Abstract
This research focuses on the relationship between fragile X syndrome (FXS) and autism spectrum disorders (ASD). Both of these populations have a tendency to avoid looking others in the eye, along with difficulties in communication with others and tend to be socially withdrawn. While it is clear that FXS and ASD share some common abnormal behaviors, the underlying brain mechanisms associated with the social and emotional deficits in these groups remain unclear. We showed pictures of emotional and non-emotional human faces to these groups while in a magnetic resonance scanner (MRI). We collected images of brain function along with measures of where on the faces the individuals were looking (e.g. eyes or mouth). The FXS group showed a similar yet less abnormal pattern of where they were looking on the face compared to the ASD group. The FXS group also showed a similar pattern of decreased brain function in the area of the brain typically used when looking at faces, the fusiform gyrus (FG). The amount of activation in the FG was associated with how much time the FXS and ASD individuals looked at the eyes, the more they looked at the eyes, the greater the FG activation. The FXS group also displayed more brain activation than both the ASD group and a group of typically developing control subjects in brain areas that might suggest increased task difficulty for the FXS group. These group differences in brain activation are important as they suggest there is some overlap in areas of brain function in FXS and ASD when looking at faces, but that these two groups also have unique activation in other brain areas. These findings largely support the idea that ASD characteristics in FXS are associated with partially different patterns of brain activation when looking at human faces compared to individuals with ASD.
Objective:
Fragile X syndrome (FXS) is the most commonly known genetic disorder associated with autism spectrum disorder (ASD). Overlapping features in these populations include gaze aversion, communication deficits, and social withdrawal. Although the association between FXS and ASD has been well documented at the behavioral level, the underlying neural mechanisms associated with the social/emotional deficits in these groups remain unclear.
Method:
We collected functional brain images and eye-gaze fixations from 9 individuals with FXS and 14 individuals with idiopathic ASD, as well as 15 typically developing (TD) individuals, while they performed a facial-emotion discrimination task.
Results:
The FXS group showed a similar yet less aberrant pattern of gaze-fixations compared to the ASD group. The FXS group also showed fusiform gyrus (FG) hypoactivation compared to the TD control group. Activation in FG was strongly and positively associated with average eye fixation and negatively associated with ASD characteristics in the FXS group. The FXS group displayed significantly greater activation than both the TD control and ASD groups in the left hippocampus (HIPP), left superior temporal gyrus (STG), right insula (INS), and left post-central gyrus (PCG).
Conclusions:
These group differences in brain activation are important as they suggest unique underlying face-processing neural circuitry in FXS versus idiopathic ASD, largely supporting the hypothesis that ASD characteristics in FXS and idiopathic ASD reflect partially divergent impairments at the neural level, at least in FXS individuals without a co-morbid diagnosis of ASD.
Keywords: fragile X syndrome, autism, face processing, brain function, fMRI
Introduction
Fragile X syndrome (FXS) affects approximately 1 in every 4,000 males and 1 in every 8,000 females (Crawford et al., 2001). The syndrome results from an expansion of the CGG trinucleotide repeat on the distal end of the X chromosome and a subsequent reduction of Fragile X Mental Retardation Protein (FMRP) expression (Pieretti et al., 1991). While FXS is a single-gene disorder, the effect of diminished or absent FMRP interacts with other proteins and developmental processes and hence has cumulative effects on other molecular, genetic and epigenetic cascades causing disruptions in the development and maintenance of both neuronal synapses and larger neuronal networks. These complex interactions lead to a wide range of severities and behavioral phenotypes across both males and females biochemically and behaviorally, making the FXS phenotype more of a spectrum disorder, (Matthew K. Belmonte et al., 2004; Matthew K Belmonte & Bourgeron, 2006; Hagerman, 1999). These behavioral characteristics overlap with the core features associated with autism spectrum disorder (ASD) and considerable research suggests that there is an association between FXS and autism (AUT), particularly in the social/emotional domain (Bailey et al., 2004; Feinstein & Reiss, 2001; Kaufmann et al., 2004). However, controversy exists regarding the nature of the underlying deficit(s) in emotional processes and social interaction in FXS and idiopathic AUT, with some suggesting that similarities at the behavioral level mask differences in the true nature of the impairments (Simon & Finucane, 1996; Turk & Cornish, 1998).
A large number of studies using functional brain imaging in ASD have found deficits and differences in brain activation patterns in response to social/emotional stimuli in areas of the ‘social brain’ (Adolphs, 2001) such as the amygdala, orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), fusiform gyrus (FG), superior temporal gyrus (STG), superior temporal sulcus (STS), inferior temporal gyrus (ITG), medial prefrontal cortex (MPFC), and insula (INS) (for review see (Penn, 2006). More recent studies on ASD have found differences in functional connectivity in both local and long-range cortical networks implicated in more automatic emotional processes and higher-level attentional and cognitive function (Matthew K. Belmonte et al., 2004; Bird et al., 2006; Critchley et al., 2004; Just et al., 2004; Kana et al., 2006). It has been hypothesized that dysfunction in modulation of amygdala activity and/or the thalamo-amygdala pathway by higher-level cortical areas, such as the MPFC and ACC, could be associated with deficits in social/emotional processes (Davidson, 2002; Davis & Whalen, 2001; Urry et al., 2006) and that integrity of reciprocal amygdala control by fronto-cortical circuits and the INS is critical for adaptive social functioning suggesting possible common underlying dysfunction in these regions and networks in ASD and FXS.
