Abstract
Shikonin derivatives, which are the active components of the medicinal plant Lithospermum erythrorhizon, exhibit many biological effects including apoptosis induction through undefined mechanisms. We recently discovered that orphan nuclear receptor Nur77 migrates from the nucleus to mitochondria, where it binds to Bcl-2 to induce apoptosis. Here, we report that certain shikonin derivatives could modulate the Nur77-Bcl-2 apoptotic pathway by increasing levels of Nur77 protein and promoting its mitochondrial targeting in cancer cells. Structural modification of acetylshikonin resulted in identification of a derivative 5,8-diacetoxyl-6-(1'-Acetoxyl-4'-methyl-3'-pentenyl)-1,4-naphthaquinones (SK07) that exhibited improved efficacy and specificity in activating the pathway. Unlike other Nur77 modulators, shikonins increased levels of Nur77 protein through their posttranscriptional regulation. The apoptotic effect of SK07 was impaired in Nur77 knockout cells and suppressed by co-treatment with leptomycin B (LMB) that inhibited Nur77 cytoplasmic localization. Furthermore, SK07 induced apoptosis in cells expressing the C-terminal half of Nur77 protein but not its N-terminal region. Our data also showed that SK07-induced apoptosis was associated with a Bcl-2 conformational change and Bax activation. Together, our results demonstrate that certain shikonin derivatives act as modulators of the Nur77-mediated apoptotic pathway and identify new shikonin-based lead that targets Nur77 for apoptosis induction.
Keywords: Nur77, acetylshikonin, mitochondria, Bcl-2, Bax, apoptosis
Introduction
Nur77 (also known as TR3 or NGFI-B), an orphan member of steroid/thyroid/retinoid receptor superfamily and an immediate-early response gene, plays a critical role in the regulation of differentiation, proliferation, apoptosis, and survival of many different cell types (1, 2). Although the growth-promoting effect of Nur77 has been described in various tumors (3, 4), Nur77 expression also mediates the killing effects of a variety of apoptotic stimuli such as synthetic retinoids, calcium ionophores, etoposide (VP-16), phorbol ester, chenodeoxycholic acid derivatives, di-nbutyltin dichloride, histone deacetylase inhibitors, cadmium, and 1,1-Bis(3'-indolyl)-1-(p-substituted phenyl)methanes in cancer cells (5-9). The apoptotic effect of Nur77 appears to be clinically relevant as the expression of the Nur77 subfamily member Nor-1 is positively correlated with survival in diffuse large B-cell lymphoma patients treated with chemotherapeutic drugs (10), and downregulation of Nur77 is associated with metastasis of primary solid tumors (11).
One of the mechanisms that regulate the opposing biological activities of Nur77, survival and death, is through its subcellular localization. Nur77 confers its growth-promoting activities through its action in the nucleus (12, 13), which requires its DNA-binding and transactivation. In contrast, the apoptotic effect of Nur77 is transcription-independent and occurs in the absence of its DNA-binding domain (5, 8, 14-16). In this case, Nur77 initiates apoptotic cascades by migrating to mitochondria where it interacts with the Bcl-2 apoptotic machinery by converting Bcl-2 from a protector to a killer (16, 17). Such a Nur77-Bcl-2 apoptotic pathway has been utilized by a variety of apoptosis-inducing agents in prostate cancer, lung cancer, colon cancer, ovarian cancer, and gastric cancer cells (2, 8, 14, 15, 17, 18). In this regard, Nur77 and Bcl-2 are often overexpressed in cancer cells, providing an excellent opportunity to preferentially induce apoptosis of these cells by inducing Nur77 migration and Bcl-2 conversion (1, 17). Such an approach may be highly effective in inducing cancer apoptosis, because it engages a simultaneous suppression of the survival function of Nur77 and Bcl-2 and activation of their pro-apoptotic potential (1, 17). Thus, agents that specifically activate the Nur77-mediated pathway may have therapeutic potential.
In an effort to identify new modulators of the Nur77-Bcl-2 apoptotic pathway, we have undertaken screening of a natural product library prepared from Chinese Herbal Medicine. Here we report that certain shikonin derivatives, active components of Chinese medicinal plant Lithospermum erythrorhizon (19, 20), could activate the Nur77-mediated apoptotic pathway in cancer cells. Further evaluation of acetylshikonin analogs demonstrated that 5,8-diacetoxyl-6-(1'-Acetoxyl-4'-methyl-3'-pentenyl)-1,4-naphthaquinones (SK07) was more active than its parental compound in inducing Nur77 expression and apoptosis. Our mechanistic studies revealed that SK07 increased levels of Nur77 protein in cancer cells through its posttranscriptional regulation and that it killed cancer cells by inducing Bcl-2 conformational change and Bax activation in a Nur77-dependent manner. Together, our studies identify new modulators of the Nur77-Bcl-2 apoptotic pathway and a potential target-based anti-cancer lead.
Material and Methods
Reagents
Lipofectamin 2000, Vybrant Apoptosis Assay Kit#2 and Trizol LS from Invitrogen (Carlsbad, CA), enhanced chemilumienescence (ECL) reagents, goat anti-rabbit and anti-mouse secondary antibody conjugated to horseradish peroxidase from Thermo (Rockford, IL), anti-mouse IgG conjugated with Cy3 from Chemicon international, polyclonal anti-Nur77 (sc-5569), anti-Hsp60 (sc-7150), anti-Bcl-2 (sc-509), anti-Bax (sc-493), anti-Bax (6A7) (sc-23959), fluorescein isothiocyanate (FITC)-labeled anti-rabbit IgG from Santa Cruz Biotechnology (Santa Cruz, CA), anti-PARP (556494), and anti-cytochrome c (556433) from BD Biosciences (San Jose, CA), monoclonal anti-β-actin antibody from Sigma (St. Louis, MO), monoclonal anti-Nur77 antibody from R&D Systems (Minneapolis, MN), anti-Bcl-2 BH3 (AP1303a) from Abgent (San Diego, CA), anti-cleaved caspase-3 (Asp175) from Cell signaling technology (Boston, MA), PVDF membranes from Millipore, cocktail of proteinase inhibitors (80-6501-23) from Amersham, were used in this study. All other chemicals used were commercial products of analytical grade obtained from Sigma.
Preparation of acetylshikonin and analogs
Lithospermum erythrorhizon grown in Liao-ning Province in Northeast China were extracted with hexane by Soxhlet apparatus and subjected to purification for shikonin derivative compounds (19, 20). Acetylshikonin (SK03) and two isomers of SK03 analogs, SK06 (5,8-diacetoxyl-2-(1'-Acetoxyl-4'-methyl-3'-pentenyl)-1,4-naphthaquinones) and SK07 (5,8-diacetoxyl-6-(1'-Acetoxyl-4'-methyl-3'-pentenyl)-1,4-naphthaquinones), were synthesized (Fig. 1A). Molecular structures were identified using spectroscopic techniques including electron ionization mass spectrometry, Fourier transform infrared spectroscopy, and NMR analysis.
