Abstract
Background
Despite a decreasing incidence of peptic ulcer disease, most previous studies report a stabile incidence of ulcer complications. We wanted to investigate the incidence of peptic ulcer complications in Sweden before and after the introduction of the proton pump inhibitors (PPI) in 1988 and compare these data to the sales of non-steroid anti-inflammatory drugs (NSAID) and acetylsalicylic acid (ASA).
Methods
All cases of gastric and duodenal ulcer complications diagnosed in Sweden from 1974 to 2002 were identified using the National hospital discharge register. Information on sales of ASA/NSAID was obtained from the National prescription survey.
Results
When comparing the time-periods before and after 1988 we found a significantly lower incidence of peptic ulcer complications during the later period for both sexes (p < 0.001). Incidence rates varied from 1.5 to 7.8/100000 inhabitants/year regarding perforated peptic ulcers and from 5.2 to 40.2 regarding peptic ulcer bleeding. The number of sold daily dosages of prescribed NSAID/ASA tripled from 1975 to 2002. The number of prescribed sales to women was higher than to males. Sales of low-dose ASA also increased. The total volume of NSAID and ASA, i.e. over the counter sale and sold on prescription, increased by 28% during the same period.
Conclusion
When comparing the periods before and after the introduction of the proton pump inhibitors we found a significant decrease in the incidence of peptic ulcer complications in the Swedish population after 1988 when PPI were introduced on the market. The cause of this decrease is most likely multifactorial, including smoking habits, NSAID consumption, prevalence of Helicobacter pylori and the introduction of PPI. Sales of prescribed NSAID/ASA increased, especially in middle-aged and elderly women. This fact seems to have had little effect on the incidence of peptic ulcer complications.
Background
Peptic ulcer complications have a high mortality, especially in elderly patients [1] and it is therefore important to understand the epidemiology of this disease in order to investigate if complications can be prevented. Despite new efficient drugs to treat peptic ulcer disease and increasing knowledge about its aetiology, the incidence of peptic ulcer complications, i.e. perforation and bleeding, have been reported by several groups to be unchanged (table 1). However, in a previous study from Lund University Hospital we found a fall in the incidence of peptic ulcer perforation from 1974 to 1992 in our primary uptake area [1]. We wanted to investigate the incidence of peptic ulcer complications in a larger population before and after the introduction of the Proton pump inhibitors (PPI) in order to investigate whether the introduction of this ulcer healing drug has influenced the incidence of these diseases.
Table 1.
Reported incidences of peptic ulcer complications (cases per 100000 inhabitants per year).
| Country (ref) | Period | Reported incidences | Comment | Studied variable | |
|---|---|---|---|---|---|
| perforation | Bleeding | ||||
| Norway (18) | 1935–1990 | About 10 | Report birth cohort specific risks for perforation | Admittance rates | |
| USA (44) | 1956–1985 | 10-5 | 7-4 | Relatively stable incidence of emergency operations for PUC | Emergency operations |
|
England and Wales (13) |
1958–1962 1979–1982 |
Increasing incidence of PUC* and NSAID use in elderly ♀ | National register | ||
| Scotland (13) | 1958–1962 1979–1982 |
Increasing incidence of PUC* and NSAID use in elderly ♀ | National register | ||
| USA (45) | 1974–1976 1984-1984 |
No rate per 100000 inhab | No rate per 100000 inhab | 13% increase in perforations and 7% increase in bleedings after H2-rec blocker introduction | Cases operated |
| Poland (11) | 1977–1996 | No rate per 100000 inhab | Constant number/year, increasing % elderly women, increasing mean age | Cases operated | |
| Hong-Kong (19) | 1979–1985 | 14–18 | Operating room logbooks |
||
| New South Wales (19) | 1979–1985 | 3–4 | Diagnosis reported to dept of heath | ||
| Finland (12,46) | 1972–1987 1987–1999 |
4–5 5–7 |
3–4 | Increasingly more ♀, increasing mean age | Cases operated, national register |
| Sweden (1) | 1974–92 | 2–11 | Significant decrease in incidence | Patients records | |
| Denmark (20) | 1974–1984 | 4–10 | 5–10 | No significant difference before/after H2-receptor blockers | Cases operated |