In contrast to the large volume of functional brain imaging studies published on ASD within the last decade (over 50 within just the last year), a paucity of similar studies have been reported in human FXS. However, a few fMRI studies have been published reporting differences in brain activation patterns between females with FXS and typically developing controls during a variety of cognitive tasks (Menon et al., 2000; Rivera et al., 2002); (Cornish et al., 2004; Greicius et al., 2004; Kwon et al., 2001; Tamm et al., 2002). While these studies present converging evidence for abnormalities in brain function during these cognitive tasks, only one study has examined social/emotional processes engaging affective neural circuitry in FXS. Garrett et al. (Garrett et al., 2004) reported a lack of the typical differentiation in activation to forward vs. angled faces in the FG in individuals with FXS during a face processing task. Although these findings suggest a possible neural basis for abnormal social perception in FXS, additional investigation is warranted given the clear deficits in these processes in FXS which are most likely directly attributable to severity of ASD characteristics in FXS. Furthermore, there is growing evidence that functional integrity and structure of the social brain and the development of adaptive social/emotional function may be effected by X-linked genetic mechanisms, such as in FXS (Carrel & Willard, 2005; Skuse, 2006).
In summary, although the association between FXS and ASD has been well-documented at the behavioral level, the common and distinct neural mechanisms underlying these disorders are largely unknown. In addition to yielding a fuller understanding of the brain-behavior relationship in FXS, a comparison between ASD and FXS, a syndrome with a known etiology, may provide additional information about social processing in ASD and thereby insights into the genetic roots of social deficits more generally. In the present study, we predicted a disrupted pattern of gaze-fixation and neural activity during face processing in FXS that would more closely resemble findings in individuals with ASD compared to the TD group. Moreover, we attempted to clarify the relationship between FXS and ASD in two ways. First, we reasoned that to the extent that the behavioral and functional brain data for the FXS and ASD groups diverged, we would have evidence for distinct underlying pathologies in the two disorders. Second, we hypothesized that the pattern of associations between neural activity, gaze-fixation, and ASD characteristics within the FXS group would provide further evidence of common underlying mechanisms reflective of deficits in social/emotional processes across both disorders.
Methods
Subjects
Nine individuals with FXS (3 males) were recruited for this study. Diagnosis of all FXS participants was confirmed by genetic tests performed by their respective clinic/hospital. ASD characteristics were assessed using the Social Communication Questionnaire (SCQ) in the FXS group (Rutter et al., 2003). Higher scores on the SCQ indicate greater endorsement of ASD characteristics, with a cut-off of 15 considered as indicative of a likely ASD. We choose to use the SCQ to assess ASD characteristics in our fragile X sample rather than the Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 2000) because none of the ADOS modules are appropriate for adolescents or adults with composite IQs <50. None of the fragile X individuals had a clinical diagnosis of autism or Asperger's syndrome; therefore the ADI-R was not used as a confirmation of ASD diagnosis in the fragile X group.
Fourteen males with ASD were recruited for this study from a list of individuals with a diagnosis of ASD in the Madison and Milwaukee area. Diagnoses of ASD were confirmed with the Autism Diagnostic Interview – Revised (ADI-R) (Lord et al., 1994) or clinical interview administered by a licensed psychologist certified in ADI-R administration. All participants in the ASD group met DSM-IV criteria for autism (n=9) or Asperger's (n=5) disorder. One participant was non-verbal and two others had minimal functional speech with pronounced echolalia; the remaining participants were verbally fluent.
Fifteen healthy, TD individuals (3 females) with no current or past psychological diagnoses served as comparison individuals. Results for the ASD and TD control groups have been already been published (Dalton et al., 2005) and so no direct comparisons between the control and autism group are presented here.
Composite IQ was determined for the FXS and ASD participants by administering either the Wide Range Intelligence Test (WRIT) (Glutting et al., 2000) or the Stanford Binet (n = 2). Neither an intelligence test nor the SCQ were administered for the TD controls.
Group characteristics are summarized in Table 1. The FXS participants were older than both the TD control (t(1,23) = 3.53, p = .002) and ASD group (t(1,21) = 2.78, p = .01). While the FXS had the lowest mean IQ of the three groups (as predicted), the FXS and AUT groups did not differ significantly on IQ (t(1,18) = 1.93, p = .07); however, the FXS group's IQ was significantly lower than the mean IQ of a standardized sample, (M = 100, SD = 15; t(1,8) = 4.26, p = .001). The FXS group had a widely variable range of SCQ scores, with 8 out of 9 individuals scoring above average compared to a similar age sample of TD controls (M = 3.81, SD = 2.27, n = 10) collected in our lab for a different study, (t(1,18) = 3.79, p = .001). Three of the individuals with FXS scored above or near (14-16) the ASD cut-off of 15. However, the participants with FXS also had a significantly lower average SCQ score compared to the ASD group, (t(1,20) = 8.39, p < .000001), with all the ASD individuals exceeding criteria for AUT and no overlap in range between the FXS and ASD group (19-32).