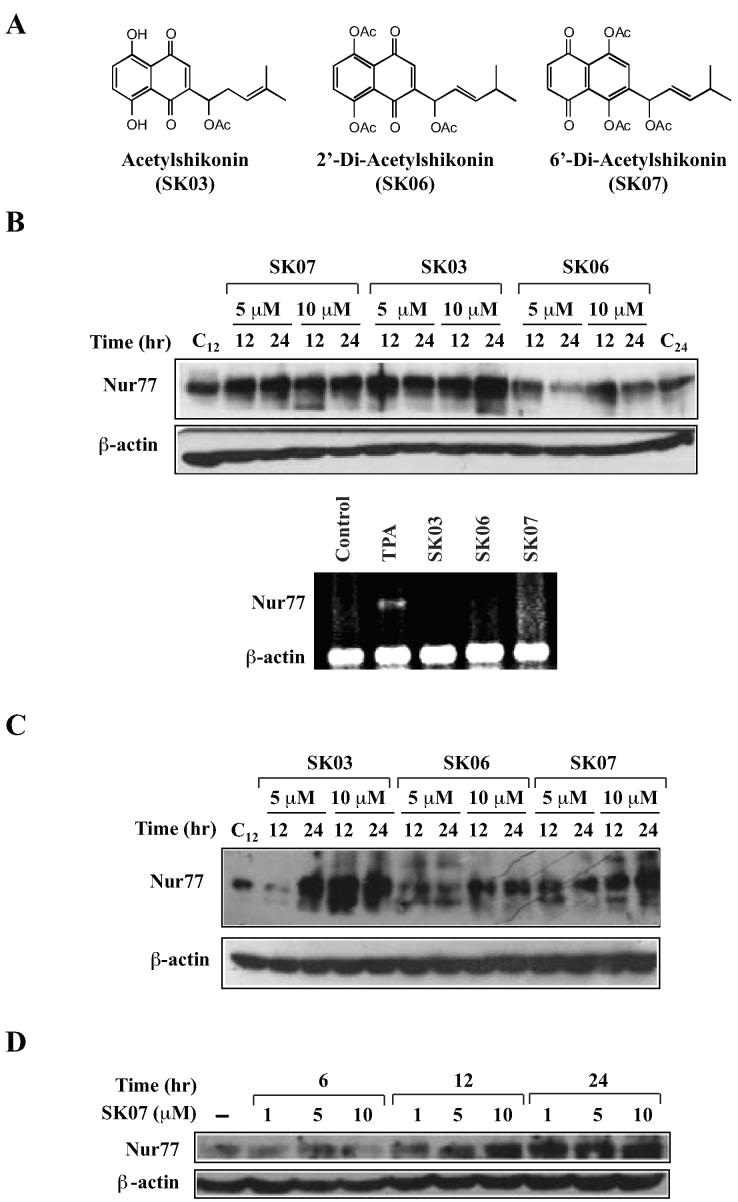
Figure 1. Induction of Nur77 protein levels by acetylshikonin and analogs.
A, structures of acytylshikonin and analogs. B, induction of Nur77 expression by shikonins in NIH-H460 lung cancer cells. Western blotting analysis was employed to evaluate the effect of shikonins on Nur77 protein levels (upper panel). NIH-H460 cells were treated with SK03, SK06, SK07 (5 or 10 µM), or vehicle for 12 or 24 hr. Nur77 protein expression was detected by using anti-Nur77 antibody. The blots were reprobed with anti-β-actin antibody for loading control. As for RT-PCR analysis (lower panel) of Nur77 mRNA expression, the same cells were treated with SK03, SK06 or SK07 (5 µM) for 3 hr, while TPA (100 ng/ml) was used as positive control. Total RNA were purified and subjected to RT-PCR analysis. Nur77 and β-actin products were simultaneously amplified by including primers for both Nur77 and β-actin in the same reaction system, in which β-actin expression level served as an internal control. C, induction of Nur77 expression by shikonins in HeLa cervical cancer cells. HeLa cells were treated with shikonins as indicated. Anti-Nur77 antibody was utilized to detect the expression of Nur77 protein by Western blotting analysis. D, dose- and time-dependent induction of Nur77. Cells were treated with vehicle or with SK07 at 1, 5 or 10 µM in serum-free medium for the indicated period of time. Nur77 expression was determined by Western blotting analysis using anti-Nur77 antibody.
Cell culture
NIH-H460 lung cancer and HeLa cervical cancer cells were cultured in RPMI1640 medium, while HEK293T human embryonic kidney, mouse embryonic fibroblasts (MEF) and MEF Nur77−/− cells were maintained in DMEM medium containing 10% fetal bovine serum (FBS) in a humidified atmosphere containing 5% CO2 at 37°C. Sub-confluent cells with exponential growth were employed throughout the experiments. Cell transfections were carried out by using Lipofectamin 2000 according to manufacture's instruction.
Cell lysis and fraction
Cell lysates were prepared by lysing cells with modified RIPA buffer containing 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 1% NP-40, 0.5% sodium deoxycholat, 0.1% SDS with a cocktail of proteinase inhibitors on ice for 30 min. For cellular fractionation, cells were lysed in cold Buffer A (10 mM HEPES-KOH pH 7.9 at 4°C, 1.5 mm MgCl2, 10 mM KCl, 0.5 mM DTT) with a cocktail of proteinase inhibitors on ice for 10 min. Cytoplasmic fraction was collected by centrifuging at 6000 × rpm for 30 seconds. Pellets containing nuclei were resuspended in cold high-salt Buffer C (20 mM HEPES-KOH pH 7.9, 25% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiothreitol), with a cocktail of proteinase inhibitors on ice for 30 min. Cellular debris was removed by centrifugation at 12000 × rpm at 4°C for 15 min. Protein concentrations were determined using the Bradford method according to the manufacturer's instruction (Bio-Rad).
Western blotting
Equal amounts of the lysates were electrophoresed on an 8% SDS-PAGE gel and transferred onto PVDF membranes, which were then blocked with 5% nonfat milk in TBST [50 mM Tris-HCl (pH 7.4), 150 mM NaCl and 0.1% Tween 20] for 1 hr, incubated with various primary antibodies for 2 hr and detected with either anti-rabbit (1:5000) or anti-mouse (1:5000) secondary antibodies for 1 hr using ECL system. The dilutions of the primary antibodies were: anti-Nur77 (sc-5569) in 1:1000, anti-PARP in 1:1000, anti-Bcl-2 in 1:1000, anti-Bax in 1:1000. The blots were reprobed with anti-β-actin antibody for loading control.
Immunofluorescence microscopy
Cells mounted on glass slides were permeabilized with PBS containing 0.1% Triton X-100 and 0.1 M glycine for 15 min, and blocked with 1% BSA in PBS for 30 min at room temperature, followed with incubation with various primary antibodies at 37 °C for 1 hr and detected by FITC-labeled anti-rabbit IgG (1:400) or anti-goat IgG conjugated with Cy3 (1:400) at room temperature for 30 min. Cells were co-stained with 4'6'-diamidino-2-phenylindole (DAPI) to visualize nuclei. The images were taken under a fluorescent microscopy (Carl Zeiss) or a LSM-510 confocal laser scanning microscope system (Carl Zeiss, Oberkochen, Germany). The primary antibodies included monoclonal anti-Nur77 antibody (R&D Systems, 1:200), anti-cytochrome c (1:500), anti-cleaved caspase-3 (1:500), anti-Hsp60 (1:500), anti-Bcl-2 (1:500), anti-Bcl-2 BH3 (1:500), and anti-Bax (6A7) (1:500).
RT-PCR Analysis
Total RNAs were isolated by Trizol LS. The first-strand synthesis was performed with RevertAidTM First Strand cDNA Synthesis Kits (Fermentas) according to the manufacturer's instructions. The primers includes those for Nur77 (forward primer: 5'-TCA TGG ACG GCT ACA CAG-3'; reverse primer: 5'-GTA GGC ATG GAA TAG CTC-3') and β-actin (forward primer: 5'-CTG GAG AAG AGC TAC GAG-3'; reverse primer: 5'-TGA TGG AGT TGA AGG TAG-3'). PCR reactions were performed in Eppendorf AG 22331 Hamburg (Eppendorf, USA). Cycling conditions were 30 sec denaturation at 94 °C, 30 sec annealing at 55 °C and 30 sec elongation at 72 °C for 35 cycles. PCR products were electrophoresed on 2% agarose gels and gel images were captured with a Gel logic 200 system (Kodak, USA).
Apoptosis assays
For DAPI staining, cells were incubated with vehicle or with different concentrations of acetylshikonin and analogs in serum-free medium for 24 hr. Detached and attached cells were collected and incubated in PBS containing 50 μg/ml DAPI and 100 μg/ml DNase-free RNase A at 37°C for 20 min. Apoptotic cells were identified as typical morphology of shrinkage of the cytoplasm, membrane blebbing, and nuclear condensation and/or fragmentation. At least 300 cells from more than 5 random microscopic fields were counted by two independent investigators. For flow cytometry analysis, cells treated with vehicle, SK07, or SK03 in serum-free medium for 24 hr were collected and stained with annexin V/PI using Vybrant Apoptosis Assay Kit#2 according to manufacture's instructions, and analyzed by flow cytometry (Beclman Coulter EPICS ALTRA).
Nur77 mutants and transfection
Two different fragments of Nur77, N-Nur77 (A/B domain) and C-Nur77 (LBD domain), were generated by PCR and inserted into EcoR I/Sal I site of pEGFP vector. The Nur77 mutants were verified by sequencing. HEK293T cells transfected with Nur77, N-Nur77 or C-Nur77 were treated with 5μM SK07 or vehicle in serum-free medium for 8 hr, and stained with DAPI. Apoptosis of transfected cells were examined as described above.
