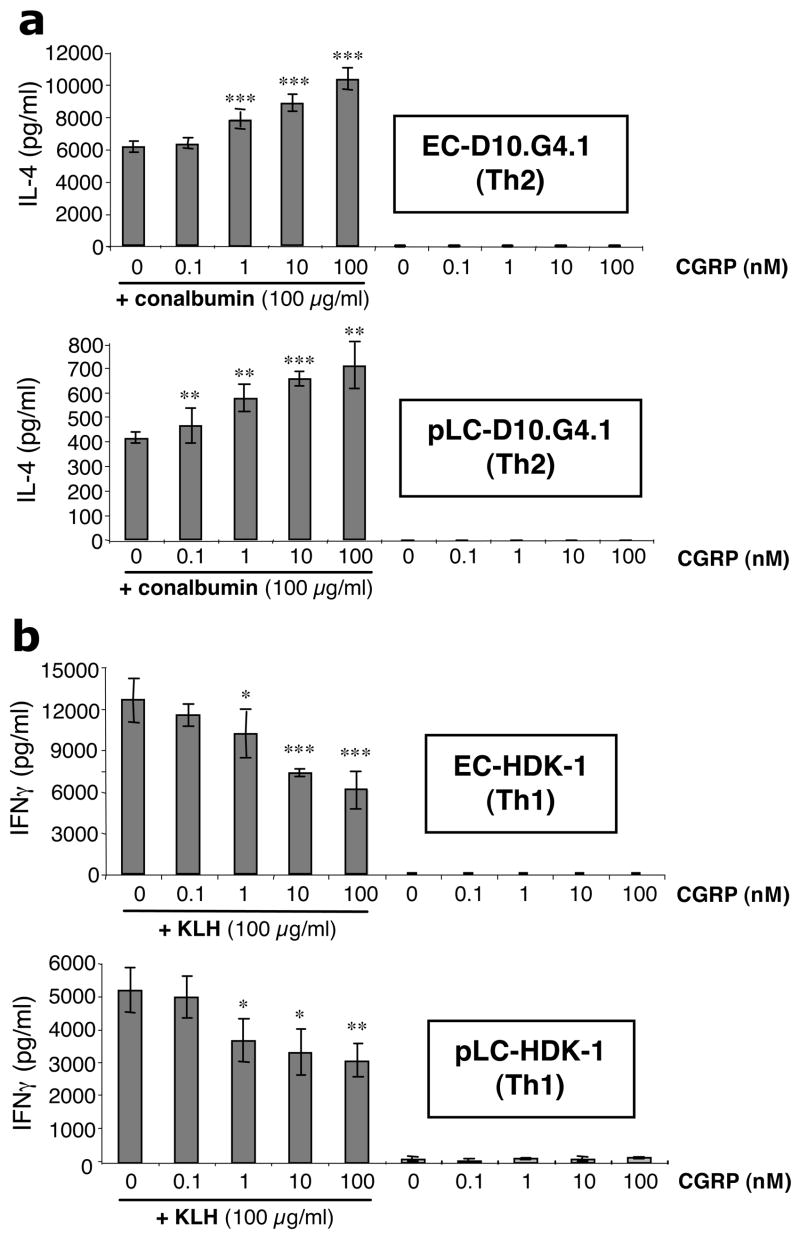
The ability of epidermal cells enriched for LC content (eEC) and ~95% pure LC (pLC) to present conalbumin to the responsive Th2 clone D10.G4.1 (27) was examined. These cells respond to presentation of conalbumin by I-Ak. As shown in Fig. 1a, CGRP treatment of eEC (upper panel) and pLC (lower panel) dose-dependently increased their ability to present conalbumin to the Th2 clone D10.G4.1. In contrast, as shown in Fig. 1b, CGRP dose-dependently induced a decrease in the response of the Th1 clone HDK-1 (28) to presentation of KLH by eEC (upper panel) and pLC (lower panel). As a control, additional experiments were performed examining the ability of substance P (a neuropeptide that co-localizes with CGRP in sensory nerves) to modulate the ability of epidermal cells enriched for Langerhans cells content to present antigen to the D10.G4.1 clone. Substance P had no affect on presentation to the clone (data not shown).
FIGURE 1.