| Finland (17) | 1977–1989 | 2–8 | 3–9 | No significant difference before/after H2-receptor blockers | Cases operated |
| Finland (15) | 1979-85-00 | 3-6-4 | No significant difference before/after H2-receptor blockers or PPI | Patients records | |
| UK (10) | 1989–99 | ♂ 10–11 ♀ 7-7 |
27–31 14–16 |
Admittance rates | |
| UK (47) | 1996–98 | ♂ 5 ♀ 4 |
Only duodenal perforations, increasing mean age in ♀ | Patients records | |
| Germany (23) | 1989–90 1999-00 |
51 49 |
Patients older and more NSAID use in the later period | Prospective | |
*PUC = peptic ulcer complications
Well-designed studies have clearly shown that NSAID and ASA contribute to the development of peptic ulcer disease and upper gastro-intestinal complications in a dose-dependent manner [2-10]
In a study from the United Kingdom, Walt et al reported increasing sales of NSAID during the 1970's and 1980's to elderly women, a cohort that has also been reported to have an increasing incidence of peptic ulcer complications [1,11-14]. Increased use of ASA and NSAID might influence the incidence of peptic ulcer complications over time. A recent Danish study report increased overall use of NSAID after the introduction of the selective COX-2 inhibitors in 1999 [15]. This phenomenon coincided with a stable hospitalization rate of peptic ulcer bleeding but a decrease in hospitalization for perforated peptic ulcer in their study. We wanted to study if a correlation between sales of NSAID and complications to peptic ulcer complications could be found in the Swedish population and thus we further studied the sales of prescribed ASA and NSAID in Sweden during the same period.
Methods
The population of Sweden increased from 8,2 millions in 1974 to 8,9 millions in 2002. The Swedish national health system is tax funded giving the whole population unrestricted access to health services.
Data on incidence of ulcer complications
In this study all cases of gastric and duodenal ulcer complications diagnosed in Sweden from 1974 to 2002 were identified using the National Hospital Discharge Register (National Board of Health and Welfare, S-10630, Stockholm, Sweden). Since 1987 it is mandatory for hospitals to report to this register. In practise, however, it has been in existence since the early seventies though not in the entire country. We compensated for this when the incidence figures from 1974 to 1986 were calculated, as described below.
The ICD codes used for the search are listed in table 2. In ICD 8 and ICD 9 there are codes named ".99" or ".9" in the last positions which represent "unspecified location without knowledge of bleeding or perforation". These codes does not exist in ICD 10 which means that the patients that were earlier coded as totally unspecified are now included in other codes. This fact could mean that incidences of ulcer complications are underestimated before 1997 when ICD 10 was introduced. During the period 1974–86 between 30–37% of all reported codes for peptic ulcer disease were reported as "totally unspecified". From 1987 to1996 this number was 16–20% according to the National Department of Health. Codes representing ulcer with bleeding as well as perforation are included in both the bleeding and the perforation groups.
Table 2.
Codes searched for in the national register
| Ulcer complication | ICD 8 (-1986) |
ICD 9 (1987–96) |
ICD 10 (1997-) |
|---|---|---|---|
| Perforated gastric ulcer | 53100, 53101 | 5311, 5312 5315, 5316 |
K251, K252 K255, K256 |
| Perforated duodenal ulcer | 53200 | 5321, 5322 5325, 5326 |
K261, K262 K265, K266 |
| Other perforated ulcer | 53300, 53400 | 5331, 5332 5335, 5336 5341, 5342 5345, 5346 |
K271, K272 K275, K276 K281, K282 K285, K286 |
| Bleeding gastric ulcer | 53190, 53193 | 5310, 5312 5314, 5316 |
K250, K252 K254, K256 |
| Bleeding duodenal ulcer | 53290 | 5320, 5322 5324, 5326 |
K260, K262 K264, K266 |
| Other bleeding ulcer | 53390, 53490 | 5330, 5332 5334, 5336 5340, 5342 5344, 5346 |
K270, K272 K274, K276 K280, K282 K284, K286 |
The data from the National Department of Health are reliable. In a study from 1994 regarding validity, they found that in 11–14% of the cases the diagnosis reported was not correct if they applied a very strict judgement, i.e. all five figures correct [16]. If they were slightly less stringent, i.e. three figures correct, the inaccurate diagnoses was about 6–8%.