Statistical analysis
Data were expressed as mean ± SD. Each assay was repeated in triplicate in three independent experiments. Statistical significance of differences between groups was analyzed by using the Student's t-test or ANOVA analysis. Values of p<0.05 were considered significant.
Results
Acetylshikonin and analogs increase levels of Nur77 protein through posttranscriptional regulation
To identify new modulators of the Nur77-mediated apoptotic pathway (17), we screened a natural product library consisting of 3,000 extracts and pure compounds prepared from known Chinese herbs with therapeutic indications (21). Our results demonstrated that several shikonin derivatives could increase levels of Nur77 protein and apoptosis in various cancer cell lines, including NIH-H460 lung cancer, HeLa cervical cancer, HepG2 liver cancer, MCF7 breast cancer, and MGC-803 gastric cancer cells (data not shown). We reported here our characterization of acetyl derivative of shikonin, SK03 (Fig. 1A). Treatment of NIH-H460 and HeLa cells with SK03 resulted in strong induction of Nur77 protein level (Fig. 1A, B and C). In NIH-H460 cells that express high basal Nur77 protein, we consistently observed that SK03 could increase Nur77 levels by about 3∼5 fold in a concentration range between 5 and 10 μM for 12 or 24 hr (Fig. 1B), while the effect of SK03 was even more significant in HeLa cells that express low basal Nur77 levels (Fig. 1C). Thus, acetylshikonin SK03 can enhance the levels of Nur77 protein in cancer cells.
In order to identify more effective and selective modulators of the Nur77-mediated apoptotic pathway, two acetylshikonin derivatives, 5,8-diacetoxyl-2-(1'-Acetoxyl-4'-methyl-3'-pentenyl)-1,4-naphthaquinones (SK06) and 5,8-diacetoxyl-6-(1'-Acetoxyl-4'-methyl-3'-pentenyl)-1,4-naphthaquinones (SK07) (Fig. 1A), were synthesized. SK06 and SK07 were triacetate shikonins that were designed to explore whether modification of pharmacophore quinine, which is the common moiety of various shikonin derivatives, could improve their regulation of Nur77 activities. Analysis of their effect on levels of Nur77 protein in NIH-H460 (Fig. 1B) and HeLa (Fig. 1C) cells showed that the ability of SK03 on increasing Nur77 protein levels was retained in SK07 but impaired in SK06. Thus, the pharmacophore quinine in shikonins is an important determinant for their modulation of Nur77 protein levels in cancer cells.
Induction of Nur77 protein levels by SK07 was dose- and time-dependent (Fig. 1D). Treatment of NIH-H460 cells with SK07 at 1, 5, and 10 μM for 6 hr did not show any effect on Nur77 expression. However, levels of Nur77 were increased when cells were treated for 12 hr. Although 10 μM was required for optimal induction of Nur77 expression when cells were treated for 12 hr, 1 μM SK07 was sufficient for maximal increase of Nur77 protein when cells were treated with the compound for 24 hr. Such a time-dependent effect of SK07 on Nur77 expression was interesting, since induction of Nur77 by growth factors and apoptotic stimuli, such as 3-Cl-AHPC, is often rapid and transient (22). We therefore determined whether shikonins could regulate Nur77 expression at transcriptional level. NIH-H460 cells were treated with vehicle or with SK03, SK06, or SK07 at 5 μM for 3 hr and their effect on Nur77 transcript was examined by RT-PCR (Fig. 1B). As a positive control, cells were treated with phorbol ester 12-O-tetradecanoyl-13-phorbol acetate (TPA, 100 ng/ml) known to induce Nur77 expression in NIH-H460 cells (4). Consistent with previous reports, TPA could significantly up-regulate Nur77 mRNA expression. However, we did not detect clear changes in Nur77 mRNA level when cells were treated with SK03, SK06, or SK07 (Fig. 1B), suggesting that induction of Nur77 protein by SK03 and SK07 is not due to their transcriptional regulation of Nur77 expression.
Role of Nur77 in shikonin-induced apoptosis
We next determined whether Nur77 expression played a role in shikonin-induced apoptosis. NIH-460 cells were treated with 5 μM SK03, SK06, SK07 or vehicle for 24 hr and apoptosis was analyzed by DAPI staining (Fig. 2A). While apoptotic cells were seldom seen in the untreated cells (7%), significant amount of cells underwent apoptosis when treated with SK03 (22%) or SK07 (33%). In contrast, SK06 exerted much less effect on apoptosis (14%). Thus, the apoptotic effect of shikonins correlated with their induction of Nur77 protein in cancer cells, suggesting that Nur77 expression contributes to their death effect.
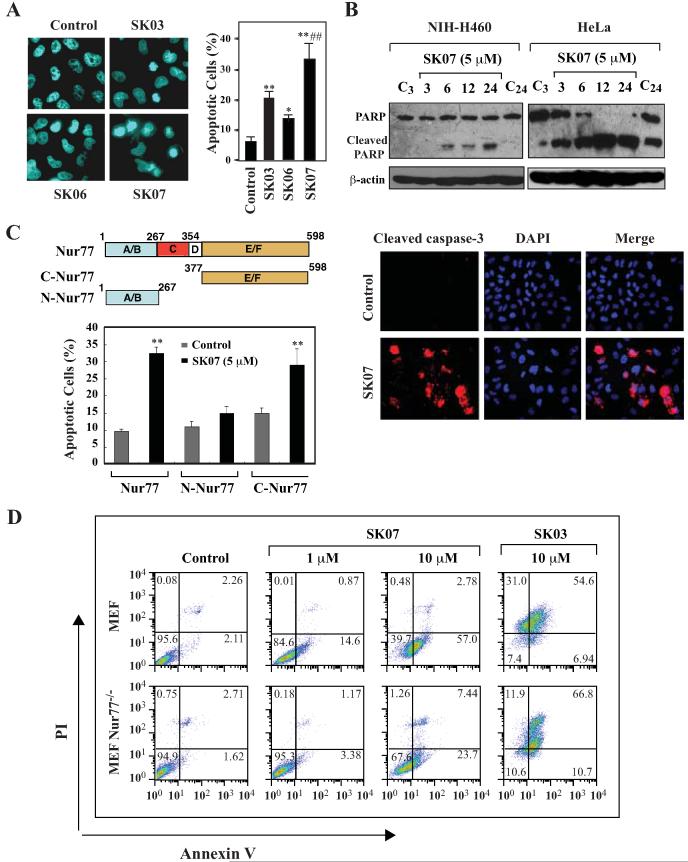
Figure 2. Induction of apoptosis by SK07 and the role of Nur77.
A, DAPI staining. NIH-H460 cells cultured in serum-free medium were treated with SK03, SK06, SK07 (5 μM), or vehicle for 24 hr and subjected to DAPI staining. Apoptotic cells were compared between different treatments. *p<0.05 (vs control); **p<0.01 (vs control); ##p<0.01 (vs SK03 or SK06). B, PARP cleavage and caspase-3 activation. NIH-H460 or HeLa cells were treated with 5 μM SK07 in serum-free medium for the indicated time. Total cell lysates were subjected to Western blotting assay for PARP cleavage using anti-PARP antibody (upper panel). As for analysis of caspase-3 activation (lower panel), NIH-H460 cells were treated with shikonins as described above and stained with an antibody recognizing the cleaved caspase-3. Nuclei were visualized by co-staining with DAPI. C, transfection of Nur77 mediates the apoptotic effect of SK07. HEK293T cells were transfected with full-length Nur77, N-Nur77 (A/B domain) or C-Nur77 (E/F domain) and subjected to SK07 treatment (5 μM) or vehicle in serum-free medium for 8 hr. Apoptotic cells examined by DAPI staining were compared between transfected cells carried different mutants. **p<0.01 (vs control). D, the apoptotic effect of SK07 is impaired in Nur77 knockout MEFs. MEF cells or MEF Nur77−/− cells were treated with vehicle or the indicated concentration of SK07 or SK03 for 24 hr and stained with PI/annexin V. Apoptosis was analyzed by FACS analysis.