Table 1.
Group descriptive statistics for age, general IQ, and autism characteristics (SCQ).
| FXS (n=9) | Group ASD (n= 14) |
TD Control (n = 15) | ||
|---|---|---|---|---|
| Age | M | 20.7 yrs | 15.9 yrs | 16.8 yrs |
| SD | 2.77 | 4.71 | 2.57 | |
| range | 17-24 | 10-25 | 13-23 | |
| IQ | M | 66.1 | 87.2 | NA |
| SD | 23.84 | 25.84 | ||
| range | 35-95 | 35-122 | ||
| SCQ | M | 9.9 | 26.1 | NA |
| SD | 4.70 | 4.27 | ||
| range | 1-16 | 19-32 |
Procedure
Adult participants and parents of child participants first read and signed a consent form that covered all aspects of the study and MRI procedures. All participants and parents were pre-screened for MRI compatibility prior to participation in the scanning protocol. All sessions began with a simulation session during which the participants and his or her parent were acclimated to the MRI environment using a mock-up of an MRI scanner. During the simulation session, the participant was also given instructions for the facial-emotion discrimination task and shown examples of the appropriate stimuli. All scans started with approximately 20 minutes of anatomical scans followed by the 7-minute functional scan during which the facial emotion discrimination task was performed. The total time in the scanner was approximately 35-45 minutes and the total time for the full session was approximately 1.5 hours.
Facial Emotion Discrimination Task
Participants were tested individually in a facial-emotion discrimination task while functional brain images were acquired. In this task, the participant was asked to decide whether a picture of a human face was emotional (displaying an emotion; happiness, fear or anger) or neutral (no obvious display of emotion) by pressing one of two buttons on an MRI compatible button-box held in the right hand (see, Dalton et al., 2005, for task details).
Eye-Movements
Eye movements, fixations, and pupil diameter were acquired using an iView system with a remote eye-tracking device (SensoMotoric Instruments, 2001) while the participant was in the scanner, concurrent with the face processing functional scan. The acquired eye data were analyzed using the iView software. This system allows the display of raw eye movements as the gaze position of the pupil over a certain length of time (gaze path) along with the amount of time spent on any given fixation point (gaze fixation). Eye fixations were defined as the amount of continuous time (with a minimum 50 ms) spent looking within a 20-pixel diameter region, therefore removing blinks, very brief saccades and off-screen fixations. None of the participants reported any eye-movement dysfunctions (e.g. strabismus). If a participant displayed an inadequate number of valid fixations (valid fixations on less than half of the trials due to excessive blinks, poor goggle alignment, etc.) their gaze-fixation data were not included in the analysis. One fragile X participant, five typically developing participants, and five autism participants displayed inadequate gaze-fixations and were dropped from the analysis, leaving roughly similar samples sizes across groups for the gaze-fixation analysis (fragile X, n=8; autism, n=9; typically developing, n=10). The total amount of time spent fixating the face in general, each eye, and the mouth region was calculated as the sum of fixations within each of those four pre-defined regions for each face. The average fixation was calculated as average of the fixation time (ms) across all trials for each feature.
Imaging
Brain MRI images were acquired with a GE Signa 3 Tesla scanner equipped with high-speed gradients and a whole-head transmit-receive quadrature birdcage headcoil (GE Medical Systems, Waukesha, WI). Structural brain images were acquired for anatomical localization of functional activity (see Dalton et al., 2005, for scan parameters and details).
Image Analyses
Differential brain activation maps were generated by comparing activation in the FXS group versus the ASD and TD control groups in a voxel-wise manner using Analysis of Functional Neural Images version 2.31 software (AFNI) (Cox, 1996), (see Dalton et al., 2005, for image processing and analysis details). To identify group differences in brain regions associated with processing faces, t-tests were performed between the FXS group and the ASD and TD control groups across all the face conditions. An additional whole-brain emotion (emotional, neutral) by orientation (straight, side) mixed-measures ANOVA was performed for the FXS group. An individual p-value threshold = 0.001 and a minimum cluster size of 50 contiguous voxels was used to control for multiple comparisons. For clusters meeting the individual p-value and cluster-size threshold combination for the interaction and main effects of interest, the average percentage signal change value was extracted for each condition and participant, and the values entered into traditional simple effects analyses to determine the source of the significant effect.