Data on sales of NSAID/ASA
Information on sales of NSAID was obtained from the National Prescription Survey (The National Corporation of Swedish Pharmacies – Apoteket AB). Data on drugs dispensed on prescription during the period 1975 – 1986 used a sample of 1/288 of all dispensed prescriptions. From 1987 – 2002, the sample was 1/25 (4%) of all dispensed prescriptions. However, medication to patients in Nursing Homes, Hospices etc. is only included if their medication had been dispensed on prescription by the pharmacies.
The composition of the combination-product Treo Comp® was changed in 1985, and the ATC-code was changed accordingly from N02BA71 (ASA, combinations with psycholeptics) to N02AA59 (codeine combinations). The DDD for Treo Comp® is 3 tablets, corresponding to 1,5 g ASA, but the DDD for ASA is 3 g. One DDD of Treo Comp® thus corresponds to 0,5 DDD ASA. The figures for Treo Comp® have been recalculated to DDD's of ASA.
Statistics
Age-standardised incidence rates were calculated using the Swedish population in 1995 as an external reference. Yearly site, age and gender specific incidence rates were calculated using data from the national population register. Time trends were investigated using linear regression analysis with log(incidence) rates as the dependent variable and the calendar year as an independent variable. An exploratory spline regression analysis of log incidence with 6 uniformly distributed knots was also performed using a piecewise linear function for time. STATA 10.1 was used for the statistical calculations. All p-values are two-sided and p-values below 5% were considered statistically significant.
The ethical committee of Lund University Hospital approved the study.
Results
Age Standardised incidence
Perforated Peptic Ulcer in Males
The incidence of gastric perforation varied from 3.5 to 7.8 and for duodenal perforation from 2.3 to 6.4 per100 000 inhabitants per year. There were slightly more gastric perforations throughout the whole period. From 1974 to 1988 there was a rise in incidence (p < 0.002) and from 1988 to 2002, a fall in incidence (p < 0.001) according to the linear regression analysis. The spline regression analyses regarding perforations in males are presented in tables 3 and 4 as well as in figures 1 and 2. The results are similar to the linear regression analysis.
Table 3.
Spline regression analysis, Gastric perforated ulcers
| Males | Females | |||
|---|---|---|---|---|
| Year | Coef. | 95% Conf. interval | Coef. | 95% Conf. interval |
| 1975 | -0.14 | -0.41 0.13 |
0.03 | -0.19 0.25 |
| 1980 | 0.01 | -0.04 0.05 |
0.06 | 0.03 0.10 |
| 1985 | 0.01 | -0.03 0.05 |
0.04 | 0.01 0.08 |
| 1990 | 0.04 | -0.001 0.08 |
0.03 | -0.003 0.06 |
| 1995 | -0.07 | -0.1 -0.03 |
-0.02 | -0.05 0.01 |
| 2000 | -0.08 | -0.11 -0.05 |
-0.10 | -0.12 -0.07 |
Table 4.
Spline regression analysis, Duodenal perforated ulcers
| Males | Females | |||
|---|---|---|---|---|
| Year | Coef. | 95% Conf. interval | Coef. | 95% Conf. interval |
| 1975 | -0.17 | -0.44 0.09 |
-0.21 | -0.58 0.16 |
| 1980 | 0.01 | -0.04 0.05 |
-0.00 | -0.06 0.06 |
| 1985 | 0.02 | -0.02 0.06 |
0.10 | 0.04 0.16 |
| 1990 | 0.03 | -0.004 0.07 |
0.05 | -0.01 0.10 |
| 1995 | -0.05 | -0.09 -0.01 |
-0.04 | -0.09 0.01 |
| 2000 | -0.11 | -0.14 -0.08 |
-0.09 | 0.13 0.05 |
Figure 1.
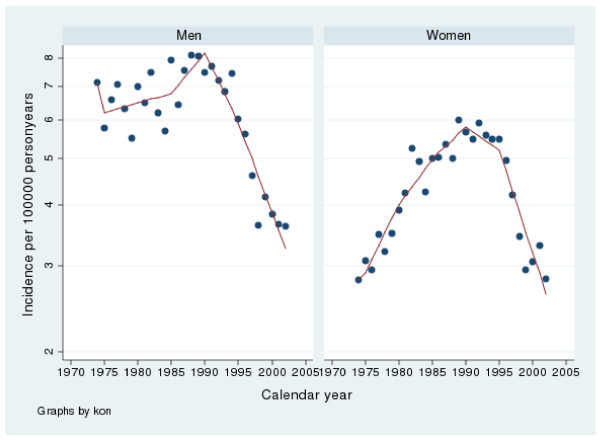
Incidence of gastric perforated peptic ulcers in Sweden from 1974 to 2002. Note that the y-axis has been truncated.