The apoptotic effect of SK07 was further demonstrated by its induction of poly (ADP-ribose) polymerase (PARP) cleavage, a sensitive apoptotic marker occurring early in the apoptotic response (23). In NIH-H460 cells, SK07-induced PARP cleavage was visible at 6 hr of posttreatment, which was increased upon longer treatments (Fig. 2B). HeLa cells were more sensitive to SK07 as PARP cleavage occurred when cells were treated with SK07 for 3 hrs, with the optimal effect seen between 12 and 24 hr (Fig. 2B). We also examined whether SK07 treatment activated caspase-3, a major executioner caspase known to cleave PARP (23). Immunostaining using an antibody specifically against the active caspase-3 showed that SK07 treatment of NIH-460 cells resulted in activation of caspase-3, as a significant amount of SK07-treated cells displayed strong immunostaining, while control cells did not (Fig. 2B).
The role of Nur77 in mediating the apoptotic effect of SK07 was also examined by studying the effect of Nur77 transfection in HEK293T cells that are resistant to SK07 (data not shown). Treatment with 5 μM SK07 did not induce a clear apoptosis of HEK293T cells. However, SK07 induced an extensive apoptosis in HEK293T cells transfected with GFP-Nur77 (about 32%) (Fig. 2C). SK07 also induced apoptosis of HEK293T cells transfected with the C-terminal region of Nur77 (C-Nur77) but not the N-terminal Nur77 region (N-Nur77). The fact that the C-terminal E/F domain of Nur77 is sufficient for mediating the apoptotic effect of SK07 suggests that DNA binding and transactivation of Nur77 are not required for mediating the apoptotic effect of SK07.
To further determine the role of Nur77 expression, we evaluated the death effect of SK07 in MEF and Nur77 knockout MEF (MEF Nur77−/−) cells by flow cytometry for annexin V and propidium iodide (PI) staining. Annexin V staining serves as a measure of phosphatidylserine externalization and cells that are annexin V+/PI− represent early apoptotic cells (24). A representative experiment (Fig. 2D) showed that early apoptotic cells (annexin V+/PI−) (14.6% and 57%) were seen in MEF treated with 1 μM and 10 μM SK07, respectively. In contrast, annexin V+/PI− staining was significantly reduced in MEF Nur77−/− cells (3.38% with 1 μM and 23.7% with 10 μM SK07 treatment). These results demonstrate that the apoptotic effect of SK07 largely depends on Nur77 expression, although other protein factors may mediate its effect when high concentration of SK07 is used. Interestingly, SK03 treatment (10 μM) resulted in a significant amount of late apoptotic cells (annexin V+/PI+) in more than half of both MEF cells (54.6%) and MEF Nur77−/− cells (66.8%). In addition, SK03 caused significant necrotic cell death in both MEF cells (31%) and MEF Nur77−/− cells (11.9%), suggesting that SK03 may induce cell death mainly through Nur77-independent pathways.
SK07 induces Nur77 nuclear export and mitochondrial targeting
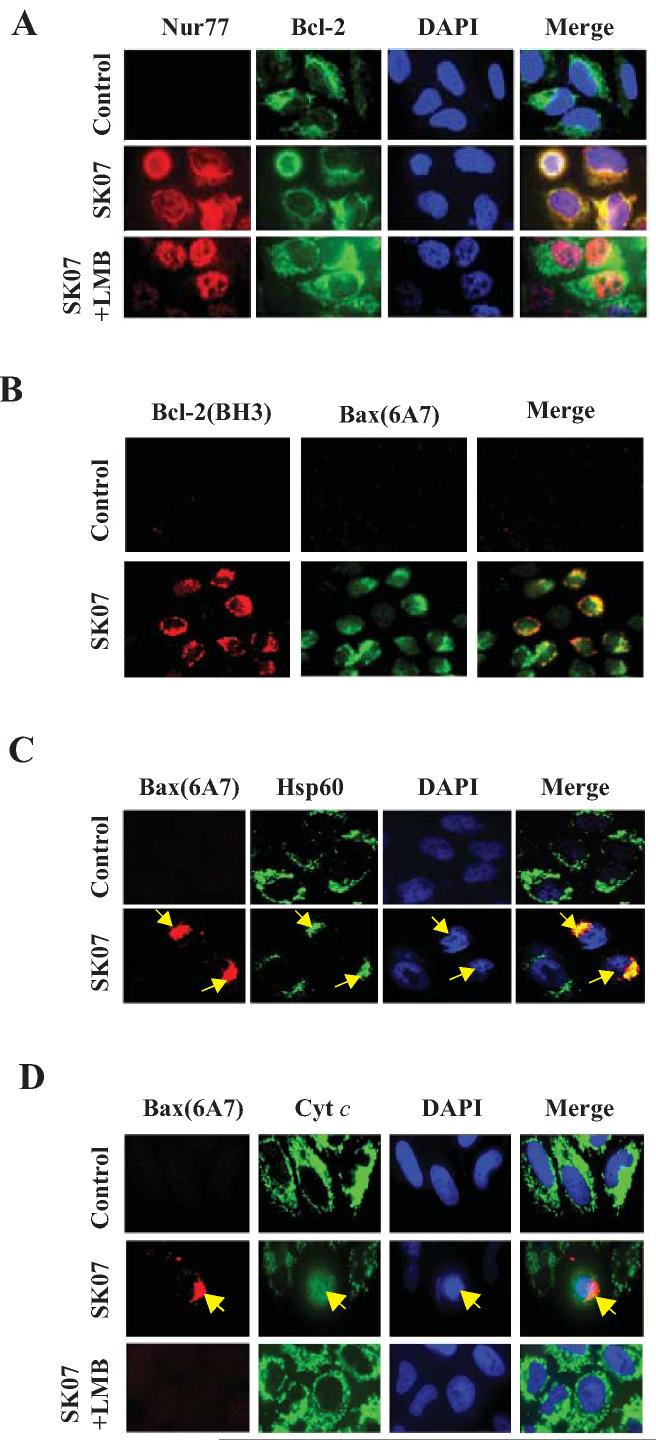
To determine whether acetylshikonin and analogs could induce cytoplasmic localization of Nur77, the hallmark of the Nur77-mediated apoptotic pathway (17), we examined their effect on the subcellular localization of Nur77 in NIH-H460 cells. As shown in Fig. 3A, Nur77 immunostaining was significantly enhanced in cells treated with SK03 and SK07, but not with SK06, consistent with their induction of Nur77 protein level. For comparison, TPA also strongly induced Nur77 immunostaining. However, TPA-induced Nur77 immunostaining was confined in the nucleus, as reported previously (4). In contrast, most of cells treated with SK03 and SK07 displayed diffused distribution of Nur77. Predominant cytoplasmic localization of Nur77 could be seen in some treated cells, which was more apparent when treated with SK07 than with SK03. These results suggested that SK03 and SK07 induced cytoplasmic localization of Nur77. Cytoplasmic localization of Nur77 observed in cells treated with SK07 might be due to its induction of Nur77 nuclear export, as leptomycin B (LMB) that is known to block CRM1-dependent nuclear export (25) significantly inhibited SK07-induced Nur77 cytoplasmic accumulation. To confirm the effect of shikonins on inducing cytoplasmic localization of Nur77, cytosolic and nuclear fractions of cells treated with SK03, SK06, or SK07 were prepared and analyzed for levels of Nur77 protein by immunoblotting. As shown in Fig. 3B, Nur77 was predominantly nuclear (92%) in control cells. However, in cells treated with SK03 and SK07, a significant amount (65% and 85%, respectively) of Nur77 protein was cytoplasmic. In contrast, only 30% of Nur77 protein resided in the cytoplasm when treated with SK06. Consistent with immunostaining results, pretreatment of cells with LMB reduced the amount of SK07-induced cytoplasmic Nur77 from 85% to 42%. Thus, SK07 is more effective than SK03 and SK06 in inducing cytoplasmic localization of Nur77.