Results
Task Accuracy and Judgment Time
The FXS group had significantly fewer correct responses (M = 32.5, SD = 8.22, accuracy = 81%) compared to the TD control group (M = 39.4, SD = 0.79, accuracy = 98.5%; t(1,18) = 2.93, p = .009), but performed at the same level of accuracy as the ASD group (M = 31.7, SD = 11.04, accuracy = 79%; t(1,18) = 0.18, p = .85). The group difference in accuracy was not a function of a speed-accuracy trade-off because the groups did not differ on judgment time. However, accuracy was positively correlated with IQ for both the FXS (r = 0.83, p =.01) and ASD (r = 0.74, p = .01) groups. Scores on the SCQ were not correlated with accuracy or judgment time for either the FXS or ASD group. These findings suggest that although the task was more difficult for the FXS group compared to the TD group, the majority of individuals (n = 6) were still able to perform the task above a chance level. The FXS and ASD groups showed similar levels of facial-emotion processing deficits.1
Gaze Fixations
The FXS group showed a fixation pattern to the eyes, mouth and face that did not differ significantly from that of the ASD group; however, average fixation to the eyes was marginally lower for the FXS group than for the TD control group (t(1,19) = 1.71, p =.10; see Figure 1).
Figure 1.
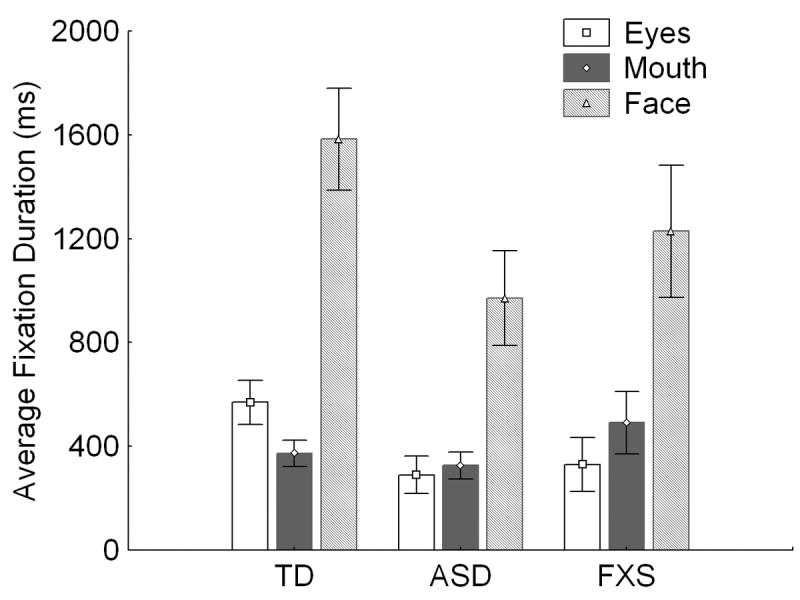
Average fixation durations. Average duration of fixation on the mouth and eye region and face in general split by group. Error bars index the SEM.
Brain Activation Maps
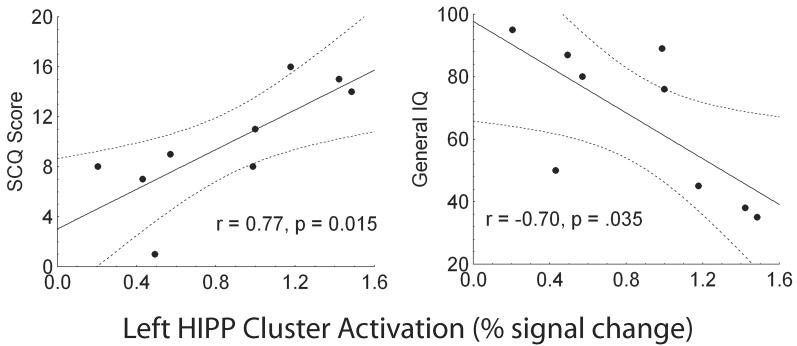
FXS minus TD control and FXS minus ASD brain activation maps were derived across all of the facial photographs to test the hypothesis that individuals with FXS show a unique pattern of brain activation while processing standard emotional facial photographs. As previously found in the ASD group, the FXS group showed a similar pattern of right FG hypoactivation to the faces compared to the TD control group (TD: M = .620, SD = .269; FXS: M = .219, SD = .131; t(1,23) = −4.16, p = .003; see Figure 2). The FXS and ASD group did not differ significantly in activation in this region of the right FG (ASD: M = .355, SD = 0.263; (t(1,21) = −1.43, p = .16). The FXS group also showed significantly greater activation to the faces compared with the TD control group and ASD group in four regions: the left HIPP (TD: t(1,23) = 5.11, p = 0.00003; ASD: t(1,21)= 3.03, p = 0.006), right INS (TD: t(1,23)= 5.32, p = 0.00002; ASD: t(1,21) = 4.29, p = 0.0003), left post central gyrus (PCG) (TD: t(1,23) = 4.77, p = 0.00008; ASD: t(1,21) = 5.82, p = 0.00009), and left STG (TD: t(1,23) = 2.41, p = 0.02; ASD: t(1,21) = 5.51, p = 0.00001; see Figure 2). Activation in the left HIPP was positively correlated with SCQ (r = 0.77, p = .015) and negatively correlated with IQ (r = −0.70, p = .035) for the FXS group (see Figure 3). The variance accounted for by SCQ in the left HIPP remained significant after partialling out IQ in a stepwise fashion (step 2: F(1,8) = 10.36, p = .01) for the FXS group. Activation in the other three regions was not correlated with IQ or scores on the SCQ for the FXS. Follow-up analyses revealed that these group differences were not mediated by interactions with the emotional expression or orientation of the facial photographs, suggesting robust group differences to facial photographs.