CGRP enhances the ability of EC and pLC to present Ag to D10.G4.1 cells (Th2), but inhibits the ability of EC and pLC to present Ag to HDK-1 cells (Th1). (a) EC (upper panel) and pLC (lower panel) from A/J mice were pre-incubated with or without CGRP (0.1–100 nM) and later pulsed with conalbumin or medium alone. After washing, EC and pLC were co-cultured with D10G4.1 cells for 24 h and supernatants then assayed for IL-4 content by ELISA. Of 7 experiments performed, 5 showed this dose-dependent increase while 2 showed no change in the response observed. (b) EC (upper panel) and pLC (lower panel) from BALB/c mice were cultured with or without CGRP (0.1–100 nM) and then pulsed with KLH or medium alone. After washing, EC and LC were co-cultured with HDK-1 cells for 72 hr and supernatants then assayed for IFN-γ production by ELISA. Each result is the mean ±SD of 3 separate plates set-up at the same time with 2 wells per condition in each plate. This effect was observed in multiple experiments and the inhibitory effect of CGRP on Ag presentation to Th1 clones has been reported previously (7). (*p <0.05; **p<0.01; ***p<0.001 compared to 0 nM CGRP + conalbumin or KLH group)
Although unlikely, the possibility that the locus of action of CGRP in these experiments is at the responding T cell rather than the APC must be excluded. In such a scenario, traces of CGRP would be carried by treated pLC to the T cells under the conditions of co-culture despite the washing of the APC populations. To exclude the possibility that the effect of CGRP on antigen presentation to D10.G4.1 cells was actually due to an effect on the responders rather than the stimulators, experiments were set-up in an identical manner as above except that D10.G4.1 cells were treated for 2.5 hours with CGRP or medium instead of APCs prior to setting up cultures. No effect was observed on the D10.G4.1 cell response under these conditions (data not shown). With regard to treatment of responding Th1 cells with CGRP, we have already reported that treatment of a Th1 T hybridoma with CGRP did not affect its response to antigen presentation by LC (5).
CGRP treatment of pLC enhances the IL-4 response while diminishing the IFN-γ response of T cells from DO11.10 cOVA transgenic mice upon presentation of cOVA
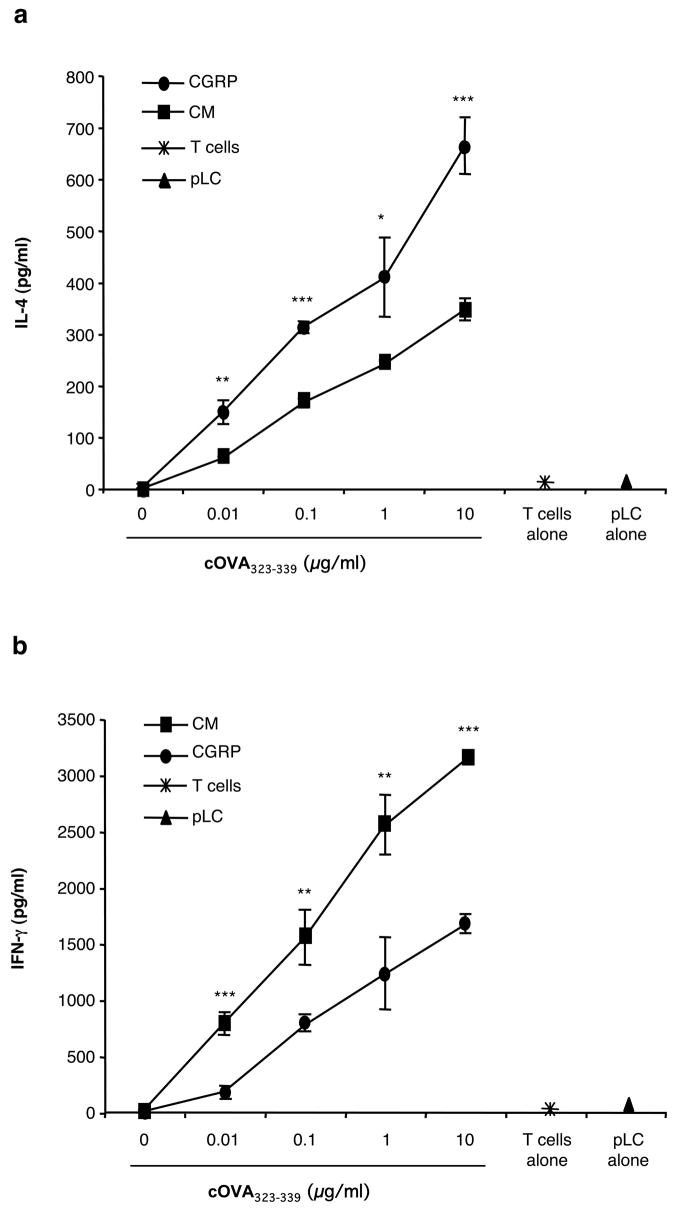
DO11.10 transgenic mice carry a MHC class II-restricted rearranged T cell receptor transgene and react to cOVA323–339 (29,30). The transgene contains rearranged T cell receptor alpha chain and T cell receptor beta chain genes and is expressed in the majority of T cells. These rearranged genes encode a cOVA-specific MHC class II (I-Ad)-restricted T cell receptor. We employed T cells from these mice as a second measure of the ability of CGRP to bias LC towards presenting Ag for a TH2 response. BALB/c pLC were cultured in CGRP or medium alone, washed and then co-cultured with DO11.10 T cells in the presence of varying concentrations of cOVA323–339. After 48 hours, conditioned supernatants were harvested and assayed for IL-4 and IFN-γ content by ELISA. As shown by the data in Figure 2a, exposure to CGRP significantly enhanced the IL-4 response of DO11.10 transgenic T cells. In contrast, as shown in Figure 2b, exposure of pLC to CGRP diminished the IFN-γ response.