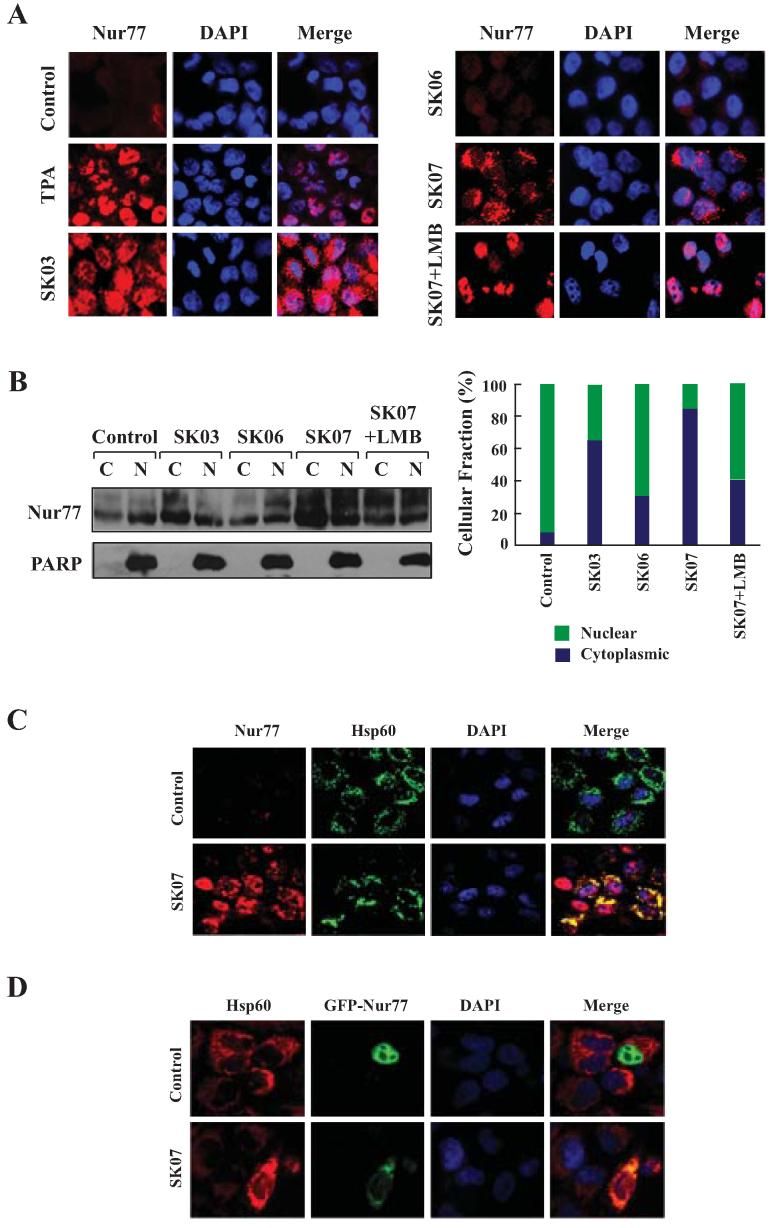
Figure 3. Acetylshikonins induce cytoplasmic localization of Nur77.
A, Nur77 translocation. NIH-H460 cells treated with SK03, SK06, or SK07 (5 μM) for 24 hr were subjected to immunostaining with anti-Nur77 antibody. Cells treated with 100 ng/mL TPA (3 hr) were used as positive control. Subcellular localization of Nur77 in cells treated with SK07 alone was compared to that in cells with cotreatment of SK07 and LMB (10 ng/mL). Cells were co-stained with DAPI to visualize the nuclei. B, cellular fractionation. Nuclear and cytoplasmic fractions were prepared from NIH-H460 cells with similar treatments as indicated in A, and subjected to Western blotting analysis. The purity of cellular fraction was confirmed by using anti-PARP antibody. Ratio of cytoplasmic Nur77 (C) to nuclear Nur77 (N) expression was quantified by BandScan 5.0. C and D, Nur77 mitochondrial targeting. NIH-H460 cells transfected with or without GFP-Nur77 were treated with 5 μM SK07 for 24 hr. Endogenous Nur77 expression was immunostained with anti-Nur77 antibody. Cells were co-stained with anti-Hsp60 antibody to recognize the mitochondria and with DAPI to visualize the nuclei. Fluorescent microscopy was used to determine the overlap of Nur77 and Hsp60.
To determine whether SK07-induced cytoplasmic Nur77 could associate with mitochondria, NIH-H460 cells treated with SK07 or vehicle were immunostained with anti-Nur77 antibody and antibody against Hsp60, a mitochondria-specific protein. Confocal microscopy analysis showed that the distribution of Nur77 was extensively overlapped with that of Hsp60 when cells were treated with SK07 (Fig. 3C). To confirm the ability of SK07 to induce Nur77 mitochondrial targeting, expression vector encoding GFP-Nur77 was transfected into NIH-H460 cells and its subcellular distribution in cells treated with or without SK07 was examined. As shown in Fig. 3D, GFP-Nur77 was exclusively nuclear in untreated cells, but it was found in the cytoplasm and colocalized with Hsp60 when cells were treated with SK07. Thus, SK07 can induce Nur77 mitochondrial targeting.
SK07-induced apoptosis is dependent on cytoplasmic localization of Nur77
To study whether the cytoplasmic localization of Nur77 was required for the killing activity of SK07, NIH-H460 cells were treated with 10 μM SK07 and subsequently stained with anti-Nur77 antibody and DAPI. Microscopy analysis showed that treatment of cells with SK07 resulted in a significant amount of cells displaying cytoplasmic localization of Nur77, many of them showing nuclear morphology typical of apoptosis (about 45%) (Fig. 4A). However, when cells were cotreated with LMB, SK07-induced Nur77 protein mainly resided in the nucleus, and cells displayed normal nuclear morphology. The inhibitory effect of LMB on SK07-induced apoptosis was confirmed by annexin V staining (Fig. 4B). Flow cytometry analysis showed that NIH-460 cells treated with 5 μM SK07 resulted in extensive early apoptosis (37.9% annexin V+/PI− cells). This level of apoptosis was dramatically reduced (4.9% annexin V+/PI− cells) when cells were cotreated with LMB. We also determined the effect of LMB on SK07-induced cytoplasmic localization of GFP-Nur77 and apoptosis. NIH-H460 cells transfected with GFP-Nur77 were exposed to 10 μM SK07 for 24 hr, and apoptosis was examined by DAPI staining. In the absence of treatment, GFP-Nur77 resided in the nucleus and no apparent apoptotic cells were detected (Fig. 4C). However, GFP-Nur77 could be found in the cytoplasm of cells treated with SK07, which was coincident with extensive nuclear condensation and fragmentation with about 80% apoptosis. In the presence of LMB, SK07-induced apoptosis was decreased to about 16%. Again, inhibition of apoptosis by LMB was accompanied by its inhibition of the cytoplasmic localization of GFP-Nur77. Together, these results demonstrate that the cytoplasmic localization of Nur77 plays a critical role in mediating the apoptotic effect of SK07.
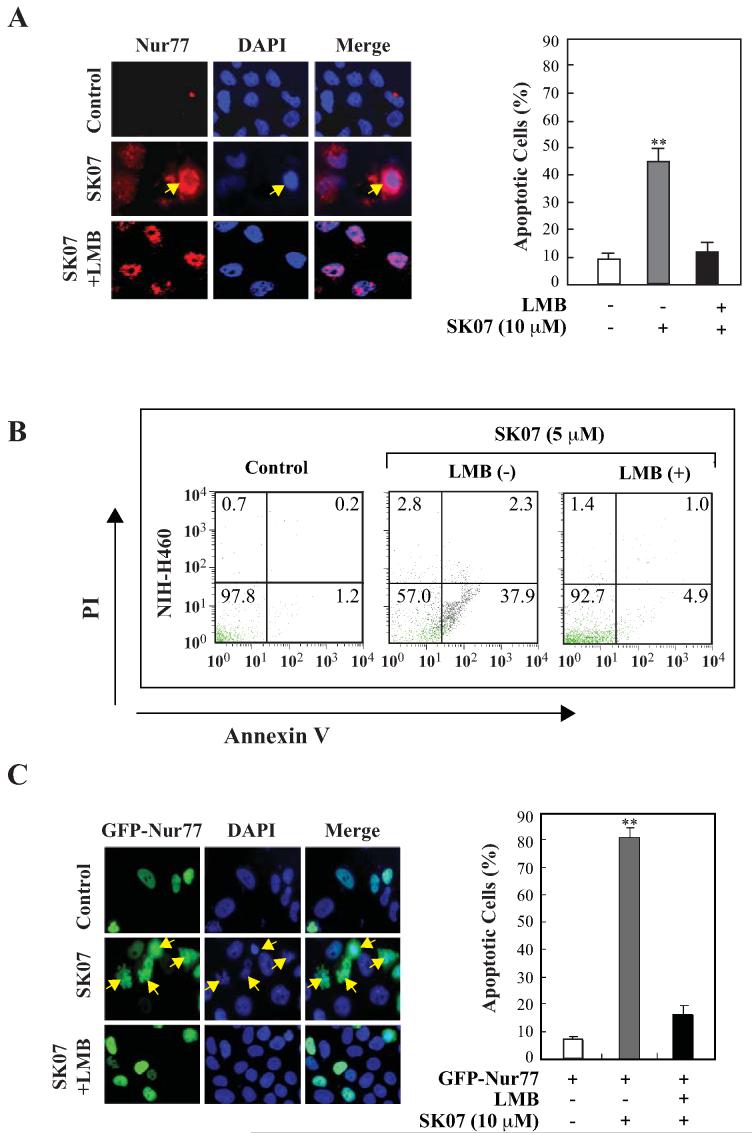
Figure 4. SK07-induced apoptosis is dependent on Nur77 expression and Nur77 nuclear export.