Figure 2.
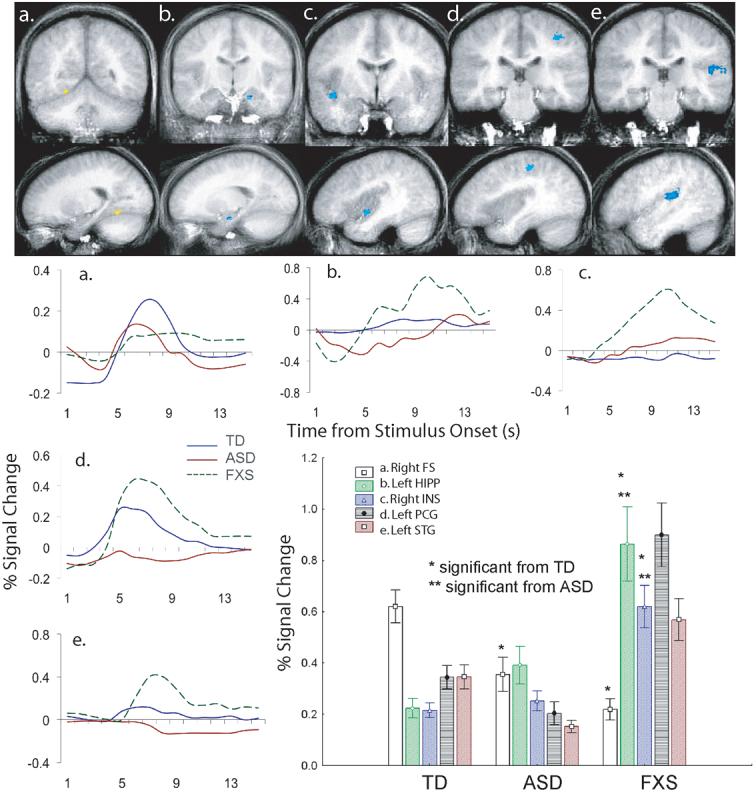
Brain cluster with significant greater brain activation across all faces in the TD versus ASD and FXS group: (a) Right Fusiform Gyrus (FG): x = 20, y = −70, z = −2; 172 voxels. Brain clusters with significantly greater brain activation across all faces in the FXS versus versus TD control and ASD groups: (b) Left Hippocampus (HIPP): x = −19, y = −13, z = −15; 116 voxels, (c) Right Insula (INS): x = 39, y = −4, z = −6; 277 voxels, (d) Left Post Central Gyrus (PCG): x = −36, y = −28, z = 48; 402 voxels, (e) Left Superior Temporal Gyrus (STG): x = −45, y = −21, z = 11; 905 voxels. All images are presented in radiological convention such that the right hemisphere is displayed on the left of each coronal image. The clusters are color- coded based on the TD control and ASD minus fragile X t-statistic values (positive values indicate TD & ASD > FXS). Averaged MR time series are presented below each cluster for the 14 seconds post stimulus onset.
Figure 3.
Scatter plot of the correlations between left HIPP cluster activation and SCQ (a) and general IQ (b) for the FXS group.
Relationship between Brain Activation and Gaze-Fixation and Autism Characteristics
Although they averaged marginally less time fixating the eyes compared with the TD control group, there was marked variability in the amount of looking time on the eyes in the FXS group. We took advantage of this variability by examining across subjects whether time spent fixating the eye region of the face predicted brain activation in the FXS group by regressing the amount of time they spent fixating the eyes on their brain activation in a voxel-wise fashion. Significant clusters of activation were extracted using a conservative threshold method (alpha = 0.001). Brain activation was strongly and positively associated with the amount of time spent fixating the eyes for the FXS group in clusters in the right and left FG (right, r = 0.96, p < 0.00001; left, r = 0.98, p < .00001; see Figure 4a-b). The amount of variance in FG activation accounted for by eye-fixations in the FXS group remained significant after partialling out variance associated with IQ in a step-wise fashion (step 2. right FG, F(1,22) = 76.9, p = .0001; step 2. left FG, F(1,22) = 175.3, p = .00001).
Figure 4.