FIGURE 2.
CGRP treatment of pLC prior to presentation of cOVA323–339 to T cells from DO11.10 transgenic mice resulted in enhanced IL-4 production and reduced IFN-γ production. Ten thousand pLC from BALB/c mice were cultured for 3 hours in 10 nM CGRP or medium alone. They were then washed and cocultured with nylon wool –enriched T cells from the spleens of DO11.10 ctransgenic mice in the absence or presence of varying concentrations of cOVA323–339. After 48 hours, the culture supernatants were harvested and assayed for IL-4 (A) and IFN-γ (B) content by ELISA. Each result is the mean ±SD of 3 separate plates set-up at the same time with 2 wells per condition in each plate. This result is representative of 2 such experiments. (*p <0.05; **p<0.01; ***p<0.001 compared to CGRP groups).
The dendritic cell line XS106 and epidermal LC express all of the components of the CGRP receptor
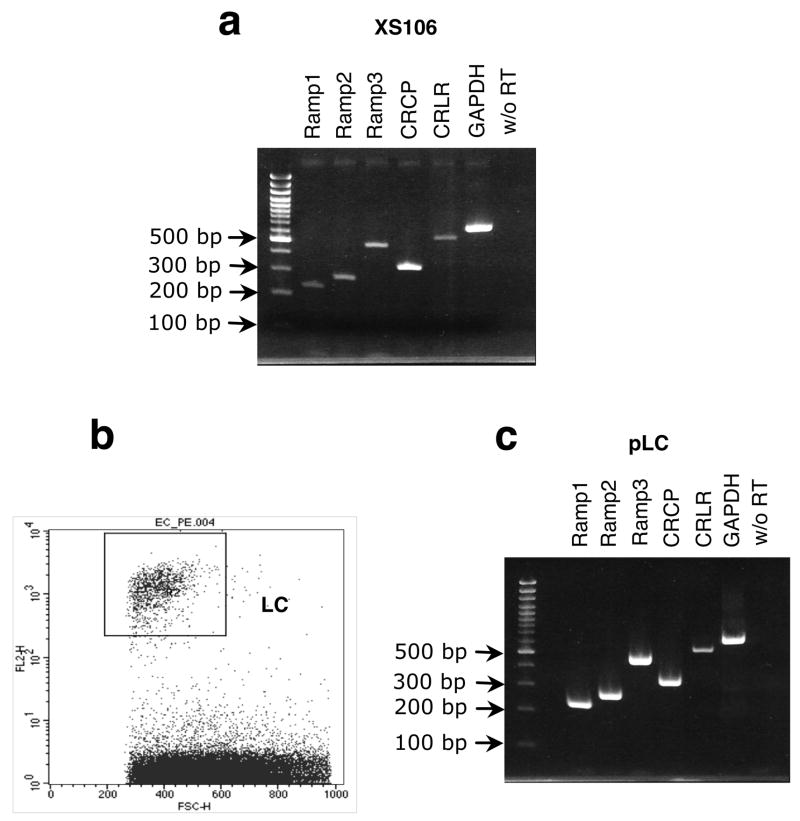
Both the dendritic cell line XS106, derived from neonatal A/J epidermis, and fresh primary LC isolated by FACS from BALB/c EC were examined for expression of mRNA for the CGRP receptor by RT-PCR. As shown by the data in Fig. 3, both XS106 cells and primary fresh LC were found to express all of the components of the CGRP receptor.
FIGURE 3.
XS106 and murine LC express mRNA for all of the components of the CGRP receptor. (a) Expression of mRNA in XS106 cells. Total RNA was extracted from XS106 cells and RT-PCR was performed using specific primers for the components of the CGRP receptor: RAMP1 (230 bp), RAMP2 (271 bp), RAMP3 (473 bp), CRCP (331 bp) and CRLR (514 bp). All amplified sequences were of the expected size. (b) Epidermal cells were obtained from BALB/c mouse epidermis and fresh LC were isolated by FACS using anti-I-Ad monoclonal antibodies. (c) Expression of components of the CGRP receptor in LC. Total RNA was extracted from fresh LC and RT-PCR was performed using the same primers. All amplified sequences were of the expected size.