A, Nur77 cytoplasmic localization and apoptosis. NIH-H460 cells were treated with 10 μM SK07 in serum-free medium in the presence or absence of LMB (10 ng/mL) for 24 hr. Subcellular localization of endogenous Nur77 was examined by immunostaining using anti-Nur77 antibody (R&D) and apoptosis was determined by DAPI staining. B, analysis of the effect of LMB on SK07-induced apoptosis by PI/annexin V staining. NIH-H460 cancer cells were treated with SK07 (5 μM) in the presence or absence of LMB (10 ng/mL) for 24 hr. Apoptosis was determined by PI/annexin V staining. C, cytoplasmic localization of transfected Nur77 and apoptosis. NIH-H460 cells transfected with GFP-Nur77 expression vector were treated with 10 μM SK07 in serum-free medium in the presence or absence of LMB (10 ng/mL) for 24 hr. Subcellular localization of GFP-Nur77 was examined by fluorescence microscopy and apoptosis was examined by DAPI staining. Quantification was conducted in nontransfected and transfected cells respectively. **p<0.01 [vs control or LMB (+)].
SK07 induces Bcl-2 conformational change
Cytoplasmic Nur77 is known to bind Bcl-2, inducing a Bcl-2 conformational change that is pro-apoptotic (16, 17). To determine whether cytoplasmic Nur77 could co-localize with Bcl-2, NIH-H460 cells treated with SK07 or vehicle were immunostained with anti-Nur77 and anti-Bcl-2 antibodies. Microscopy analysis showed that the distribution pattern of SK07-induced cytoplasmic Nur77 overlapped extensively with that of Bcl-2 (Fig. 5A). Cotreatment with LMB inhibited the colocalization of Nur77 and Bcl-2 by preventing Nur77 cytoplasmic localization. In transfection experiment, we also observed that SK07-induced cytoplasmic GFP-Nur77 colocalized with Bcl-2 expression (data not shown). The colocalizaiton of cytoplasmic Nur77 with Bcl-2 suggests that SK07-induced Nur77 might interact with Bcl-2.
Figure 5. SK07 induces Bcl-2 conformational change and Bax activation.
A, colocalization of Nur77 with Bcl-2. NIH-H460 cells treated with vehicle or 5 μM SK07 in the presence or absence of LMB (10 ng/mL) for 24 hr were subjected to co-staining with anti-Nur77 and anti-Bcl-2 antibodies. Fluorescent microscopy was used to analyze the subcellular localization of Nur77 and Bcl-2. B, Bcl-2 conformational change and Bax activation. NIH-H460 cells treated with 5 μM SK07 were examined for Bcl-2 conformational change by anti-Bcl-2(BH3) antibody. Its association with Bax activation was analyzed by co-staining with anti-Bax (6A7) antibody. Cells were also stained with DAPI to visualize the nucleus. C, translocation of Bax to mitochondria. NIH-H460 cells treated with 5 μM SK07 were examined for Bax activation by antibody against Bax (6A7). Cells were co-stained with anti-Hsp60 antibody to reveal Bax colocalization with mitochondria. DAPI staining was employed to visualize the nuclei. The colocalization of active Bax with mitochondria and their association with apoptotic nuclear morphology were compared. D, Bax activation and cytochrome c release. NIH-H460 cells treated with 5 μM SK07 were stained with anti-Bax (6A7) and anti-cytochrome c (cyt c) antibodies. Bax activation was compared to its induction of cytochrome c release as indicated by diffusive cyt c staining. LMB was used to determine its effect on both Bax activation and cyt c release.
Interaction of Nur77 with Bcl-2 induces a Bcl-2 conformational change with its BH3 domain exposed (17). Such a Bcl-2 conformation is pro-apoptotic and can be detected by anti-Bcl-2 antibody against the BH3 domain of Bcl-2, anti-Bcl-2(BH3), (17). To study whether SK07 induced a Bcl-2 conformational change, NIH-H460 cells treated with SK07 or vehicle were immunostained with the anti-Bcl-2(BH3) antibody. Although Bcl-2 is highly expressed in NIH-H460 cells, which could be stained with anti-Bcl-2 antibody against the whole Bcl-2 protein (Fig. 5A), it was not stained by the anti-Bcl-2(BH3) antibody (Fig. 5B), suggesting that Bcl-2 was anti-apoptotic in untreated NIH-H460 cells. However, when cells were treated with SK07, we observed a strong immunostaining by the anti-Bcl-2(BH3) antibody (Fig. 5B). Thus, SK07 is able to induce a Bcl-2 conformational change, likely through Nur77/Bcl-2 interaction.
SK07 induces Bax activation.
An unresolved issue in the Nur77-Bcl-2 apoptotic pathway is whether Bcl-2 conformational change is sufficient to induce apoptosis or whether exposed BH3 domain of Bcl-2 acts as a BH3-only protein that induces apoptosis through activation of pro-apoptotic Bcl-2 family members and/or inhibition of anti-apoptotic members (17, 26). Thus, we determined whether Bax activation was involved in SK07-induced apoptosis by immunostaining NIH-H460 cells with anti-Bax (6A7) antibody that recognizes activated Bax. Untreated NIH-460 cells were not immunostained by the anti-Bax (6A7)antibody, suggesting that Bax was inactive in the cells (Fig. 5B). However, cells treated with SK07 displayed strong immunostaining with the anti-Bax (6A7) antibody, demonstrating that SK07 could activate Bax. Our observation that SK07-treated cells stained by both anti-Bcl-2(BH3) and anti-Bax (6A7) antibodies suggests that SK07-induced Bcl-2 conformational change may play a role in Bax activation.
Bax resides predominantly in the cytosol before its activation and apoptosis induction. During early apoptosis, Bax undergoes conformational change and oligomerization, resulting in its translocation from the cytosol to mitochondria (27). We then examined whether Bax could associate with mitochondria in SK07-treated cells. Microscopy analysis demonstrated an extensive colocalization of active Bax with mitochondrial specific protein Hsp60 (Fig. 5C). Furthermore, cells with active Bax immunostaining often displayed nuclear condensation (Fig. 5C), suggesting that SK07-induced apoptosis might involve Bax activation. To further confirm this association, NIH-H460 cells were co-stained with anti-Bax (6A7) and anti-cytochrome c antibodies. In control cells, cytochrome c displayed a punctate immunostaining pattern, consistent with its mitochondrial localization (Fig. 5D). However, cytochrome c was diffusely distributed in SK07-treated cells with active Bax immunostaining. Thus, SK07-induced Bax activation was associated with cytochrome c release. LMB co-treatment, which prevented Nur77 nuclear export and Bcl-2 conformational change, almost completely inhibited SK07-induced Bax activation and cytochrome c release (Fig. 5D).
SK07 does not affect Bcl-2 and Bax expression
Since it has been widely reported that the ratio between Bcl-2 and Bax determines cell survival or death (26), we next examined whether the apoptotic effect of SK07 was due to its effect on Bcl-2 and Bax expression. The result showed that SK07 caused no apparent change in level of either Bcl-2 or Bax for treatment time ranging from 3 to 24 hr (Fig. 6). Thus, SK07 induces apoptosis through induction of Bcl-2 conformational change and Bax activation rather than affecting Bcl-2/Bax ratio.
Figure 6. SK07 does not affect Bax and Bcl-2 expression levels.