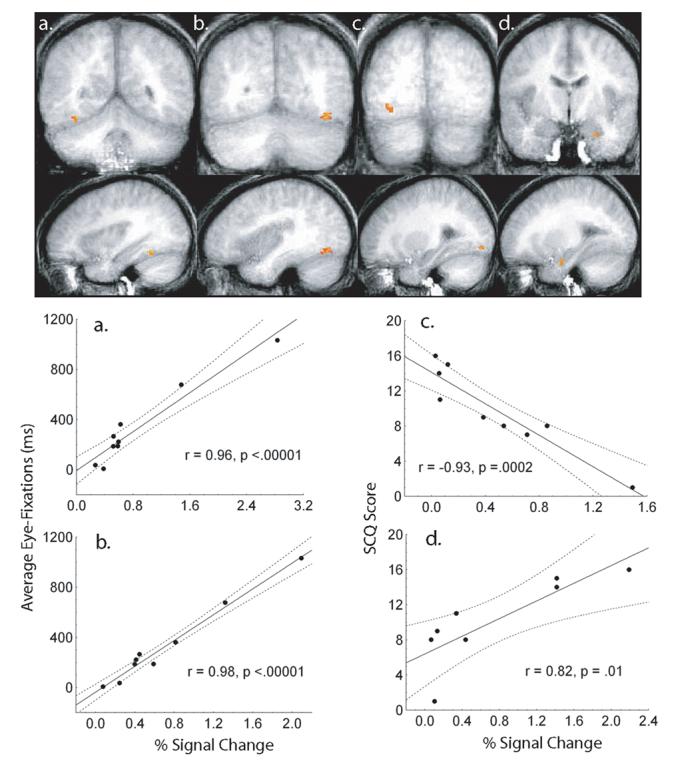
Brain activation clusters associated with average eye-fixation time for the FXS group. (a) Right FG: x = 33, y = −51, z = −8; 70 voxels, (b) Left FG: x = −34, y = −62, z = −8; 239 voxels. Scatter plots depicting the relationship between brain activation and average eye-fixation are presented below each cluster. Brain activation clusters associated with AUT characteristics (SCQ score) for the FXS group. (c) Right FG: x = 29, y = −72, z = −3; 103 voxels;. (d) Left Amygdala: x = −23, y = −7, z = −18; 82 voxels. Scatter plots depicting the relationship between brain activation and average autism characteristics are presented below each cluster.
A similar analysis was performed across subjects to determine whether ASD characteristics predicted brain activation in the FXS group by regressing their SCQ scores on their brain activation patterns in a voxel-wise fashion. Significant clusters of activation were extracted using a conservative threshold method (alpha = 0.001). Brain activation was strongly and negatively associated with SCQ scores for the FXS group in a cluster in the right FG (r = −0.93, p = .0001; see Figure 4c). This effect remained significant after removing variance associated with IQ in a step-wise fashion (step 2: F(1,22) = 24.69, p = .002). Because we had a priori predictions regarding SCQ score and amygdala activation, a less conservative threshold (alpha = .05) was used focusing only on SCQ and amygdala activation. A region in the left amygdala was strongly and positively associated with SCQ score in the FXS group (r = 0.82, p = .01; see Figure 4d), however, this association dropped below significance once variance associated with IQ was removed (step 2: F(1,22) = 0.89, p = .38).
Discussion
As predicted, the FXS group showed a similar pattern of diminished gaze-fixations as seen in the ASD group. Also as predicted, the TD control group had significantly greater activation in the right FG in response to faces compared to both the FXS and ASD groups. Average eye fixation was strongly and positively associated with both right and left FG activation in the both the FXS and ASD groups. These correlations remained significant even after variance associated with IQ was removed in the FXS group, suggesting the association between gaze fixation and FG activation is independent of cognitive impairment and an extremely robust effect. Activation in a region of the right FG was also strongly and negatively associated with ASD characteristics, independent of IQ in the FXS group. This convergence of brain activation pattern between FXS and ASD suggests that FG hypoactivation may be specific to social/emotional deficits core to ASD (i.e. diminished gaze-fixation) rather than a general deficit in cognitive function.
Importantly, the FXS group displayed significantly greater activation than both the TD control and ASD groups in the left HIPP, left STG, right INS, and left PCG. These relative increases in brain activation may be explained as reduced functional habituation to emotionally salient stimuli (HIPP), fear-specific and compensatory brain activation for emotional face processing (STG), increased anxiety/orienting to the emotional faces (right INS), and an enhanced cortical motor response during the task (PCG). These group differences in brain activation pattern associated with the facial-emotion discrimination task are important as they suggest unique underlying face-processing neural circuitry in FXS versus idiopathic ASD.
Interestingly, hippocampal activation to the faces was positively associated with ASD characteristics in our FXS sample, even after variance associated with IQ was removed. This suggests that ASD characteristics play a significant role in hippocampal activation to human faces in FXS. However, it remains unclear how this relates to ASD characteristics in idiopathic ASD, as our ASD sample did not display a similarly elevated HIPP activation to the faces. This divergence between FXS and idiopathic ASD in brain activation to social stimuli and it's association with ASD characteristics implies that the social impairments observed in FXS, including gaze aversion, shyness, and aberrant social greeting behavior, may derive from partially distinct neural circuitry associated with social anxiety in the case of FXS versus more general social impairment or indifference in ASD (Bird et al., 2006). Further support for this notion comes from the finding that while ASD characteristics were associated with amgydala activity in our FXS sample the effect was partially mediated by IQ. These findings suggest that social/emotional deficits in FXS and idiopathic ASD are associated with divergent activation patterns in lower-level affective neural circuitry.