CGRP inhibits the stimulated production of CXCL9 and CXCL10 by XS106 cells and pLC
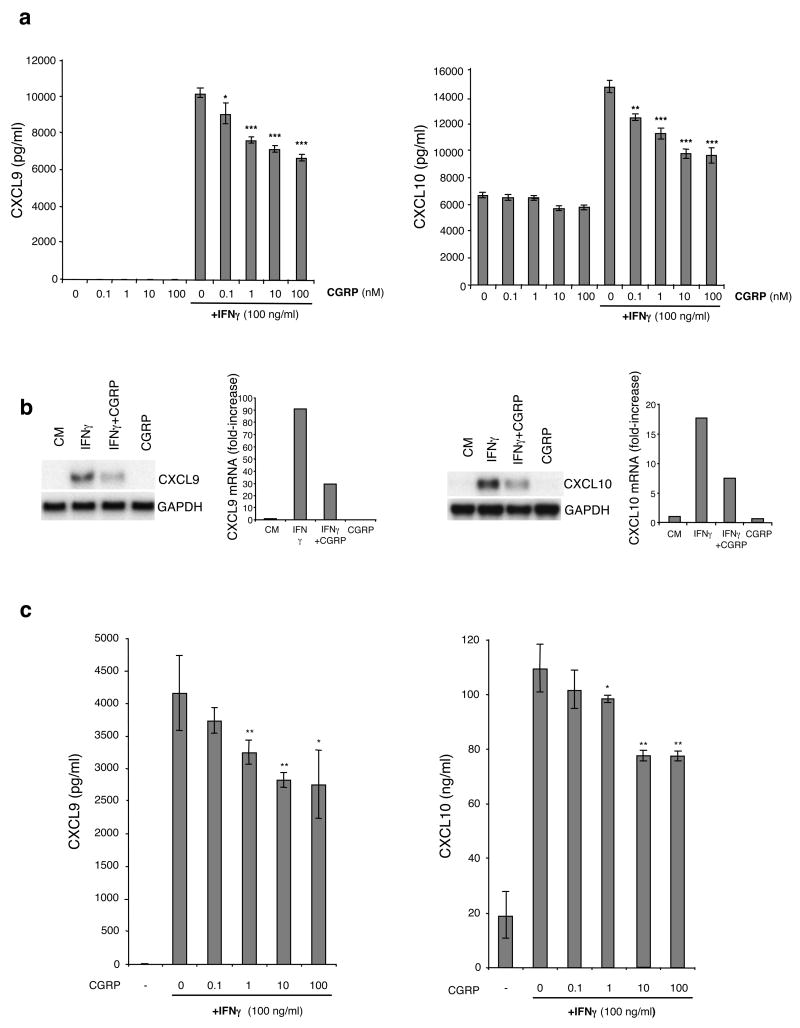
We next examined the ability of CGRP to modulate the production of Th1 chemokines. XS106 cells were stimulated with 100 ng/ml of IFN-γ in the presence or absence of various concentrations of CGRP. After 72 hours of culture, supernatants were harvested and CXCL9 and CXCL10 content assayed by ELISA. As shown by the data in Fig. 4a, exposure to IFN-γ led to significant production of these chemokines and production of each chemokine was reduced in a dose-dependent fashion by the presence of CGRP. This finding was confirmed at the mRNA level by Northern blotting (Fig. 4b). CGRP also inhibited the production of both CXCL9 and CXCL10 by pLC stimulated with IFN-γ in a similar manner (Fig. 4c). The ability of CGRP to inhibit the stimulated release of CXCL9 and CXCL10 from XS106 cells could be blocked by the presence of the type 1 CGRP receptor antagonist CGRP8–37 (data not shown).
FIGURE 4.
CGRP inhibits IFN-γ-induced production of CXCL9 and CXCL10 by XS106 cells and pLC and decreases the level of mRNA expression of CXCL9 and CXCL10 in XS106 cells. (a) XS106 cells were stimulated with 100 ng/ml of IFN-γ in the presence or absence of varying concentration ofCGRP. Supernatants were harvested after 72 h and CXCL9 and CXCL10 content was assessed by ELISA. (b). XS106 cells were stimulated with or without 100 ng/ml of IFN-γ in the presence or absence of 10 nM of CGRP. Sixteen hours later, total RNA was extracted and expression of CXCL9 and CXCL10 mRNA was analyzed by Northern blot. The intensity of the transcript was normalized to the intensity of GAPDH mRNA using a phosphor imaging system with results expressed as fold-increase compared to the level obtained with medium alone. One representative experiment of 3 is shown. (c) pLC from BALB/c mice were cultured with varying concentrations of CGRP and then stimulated with 100 ng/ml of IFN-γ Supernatants were collected for 48 h and analyzed for CXCL9 and CXCL10 production by ELISA. Each result is the mean ±SD of 3 separate plates set-up at the same time with 2 wells per condition in each plate. (*p <0.05; **p<0.01; ***p<0.001 compared to 0 nM CGRP + IFN-γ)
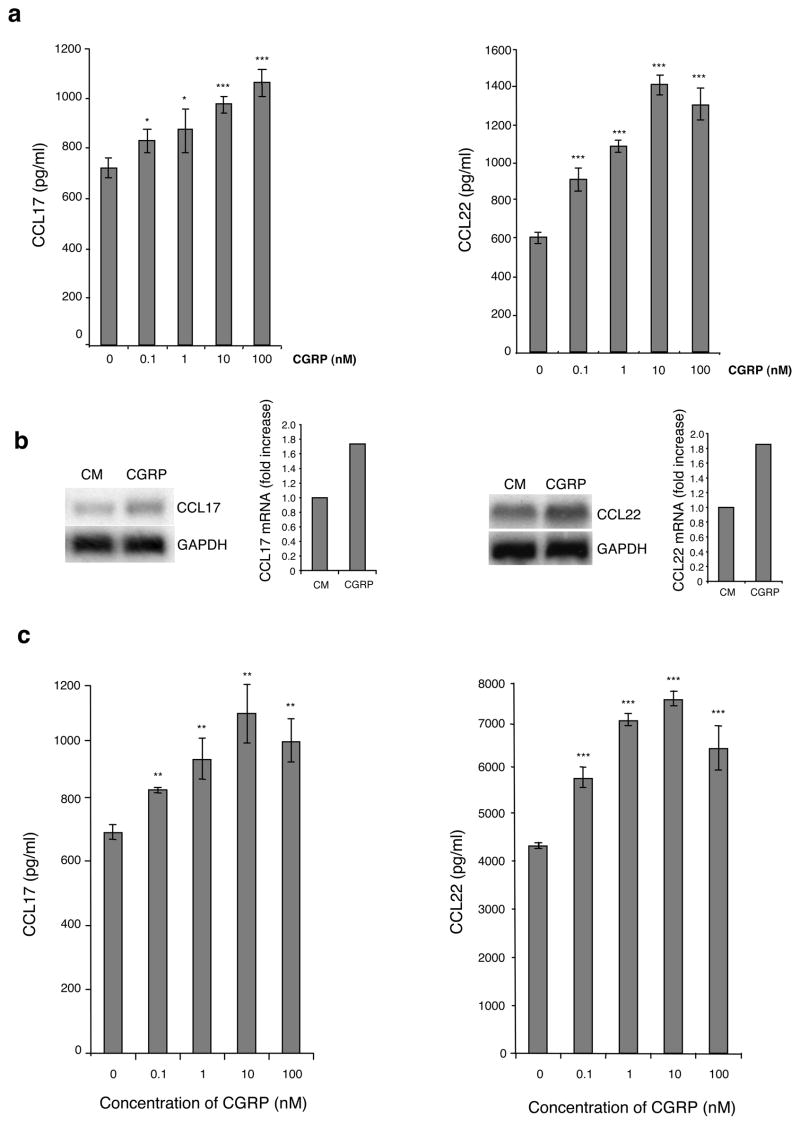
CGRP induces production of CCL17 and CCL22 by XS106 cells and pLC