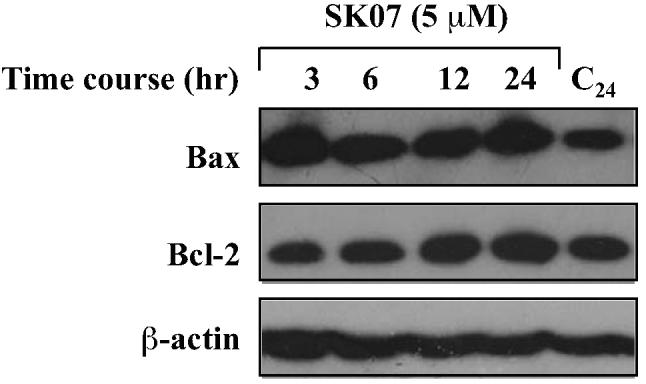
Cells were treated with vehicle or 5 μM SK07 for the indicated period of time. Total cell lysates were prepared and examined for Bcl-2 and Bax expression by Western blotting analysis. β-actin expression was used as a loading control.
Discussion
Traditional Chinese herbal medicines, which constitute numerous chemically unique and biomedically powerful secondary metabolites, are a rich source of therapeutic leads (21). Recent examples include the discoveries of paclitaxel and camptothecin (28, 29). Shikonin derivatives, mainly contained in the roots of Chinese plant Lithospermum erythrorhizon, are the naturally occurring naphthoquinones that embody numerous active compounds with a unique pharmacophore quinone moiety (19). These compounds are promising drug candidates as they have been reported to have multiple anti-cancer effects in vitro and in animals (19, 20). However, rational design of shikonin analogs is necessary in order to reduce the non-specific toxicity of shikonins, which has prevented them from clinical development.
Nur77 is a unique orphan member of the nuclear receptor superfamily (1). It is perhaps the most potent pro-apoptotic member in the nuclear receptor superfamily (16, 17). Recently, we demonstrated a new paradigm in cancer cell apoptosis through Nur77 mitochondrial targeting (17, 30), which was later confirmed by other groups (15, 16, 18). We showed that apoptosis of cancer cells induced by certain apoptotic agents requires Nur77 expression and its translocation from the nucleus to mitochondria where Nur77 interacts with Bcl-2, resulting in a Bcl-2 conformational change that converts it into a cytodestructive molecule (17). These findings suggest that agents that induce cytoplasmic localization of Nur77 and its interaction with Bcl-2 may preferentially kill cancer cells with elevated Bcl-2 levels.
In the course of identifying new agents that modulate the Nur77-mediated apoptotic pathway, we report here our finding that certain shikonin derivatives could activate the Nur77 pathway through their induction of Nur77 expression, nuclear export, mitochondrial targeting, Bcl-2 conformational change and Bax activation. These results are consistent with previous report that shikonin-like compounds can induce apoptosis through mitochondria and caspase pathway with unknown mechanisms (31). Our current finding that certain shikonins could regulate the Nur77-mediated apoptotic pathway set a stage for rational design of shikonin analogs with improved anti-cancer efficacy and specificity. It is generally believed that the non-specific toxicity of shikonins is caused by electronic transferation of hydroxyl groups between 1-α and C-11 positions and subsequently occurring alkylation (20). Our current study indicates that certain esteration of 1-α carbonyl group of acetylshikonin like SK07 can enhance apoptosis by promoting Nur77 expression and migration. We found that introduction of acetyl groups into the pharmacophore quinoid moiety of SK03 exerted great effect on its modulation of Nur77 activity. Induction of Nur77 protein levels by SK03 in cancer cells was retained in SK07 (Fig. 1, B and C). Interestingly, the ability of inducing Nur77 cytoplasmic localization by SK03 was significantly enhanced in SK07 but reduced in SK06 (Fig. 3, A and B). The distinct abilities to induce Nur77 expression and Nur77 cytoplasmic translocation shown in different shikonin derivatives (SK07 and SK06) suggest that quinoid modification is an important determinant for their modulation of Nur77 activities, including its stability and nuclear export.
Our results demonstrated that increase of Nur77 protein levels and its cytoplasmic localization were associated with the apoptotic effect of shikonins. Treatment of NIH-H460 cells with SK07, which induced Nur77 expression and nuclear export, resulted in cytochrome c release from the mitochondria (Fig. 5D), caspase-3 activation and PARP cleavage (Fig. 2B). SK07-induced apoptosis was dependent on Nur77 expression level as its killing effect was largely impaired in MEF Nur77−/− cells when compared to MEF cells (Fig. 2D) and enhanced by Nur77 transfection (Fig. 2C and 4C). The apoptotic effect of SK07 could be observed in other cancer cells like HeLa cells (Fig. 1C and Fig. 2B, and data not shown), consistent with the role of Nur77 expression in different types of cancer cells (8, 14, 15, 18). Our results also demonstrated that SK07-induced apoptosis was associated with cytoplasmic localization of Nur77 since cytoplasmic accumulation of both endogenous Nur77 and transfected Nur77 was accompanied with nuclear condensation and fragmentation (Fig. 4, A and C). In addition, LMB blockage of Nur77 cytoplasmic localization significantly inhibited SK07-induced Bax activation, cytochrome c release (Fig. 5D), and apoptosis (Fig. 4). Consistently, deletion of the DNA-binding domain from Nur77 did not affect the apoptotic effect of SK07 in HEK293T cells (Fig. 2C).
In studying the mechanism by which cytoplasmic Nur77 induced apoptosis, we found that SK07-induced cytoplasmic Nur77 targeted mitochondria (Fig. 3, C and D) probably through its interaction with Bcl-2 (Fig. 5A), leading to Bcl-2 conformational change that exposes its BH3 domain (Fig. 5B). These results confirmed our previous finding that cytoplasmic Nur77 could target mitochondria and induce Bcl-2 conversion (17). Interestingly, our result showed that SK07-induced Bcl-2 conformational change was associated with Bax activation (Fig. 5B). This observation is significant, as it would suggest that the converted Bcl-2 requires Bax activation for apoptosis indcution. Although it is unclear how converted Bcl-2 activates Bax, it can be envisioned that the converted Bcl-2 with its BH3 domain exposed may act directly or indirectly on Bax, resulting in Bax activation and apoptosis.
Besides its improved efficacy in inducing apoptosis, SK07 may have improved selectivity towards the Nur77-mediated apoptotic pathway. Shikonins are known to modulate multiple signal transduction pathways, including inhibition of DNA topoisomerases (31), induction of reactive oxygen species (ROS) release (32), and inhibition of survival pathways involving ERK, Akt and NF-κB activities (33). Interestingly, the apoptotic effect of SK07 was largely impaired in Nur77 knock-out cells (Fig. 2D). By contrast, SK03, the parent compound of SK07, induced a significant amount of late apoptotic and necrotic death cells in both MEF and MEF Nur77−/− cells (Fig. 2D), suggesting that its biological activities largely depended on Nur77-independent pathways. Improved Nur77 selectivity, as that observed with SK07, may be therapeutic desirable as Nur77 is often overexpressed in cancer cells comparing to normal cells. Such a tumor selectivity can be further ensured by the fact that the Nur77-mediated apoptotic pathway targets Bcl-2 whose expression is also often elevated in cancer cells (17). Thus, SK07 may represent a new drug lead that induces cancer cell death by targeting the Nur77-Bcl-2 apoptotic pathway.
Acknowledgments
We thank Dr. Pinchas Cohen for providing Nur77 knockout MEFs and Dr. Ying Su for technical assistance. This work is in part supported by grants to J-Z Zeng and X-k Zhang from 863 Program of China (No. 2007AA09Z404), National Natural Science Foundation of China (No. 30471939), Shanghai Key Project (No. 04DZ19115), Program of Shanghai Subject Chief Scientist (No. OZ041915), Key Science and Technology Planning Project of Fujian Province (No. 2007I0023), NIH (CA87000, GM60544, CA109345), the Susan G. Komen Breast Cancer Foundation, the U.S. Army Medical Research and Material Command, the California Tobacco-Related Diseases Research Program, and the California Breast Cancer Research Program.