There are a number of limitations of this study that raise critical issues in fMRI research on the relationship between FXS and ASD. First, it is important to point out that none of our FXS participants had a clinical diagnosis of ASD, although they all displayed at least one ASD characteristic with a group average of 9 characteristics, well above what would be expected in the typically developing population. Future research should strive to obtain samples of FXS individuals both with and without comorbid ASD as these two groups may differ at the neurological level. Second, while our FXS sample contained both male and female participants, we lacked the requisite statistical power to test for gender effects. However, while our AUT group contained all males, our TD sample did include three females. The issue of gender is important in this research and in fMRI research on ASD in general. While obtaining valid fMRI data from male participants with FXS is challenging, and females with ASD are out numbered by males 4:1, future fMRI research on FXS and ASD should strive to over come these obstacles as gender issues have been largely ignored in the fMRI research in these populations. Third, there is the issue of matching these groups on age and IQ. While it would be optimal to include both children and adults in this research, based on our experience, it would be extremely difficult to obtain valid fMRI data using male children with FXS, therefore, future fMRI research on FXS will most likely be restricted to adolescents and adults with FXS, as is currently the norm. The issue of matching on IQ is even more problematic as mental retardation is a hallmark of FXS. Covarying on IQ may be removing other defining characteristics associated with this sample and so is not optimal. Furthermore, IQ and gender are confounded in FXS, with males more affected than females. The solution to these issues remains controversial and future research should be mindful of dealing with them. Finally, caution should be taken about drawing inferences about brain activation during face processing in FXS based on these results given the small number of FXS participants. While this is a small sample study (as is common in fMRI studies on FXS), and despite the limitations outlined above, it nonetheless provides valuable insight into the relationship between ASD and FXS and demonstrates that this type of research can and should be pursued. This is the first fMRI study to our knowledge to successfully include more severely affected FXS males.
In summary, our findings suggest complex relationships between ASD characteristics, brain function and behavior during human facial-emotion processing in FXS and largely support the hypothesis that ASD characteristics in FXS and idiopathic ASD reflect partially divergent impairments at the neural level, at least in FXS individuals without a co-morbid diagnosis of ASD. However, there was a wide variability in both ASD symptom severity and task performance in our FXS group, suggesting that like ASD, social and emotional deficits in FXS fall along a wide spectrum and so group differences should be interpreted with caution. Our data also suggest that a combination of functional brain imaging and eye tracking can be used to parse heterogeneity and develop endophenotypic markers in FXS individuals. Future research can relate these endophenotypic measures to molecular markers in this syndrome.
Acknowledgements
Sincerest thanks to all the individuals and families who participated in this research and to Michael Anderle and Ronald Fisher for technical assistance in data acquisition and Andrea McDuffie for participant recruitment and acclimation to the MRI environment. We also wish to thank faculty and staff at the Waisman Center and Waisman Laboratory for Brain Imaging and Behavior for administrative and technical support. This work was supported by a National Institute of Mental Health (NIMH) Studies to Advance Autism Research and Treatment (STAART) grant U54MH066398 Project IV (R.J. Davidson, project PI; H. Tager-Flusberg, Center Director), a National Alliance for Research on Schizophrenia and Affective Disorders (NARSAD) Distinguished Investigator Award to R.J. Davidson, an NIH core grant P30 HD03352 (Marsha Mailick Seltzer, PI) and an NICHD training grant T32 HD07489 (Leonard Abbeduto, PI).
Grant Sponsor: NIH STAART; Grant number: U54MH066398
Footnotes
Three individuals with AUT also performed the task below chance level. All subsequent analyses were performed with and without the three FXS and three AUT participants included. Similar statistically significant group effects (FXS vs. TD and FXS vs. AUT) were found for task accuracy, brain activation to faces, and the relationship between brain activation and gaze-fixation and autism characteristics both with and without the non-responders included in the analyses.