We also evaluated the ability of CGRP to induce release of the Th2 chemokines CCL17 and CCL22. XS106 cells were cultured in media containing 0–100 nM CGRP or medium alone. Supernatants were collected after 72 hours of culture and assayed for CCL17 and CCL22 content by ELISA. As shown by the data in Fig. 5a, CGRP dose-dependently increased production of CCL17 and CCL22 by XS106 cells. This effect could also be observed at the mRNA level by Northern blotting (Fig. 5b). CGRP treatment of pLC in the same manner also increased CCL17 and CCL22 production (Fig. 5c). Interestingly, this effect of CGRP could not be inhibited by CGRP8–37 (data not shown). Thus, it is likely that this latter effect is not mediated by the CGRP1 receptor. CGRP is known to also activate the calcitonin like-receptor when associated with RAMP3 in transfected cell models (31) and it is likely acting on XS106 cells and LC in the same manner.
FIGURE 5.
CGRP enhances production of the Th2 chemokines CCL17 and CCL22 by LC and increases the level of mRNA expression of CCL17 and CCL22. (a) XS106 cells were cultured with or without CGRP (0–100 nM). Supernatants were harvested at 72 hr and CCL17 and CCL22 content assessed by ELISA. (b) XS106 cells were cultured with or without 10 nM of CGRP. Sixteen hours later, total RNA was extracted and CCL17 and CCL22 mRNA expression was analyzed by Northern blot. The intensity of the transcript for CXCL9 and CXCL10 was normalized to the intensity of GAPDH mRNA using a phosphor imaging system with results expressed as fold-increase over the level obtained with medium alone. One representative experiment of 3 is shown. (c) pLC from BALB/c mice were cultured with varying concentrations of CGRP. Supernatants were harvested at 48 hr and CCL17 and CCL22 content assessed by ELISA. Each result is the mean ±SD of 3 separate plates set-up at the same time with 2 wells per condition in each plate. (*p <0.05; **p<0.01; ***p<0.001 compared to 0 nM CGRP)
Discussion
LC reside in the suprabasilar portion of the epidermis and have been shown to be capable of presenting haptens, immunogenic peptides, and tumor Ags for T cell-dependent immune responses (32–35). As mentioned above, LC have been considered to be professional APC that play an important role in the initiation of cutaneous immunity. However, several lines of evidence have challenged that view. In experimental models of leishmaniasis, LC are not to be responsible for induction of immunity and, instead, may induce tolerance (36). Similarly, in murine models of herpes simplex infection, LC do not appear to be responsible for induction of immunity (37,38). Of particular interest, as mentioned above, Kaplan and collaborators employed transgenic mice in which regulatory elements from human langerin were used to drive expression of diphtheria toxin resulting in elimination of epidermal LC (4). In this system, elimination of LC resulted in enhanced contact hypersensitivity responses, suggesting that in situ LC downregulate the expression of contact hypersensitivity (4).