References
- 1.Zhang XK. Targeting Nur77 translocation. Expert Opin Ther Tar. 2007;11:69–79. doi: 10.1517/14728222.11.1.69. [DOI] [PubMed] [Google Scholar]
- 2.Moll UM, Marchenko N, Zhang XK. p53 and Nur77/TR3 - transcription factors that directly target mitochondria for cell death induction. Oncogene. 2006;25:4725–43. doi: 10.1038/sj.onc.1209601. [DOI] [PubMed] [Google Scholar]
- 3.Chen GQ, Lin B, Dawson MI, Zhang XK. Nicotine modulates the effects of retinoids on growth inhibition and RAR beta expression in lung cancer cells. Int J Cancer. 2002;99:171–8. doi: 10.1002/ijc.10304. [DOI] [PubMed] [Google Scholar]
- 4.Wu Q, Liu S, Ye XF, Huang ZW, Su WJ. Dual roles of Nur77 in selective regulation of apoptosis and cell cycle by TPA and ATRA in gastric cancer cells. Carcinogenesis. 2002;23:1583–92. doi: 10.1093/carcin/23.10.1583. [DOI] [PubMed] [Google Scholar]
- 5.Stasik I, Rapak A, Kalas W, Ziolo E, Strzadala L. Ionomycin-induced apoptosis of thymocytes is independent of Nur77 NBRE or NurRE binding, but is accompanied by Nur77 mitochondrial targeting. BBA-Mol Cell Res. 2007;1773:1483–90. doi: 10.1016/j.bbamcr.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Gennari A, Bleumink R, Viviani B, et al. Identification by DNA macroarray of nur77 as a gene induced by di-n-butyltin dichloride: its role in organotin-induced apoptosis. Toxicol Appl Pharmacol. 2002;181:27–31. doi: 10.1006/taap.2002.9357. [DOI] [PubMed] [Google Scholar]
- 7.Liu S, Wu Q, Ye XF, Cai JH, Huang ZW, Su WJ. Induction of apoptosis by TPA and VP-16 is through translocation of TR3. World J Gastroenterol. 2002;8:446–50. doi: 10.3748/wjg.v8.i3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson AJ, Arango D, Mariadason JM, Heerdt BG, Augenlicht LH. TR3/Nur77 in colon cancer cell apoptosis. Cancer Res. 2003;63:5401–7. [PubMed] [Google Scholar]
- 9.Chintharlapalli S, Burghardt R, Papineni S, Ramaiah S, Yoon K, Safe S. Activation of Nur77 by selected 1,1-Bis(3'-indolyl)-1-(p-substituted phenyl)methanes induces apoptosis through nuclear pathways. J Biol Chem. 2005;280:24903–14. doi: 10.1074/jbc.M500107200. [DOI] [PubMed] [Google Scholar]
- 10.Shipp MA, Ross KN, Tamayo P, et al. Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat Med. 2002;8:68–74. doi: 10.1038/nm0102-68. [DOI] [PubMed] [Google Scholar]
- 11.Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 12.Kolluri SK, Bruey-Sedano N, Cao X, et al. Mitogenic effect of orphan receptor TR3 and its regulation by MEKK1 in lung cancer cells. Mol Cell Biol. 2003;23:8651–67. doi: 10.1128/MCB.23.23.8651-8667.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng H, Qin L, Zhao D, et al. Orphan nuclear receptor TR3/Nur77 regulates VEGF-A-induced angiogenesis through its transcriptional activity. J Exp Med. 2006;203:719–29. doi: 10.1084/jem.20051523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong JH, Park JS, Moon B, et al. Orphan nuclear receptor Nur77 translocates to mitochondria in the early phase of apoptosis induced by synthetic chenodeoxycholic acid derivatives in human stomach cancer cell line SNU-1. Ann NY Acad Sci. 2003;1010:171–7. doi: 10.1196/annals.1299.029. [DOI] [PubMed] [Google Scholar]
- 15.Sibayama-Imazu T, Fujisawa Y, Masuda Y, et al. Induction of apoptosis in PA-1 ovarian cancer cells by vitamin K(2) is associated with an increase in the level of TR3/Nur77 and its accumulation in mitochondria and nuclei. J Cancer Res Clin. 2008;134:803–12. doi: 10.1007/s00432-007-0349-z. [DOI] [PubMed] [Google Scholar]
- 16.Thompson J, Winoto A. During negative selection, Nur77 family proteins translocate to mitochondria where they associate with Bcl-2 and expose its proapoptotic BH3 domain. J Exp Med. 2008;205:1029–36. doi: 10.1084/jem.20080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin B, Kolluri SK, Lin F, et al. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004;116:527–40. doi: 10.1016/s0092-8674(04)00162-x. [DOI] [PubMed] [Google Scholar]
- 18.Maddika S, Booy EP, Johar D, Gibson SB, Ghavami S, Los M. Cancer-specific toxicity of apoptin is independent of death receptors but involves the loss of mitochondrial membrane potential and the release of mitochondrial cell-death mediators by a Nur77-dependent pathway. J Cell Sci. 2005;118:4485–93. doi: 10.1242/jcs.02580. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Yang L, Oppenheim JJ, Howard MZ. Cellular pharmacology studies of shikonin derivatives. Phytother Res. 2002;16:199–209. doi: 10.1002/ptr.1100. [DOI] [PubMed] [Google Scholar]
- 20.Papageorgiou V, Assimopoulou AN, Couladouros EA, Hepworth D, Nicolaou KC. The chemistry and biology of alkannin, shikonin, and related naphthazarin natural products. Angew Chem Int Ed. 1999;38:270–300. doi: 10.1002/(SICI)1521-3773(19990201)38:3<270::AID-ANIE270>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 21.Zeng JZ, Sun DF, Wang L, et al. Hypericum sampsonii induces apoptosis and nuclear export of retinoid X receptor-alpha. Carcinogenesis. 2006;27:1991–2000. doi: 10.1093/carcin/bgl046. [DOI] [PubMed] [Google Scholar]
- 22.Dawson MI, Harris DL, Liu G, et al. Antagonist analogue of 6-[3'-(1-adamantyl)-4'-hydroxyphenyl]-2-naphthalenecarboxylic acid (AHPN) family of apoptosis inducers that effectively blocks AHPN-induced apoptosis but not cell-cycle arrest. J Med Chem. 2004;47:3518–36. doi: 10.1021/jm030524k. [DOI] [PubMed] [Google Scholar]
- 23.Kaufmann SH, Desnoyers S, Ottaviano Y, Davidson NE, Poirier GG. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993;53:3976–85. [PubMed] [Google Scholar]
- 24.Bacso Z, Everson RB, Eliason JF. The DNA of annexin V-binding apoptotic cells is highly fragmented. Cancer Res. 2000;60:4623–8. [PubMed] [Google Scholar]
- 25.Cao X, Liu W, Lin F, et al. Retinoid X receptor regulates Nur77/TR3-dependent apoptosis by modulating its nuclear export and mitochondrial targeting. Mol Cell Biol. 2004;24:9705–25. doi: 10.1128/MCB.24.22.9705-9725.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. eath switch. Nat Rev Cancer. 2002;2:647–56. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 27.Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–92. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed AA, Mills AD, Ibrahim AE, et al. The extracellular matrix protein TGFBI induces microtubule stabilization and sensitizes ovarian cancers to paclitaxel. Cancer Cell. 2007;12:514–27. doi: 10.1016/j.ccr.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ulukan H, Swaan PW. Camptothecins: a review of their chemotherapeutic potential. Drugs. 2002;62:2039–57. doi: 10.2165/00003495-200262140-00004. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Kolluri SK, Gu J, et al. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science. 2000;289:1159–64. doi: 10.1126/science.289.5482.1159. [DOI] [PubMed] [Google Scholar]
- 31.Yang F, Chen Y, Duan W, Zhang C, Zhu H, Ding J. SH-7, a new synthesized shikonin derivative, exerting its potent antitumor activities as a topoisomerase inhibitor. Int J Cancer. 2006;119:1184–93. doi: 10.1002/ijc.21943. [DOI] [PubMed] [Google Scholar]
- 32.Gao D, kakuma M, Oka S, Sugino K, Sakurai H. Reaction of beta-alkannin (shikonin) with reactive oxygen species: detection of beta-alkannin free radicals. Bioorg Med Chem. 2000;8:2561–9. doi: 10.1016/s0968-0896(00)00191-7. [DOI] [PubMed] [Google Scholar]
- 33.Hashimoto S, Xu M, Masuda Y, et al. beta-hydroxyisovalerylshikonin inhibits the cell growth of various cancer cell lines and induces apoptosis in leukemia HL-60 cells through a mechanism different from those of Fas and etoposide. J Biochem. 1999;125:17–23. doi: 10.1093/oxfordjournals.jbchem.a022255. [DOI] [PubMed] [Google Scholar]