References
- Adolphs R. The neurobiology of social cognition. Current Opinion in Neurobiology. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Roberts JE, Hooper SR, Hatton DD, Mirrett PL, Schaaf JM. Research on fragile X syndrome and autism: Implications for the study of genes, environments, and developmental language disorders. In: Rice ML, Warren SF, editors. Developmental Language Disorders: From Phenotypes to Etiologies. Lawrence Erlbaum Associates, Inc; Manwah, NJ: 2004. pp. 121–150. [Google Scholar]
- Belmonte MK, Allen G, Becke-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. Journal of Neuroscience. 2004;24:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte MK, Bourgeron T. Fragile X syndrome and autism at the intersection of genetic and neural networks. Nature Neuroscience. 2006;9(10):1221–1225. doi: 10.1038/nn1765. [DOI] [PubMed] [Google Scholar]
- Bird G, Catmur C, Silani G, Frith C, Frith U. Attention does not modulate neural responses to social stimuli in autism spectrum disorders. NeuroImage. 2006;31:1614–1624. doi: 10.1016/j.neuroimage.2006.02.037. [DOI] [PubMed] [Google Scholar]
- Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- Cornish K, Swainson R, Cunnington R, Wilding J, Morris P, Jackson G. Do women with fragile X syndrome have problems in switching attention: Preliminary findings from ERP and fMRI. Brain and Cognition. 2004;54:235–239. doi: 10.1016/j.bandc.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computer and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crawford DC, Acuna JM, Sherman SL. FMR1 and the fragile X syndrome: Human genome epidemiology review. Genetics in Medicine. 2001;3:359–371. doi: 10.1097/00125817-200109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze-fixation and the neural circuity of face processing in autism. Nature Neuroscience. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: Role of prefrontal cortex and amygdala. Biological Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Feinstein C, Reiss AL. Autism: The point of view from fragile X studies. Journal of Autism and Developmental Disorders. 2001;28:393–405. doi: 10.1023/a:1026000404855. [DOI] [PubMed] [Google Scholar]
- Garrett AS, Menon V, MacKenzie K, Reiss AL. Here's looking at you, kid: Neural systems underlying face and gaze processing in fragile X syndrome. Archives of General Psychiatry. 2004;61:281–288. doi: 10.1001/archpsyc.61.3.281. [DOI] [PubMed] [Google Scholar]
- Glutting J, Adams W, Sheslow D. Wide Range Intelligence Test. Wide Range Inc; Wilmington, Delaware, USA: 2000. [Google Scholar]
- Greicius MD, Boyett-Anderson JM, Menon V, Reiss AL. Reduced basal forebrain and hippocampal activation during memory encoding in girls with fragile X syndrome. NeuroReport. 2004;15(10):1579–1583. doi: 10.1097/01.wnr.0000134472.44362.be. [DOI] [PubMed] [Google Scholar]
- Hagerman R. Neurodevelopmental disorders. Oxford Univeristy Press; Oxford: 1999. [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127(8):1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory control in high-functioning autism: Decreased activation and underconnectivity in inhibition networks. Biological Psychiatry. 2006 doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann WE, Cortell R, Kau A,SM, Bukelis I, Tierney E, Gray RM, et al. Autism spectrum disorder in fragile X syndrome: Communication, social interaction, and specific behaviors. American Journal of Medical Genetics. 2004;129A:225–234. doi: 10.1002/ajmg.a.30229. [DOI] [PubMed] [Google Scholar]
- Kwon H, Menon V, Eliez S, Warsofsky IS, White CD, Dyer-Friedman J, et al. Functional neuranatomy of visuospatial working memory in fragile X syndrome: Relation to behavioral and molecular measures. American Journal of Psychiatry. 2001;158:1040–1051. doi: 10.1176/appi.ajp.158.7.1040. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EHJ, Leventhal BL, DiLavore PC, et al. The Autism Diagnostic Observation Schedule - Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism & Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, LeCouteur A. Autism Diagnostic Interview - Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism & Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Menon V, Rivera SM, White CD, Glover GH, Reiss AL. Dissociating prefrontal and parietal cortex activation during arithmetic processing. NeuroImage. 2000;12:357–365. doi: 10.1006/nimg.2000.0613. [DOI] [PubMed] [Google Scholar]
- Penn HE. Neurobiological correlates of autism: a review of recent research. Child Neuropsychology. 2006;12(1):57–79. doi: 10.1080/09297040500253546. [DOI] [PubMed] [Google Scholar]
- Pieretti M, Zhang F, Fu Y-H, Warren ST, Oostra BA, Caskey CT, et al. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66:817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- Rivera SM, Menon V, White CD, Glaser B, Reiss AL. Functional brain activation during arithmetic processing in females with fragile X syndrome is related to FMR1 protein expression. Developmental Psychobiology. 2002;39:107–123. doi: 10.1002/hbm.10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. Social Communication Questionnaire. Western Psychological Services; Los Angeles: 2003. [Google Scholar]
- Simon EW, Finucane BM. Facial emotion identification in males with fragile X syndrome. American Journal of Medical Genetics. 1996;67:77–80. doi: 10.1002/(SICI)1096-8628(19960216)67:1<77::AID-AJMG13>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Skuse D. Genetic influences on the neural basis of social cognition. Philosophical Transactions of the Royal Society of London. 2006;361:2129–2141. doi: 10.1098/rstb.2006.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L, Menon V, Johnston CK, Hessl DR, Reiss AL. fMRI study of cognitive interference processing in females with fragile X syndrome. Journal of Cognitive Neuroscience. 2002;14:160–171. doi: 10.1162/089892902317236812. [DOI] [PubMed] [Google Scholar]
- Turk J, Cornish KM. Face recognition and emotional perception in boys with fragile X syndrome. Journal of Intellectual Disability Research. 1998;42:490–499. doi: 10.1046/j.1365-2788.1998.4260490.x. [DOI] [PubMed] [Google Scholar]
- Urry HL, Reekum C. v., Johnstone T, Kalin NH, Thurow ME, Schaefer HS, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]