We have hypothesized that the observation that LC are frequently anatomically-associated with epidermal nerves containing CGRP may be relevant to the possibility that in the steady-state LC subserve a primarily downregulatory or immunosuppressive role, perhaps to prevent unwanted immune reactivity against self-Ags or commensal microorganisms. We and others have previously shown that CGRP treatment of LC in vitro inhibits their ability to present Ag in several assays in vitro (5,7). Additionally, treatment of CGRP ex vivo inhibits their ability to used to immunize naïve mice upon subcutaneous injection and immunization of mice with a contact sensitizer applied to a site injected intradermally with CGRP leads to a suppressed degree of sensitivity (6). We have now extended these observations to demonstrate that CGRP biases LC towards favoring functioning for Th2–type immunity.We found that CGRP treatment of LC augmented their ability to present Ag to a Th2 clone while inhibiting Ag presentation to a Th1 clone. Similarly, treatment of LC with CGRP led to a decreased ability to present cOVA 323–339 to DO11.10 transgenic T cells for a Th1 response (IFN-γ production) while augmenting a Th2 response (IL-4 production). CGRP also induced production of the Th2 chemokines CCL17 and CCL22 by XS106 cells and pLC. In the case of the Th1 chemokines CXCL9 and CXCL10, CGRP treatment resulted in inhibition of the stimulated production of these chemokines. With XS106 cells, we examined mRNA levels of these chemokines and the mRNA levels correlated with the effects of CGRP on expression of each of the chemokines at the protein level. To examine the possibility that any of these effects could be due to changes in viability of LC or T cells induced by exposure of LC to CGRP, we examined the viability of pLC in culture over time in 0, 0.1, 1, 10 and 100 nM CGRP. No significant differences in viability were observed (data not shown). To examine possible differences in viability of HDK-1 cells when Ag presentation is performed with LC treated with CGRP, we set-up experiments where LC were employed with and without pre-treatment with CGRP prior to being used to present KLH to HDK-1 cells. At the end of 72-hours, HDK-1 cells were enumerated by performing FACS analysis for CD3. No significant difference in the number of HDK-1 cells was observed between the two conditions (data not shown).
It is likely that exposure of LC to CGRP from associated nerves in situ in the epidermis maintains LC in a state that favors activation of Th2 mechanisms over Th1 mechanisms and this effect may account, at least in part, for the immunosuppressive activities of LC with regard to Th1-type immunity. It has long been known that culture of epidermal cells in vitro leads to enhanced LC Ag presenting capability for some assays of APC function (39). While exposure to granulocyte-macrophage colony stimulating factor and other stimulatory cytokines in culture (from contaminating keratinocytes) may account for some of this maturation, removal from an environment containing CGRP may also play a role. As a whole, these results support the concept of a locus of interaction between the nervous system and the immune system within the skin by which the nervous system is able to regulate the character of the cutaneous immune response. Furthermore, these findings might suggest practical new avenues for manipulation of cutaneous immunity through activation or inhibition of CGRP receptors.
Footnotes
This work was supported by NIH grant 5R01 AR042429 (RDG and JW), a grant from the Dana Foundation, a gift from the Jacob L. and Lillian Holtzmann Foundation, a grant from the Edith C Blum Foundation, contributions from the Carl and Fay Simons Family Trust and contributions from the Ann L. and Herbert J. Siegel Philanthropic Fund.
Abbreviations: LC, Langerhans cell; CGRP, calcitonin gene-related peptide; MIG, monokine induced by IFN-γ; IP-10, interferon-inducible protein 10; TARC, thymus and activation-regulated chemokine; MDC, macrophage-derived chemokine; KLH, keyhole limpet hemocyanin; cOVA, chicken ovalbumin; EC, epidermal cells; eEC, LC-enriched epidermal cells; pLC, purified Langerhans cells; RAMP, receptor activity modifying; CRLR, calcitonin receptor-like receptor; CRCP, calcitonin gene-related peptide-receptor component protein.
References
- 1.Romani N, Ebner S, Tripp CH, Flacher V, Koch F, Stoitzner P. Epidermal Langerhans cells—changing views on their function in vivo. Immunol Lett. 2006;106:119–125. doi: 10.1016/j.imlet.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Granstein RD, Ding W, Huang J, Holzer A, Gallo RL, Di Nardo A, Wagner JA. Augmentation of cutaneous immune responses by ATP gamma S: purinergic agonists define a novel class of immunologic adjuvants. J Immunol. 2005;174:7725–7731. doi: 10.4049/jimmunol.174.12.7725. [DOI] [PubMed] [Google Scholar]
- 3.Merad M, Hoffmann P, Ranheim E, Slaymaker S, Manz MG, Lira SA, Charo I, Cook DN, Weissman IL, Strober S, Engleman EG. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nat Med. 2004;10:510–517. doi: 10.1038/nm1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal Langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Hosoi J, Murphy GF, Egan CL, Lerner EA, Grabbe S, Asahina A, Granstein RD. Regulation of Langerhans cell function by nerves containing CGRP. Nature. 1993;363:159–163. doi: 10.1038/363159a0. [DOI] [PubMed] [Google Scholar]
- 6.Asahina A, Hosoi J, Beissert S, Stratigos A, Granstein RD. Inhibition of the induction of delayed-type and contact hypersensitivity by calcitonin gene-related peptide. J Immunol. 1995;154:3056–3061. [PubMed] [Google Scholar]
- 7.Asahina A, Moro O, Hosoi J, Lerner EA, Xu S, Takashima A, Granstein RD. Specific induction of cAMP in Langerhans cells by calcitonin gene-related peptide: relevance to functional effects. Proc Natl Acad Sci USA. 1995;92:8323–8327. doi: 10.1073/pnas.92.18.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakae S, Iwakura Y, Suto H, Galli SJ. Phenotypic differences between Th1 and Th17 cells and negative regulation of Th1 cell differentiation by IL-17. J Leukoc Biol. 2007;81:1258–1268. doi: 10.1189/jlb.1006610. [DOI] [PubMed] [Google Scholar]
- 9.Cosmi L, Annunziato F, Maggi E, Romagnani S, Manetti R. Chemoattractant receptors expressed on type 2 T cells and their role in disease. Int Arch Allergy Immunol. 2001;125:273–279. doi: 10.1159/000053827. [DOI] [PubMed] [Google Scholar]
- 10.Fujita H, Asahina A, Sugaya M, Nakamura K, Gao P, Fujiwara H, Tamaki K. Differential production of Th1- and TH2-type chemokines by mouse Langerhans cells and splenic dendritic cells. J Invest Dermatol. 2005;124:343–350. doi: 10.1111/j.0022-202X.2004.23607.x. [DOI] [PubMed] [Google Scholar]
- 11.Robertson MJ. Role of chemokines in the biology of natural killer cells. J Leukoc Biol. 2002;71:73–83. [PubMed] [Google Scholar]
- 12.Lundberg P, Openshaw H, Wang M, Yang HJ, Cantin E. Effects of CXCR3 signaling on development of fatal encephalitis and corneal and periocular skin disease in HSV-infected mice are mouse-strain dependent. Invest Ophthalmol Vis Sci. 2007;48:4162–4170. doi: 10.1167/iovs.07-0261. [DOI] [PubMed] [Google Scholar]
- 13.Liu L, Callahan MK, Huang D, Ransohoff RM. Chemokine receptor CXCR3: an unexpected enigma. Curr Top Dev Biol. 2005;68:149–181. doi: 10.1016/S0070-2153(05)68006-4. [DOI] [PubMed] [Google Scholar]
- 14.Garcia G, Godot V, Humbert M. New chemokine targets for asthma therapy. Curr Allergy Asthma Rep. 2005;5:155–160. doi: 10.1007/s11882-005-0090-0. [DOI] [PubMed] [Google Scholar]
- 15.Ishida T, Ueda R. CCR4 as a novel molecular target for immunotherapy of cancer. Cancer Sci. 2006;97:1139–1146. doi: 10.1111/j.1349-7006.2006.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu S, Ariizumi K, Caceres-Dittmar G, Edelbaum D, Hashimoto K, Bergstresser PR, Takashima A. Successive generation of antigen-presenting, dendritic cell lines from murine epidermis. J Immunol. 1995;154:2697–2705. [PubMed] [Google Scholar]
- 17.Xu S, Ariizumi K, Edelbaum D, Bergstresser PR, Takashima A. Cytokine-dependent regulation of growth and maturation in murine epidermal dendritic cell lines. Eur J Immunol. 1995;25:1018–1024. doi: 10.1002/eji.1830250424. [DOI] [PubMed] [Google Scholar]
- 18.Schuhmachers G, Xu S, Bergstresser PR, Takashima A. Identity and functional properties of novel skin-derived fibroblast lines (NS series) that support the growth of epidermal-derived dendritic cell lines. J Invest Dermatol. 1995;105:225–230. doi: 10.1111/1523-1747.ep12317512. [DOI] [PubMed] [Google Scholar]
- 19.Simon JC, Cruz PD, Jr, Bergstresser PR, Tigelaar RE. Low dose ultraviolet B-irradiated Langerhans cells preferentially activate CD4+ cells of the T helper 2 subset. J Immunol. 1990;145:2087–2091. [PubMed] [Google Scholar]
- 20.Nakashima K, Kokubo T, Shichijo M, Li YF, Yura T, Yamamoto N. A novel Syk kinase-selective inhibitor blocks antigen presentation of immune complexes in dendritic cells. Eur J Pharmacol. 2004;505:223–283. doi: 10.1016/j.ejphar.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 21.Kodali S, Ding W, Huang J, Seiffert K, Wagner JA, Granstein RD. Vasoactive intestinal peptide modulates Langerhans cell immune function. J Immunol. 2004;173:6082–6088. doi: 10.4049/jimmunol.173.10.6082. [DOI] [PubMed] [Google Scholar]
- 22.Schinke T, Liese S, Priemel M, Haberland M, Schilling AF, Catala-Lehnen P, Blicharski D, Rueger JM, Gagel RF, Emeson RB, Amling M. Decreased bone formation and osteopenia in mice lacking alpha-calcitonin gene-related peptide. J Bone Miner Res. 2004;19:2049–2056. doi: 10.1359/JBMR.040915. [DOI] [PubMed] [Google Scholar]
- 23.Park MK, Hoffmann KF, Cheever AW, Amichay D, Wynn TA, Farber JM. Patterns of chemokine expression in models of Schistosoma mansoni inflammation and infection reveal relationships between type 1 and type 2 responses and chemokines in vivo. Infect Immun. 2001;69:6755–6768. doi: 10.1128/IAI.69.11.6755-6768.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuroda E, Sugiura T, Okada K, Zeki K, Yamashita U. Prostaglandin E2 up-regulates macrophage-derived chemokine production but suppresses IFN-inducible protein-10 production by APC. J Immunol. 2001;166:1650–1658. doi: 10.4049/jimmunol.166.3.1650. [DOI] [PubMed] [Google Scholar]
- 25.Xiao T, Fujita H, Saeki H, Mitsui H, Sugaya M, Tada Y, Kakinuma T, Torii H, Nakamura K, Asahina A, Tamaki K. Thymus and activation-regulated chemokine (TARC/CCL17) produced by mouse epidermal Langerhans cells is upregulated by TNF-alpha and IL-4 and downregulated by IFN-gamma. Cytokine. 2003;23:126–132. doi: 10.1016/s1043-4666(03)00221-7. [DOI] [PubMed] [Google Scholar]
- 26.Kuroda E, Sugiura T, Okada K, Zeki K, Yamashita U. Prostaglandin E2 up-regulates macrophage-derived chemokine production but suppresses IFN-inducible protein-10 production by APC. J Immunol. 2001;166:1650–1658. doi: 10.4049/jimmunol.166.3.1650. [DOI] [PubMed] [Google Scholar]
- 27.Elliott KA, Osna NA, Scofield MA, Khan MM. Regulation of IL-13 production by histamine in cloned murine T helper type 2 cells. Int Immunopharmacol. 2001;1:1923–1937. doi: 10.1016/s1567-5769(01)00117-5. [DOI] [PubMed] [Google Scholar]
- 28.Matsue H, Edelbaum D, Hartmann AC, Morita A, Bergstresser PR, Yagita H, Okumura K, Takashima A. Dendritic cells undergo rapid apoptosis in vitro during antigen-specific interaction with CD4+ T cells. J Immunol. 1999;162:5287–5298. [PubMed] [Google Scholar]
- 29.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 30.Hsieh CS, Heimberger AB, Gold JS, O’Garra A, Murphy KM. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T-cell-receptor transgenic system. Proc Natl Acad Sci USA. 1992;89:6065–6069. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikitenko LL, Blucher N, Fox SB, Bicknell R, Smith DM, Rees MC. Adrenomedullin and CGRP interact with endogenous calcitonin-receptor-like receptor in endothelial cells and induce its desensitisation by different mechanisms. J Cell Sci. 2006;119:910–922. doi: 10.1242/jcs.02783. [DOI] [PubMed] [Google Scholar]
- 32.Stingl G, Wolff-Schreiner EC, Pichler WJ, Gschnait F, Knapp W, Wolff K. Epidermal Langerhans cells bear Fc and C3 receptors. Nature. 1977;268:245–246. doi: 10.1038/268245a0. [DOI] [PubMed] [Google Scholar]
- 33.Romani N, Lenz A, Glassel H, Stossel H, Stanzl U, Majdic O, Fritsch P, Schuler G. Cultured human Langerhans cells resemble lymphoid dendritic cells in phenotype and function. J Invest Dermatol. 1989;93:600–609. doi: 10.1111/1523-1747.ep12319727. [DOI] [PubMed] [Google Scholar]
- 34.Grabbe S, Granstein RD. Modulation of antigen-presenting cell function as a potential regulatory mechanism in tumor-host immune reactions. In Vivo. 1993;7:265–270. [PubMed] [Google Scholar]
- 35.Lappin MB, Kimber I, Norval M. The role of dendritic cells in cutaneous immunity. Arch Dermatol Res. 1996;288:109–121. doi: 10.1007/BF02505819. [DOI] [PubMed] [Google Scholar]
- 36.Ritter U, Osterloh A. A new view on cutaneous dendritic cell subsets in experimental leishmaniasis. Med Microbiol Immunol. 2007;196:51–59. doi: 10.1007/s00430-006-0023-0. [DOI] [PubMed] [Google Scholar]
- 37.Bosnjak L, Miranda-Saksena M, Koelle DM, Boadle RA, Jones CA, Cunningham AL. Herpes simplex virus infection of human dendritic cells induces apoptosis and allows cross-presentation via uninfected dendritic cells. J Immunol. 2005;174:2220–2227. doi: 10.4049/jimmunol.174.4.2220. [DOI] [PubMed] [Google Scholar]
- 38.Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, Iwasaki A. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schuler G, Steinman RM. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985;161:526–546. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]