Figure 2.
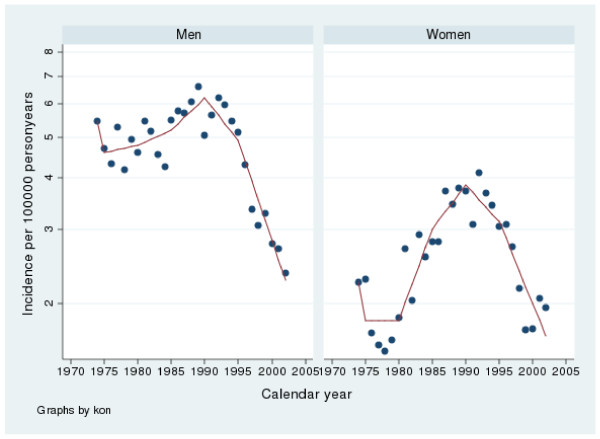
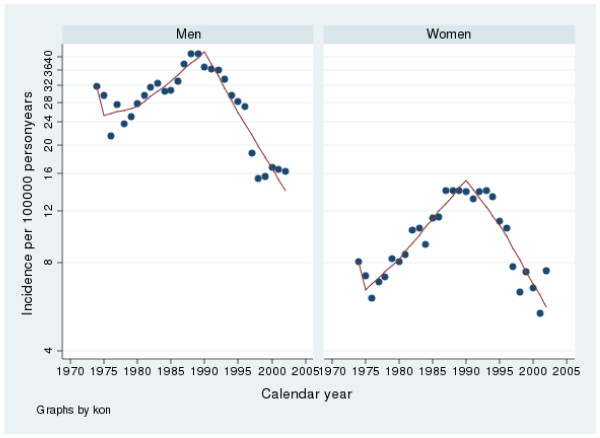
Incidence of duodenal perforated peptic ulcers in Sweden from 1974 to 2002. Note that the y-axis has been truncated.
Bleeding Peptic Ulcer in Males
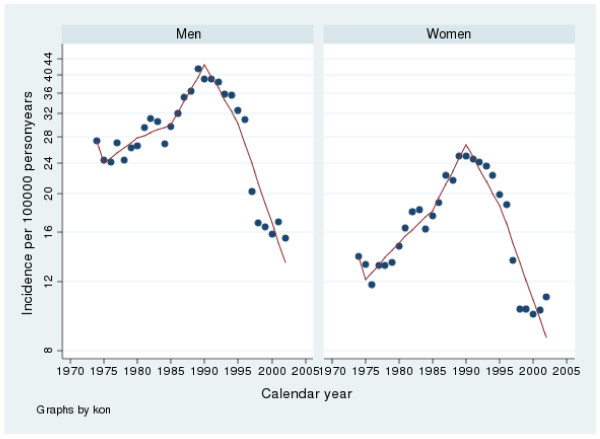
The incidence of bleeding gastric ulcer varied from 14.9 to 40.2 and for bleeding duodenal ulcer between 15.0 to 40.0 per100 000 inhabitants per year. From 1974 to 1988 there was a rise in incidence (p < 0.001) and from 1988 and 2002 a fall in incidence (p < 0.001) according to the linear regression analysis. The spline regression analyses regarding bleeding in males are presented in tables 5 and 6 as well as in figures 3 and 4. The results are similar to the linear regression analysis
Table 5.
Spline regression analysis, Gastric bleeding ulcers
| Males | Females | |||
|---|---|---|---|---|
| Year | Coef. | 95% Conf. interval | Coef. | 95% Conf. interval |
| 1975 | -0.13 | -0.38 0.12 |
-0.14 | -0.42 0.15 |
| 1980 | 0.03 | -0.14 0.07 |
0.04 | -0.005 0.09 |
| 1985 | 0.01 | -0.02 0.53 |
0.04 | -0.005 0.08 |
| 1990 | 0.07 | 0.03 0.11 |
0.08 | 0.03 0.12 |
| 1995 | -0.07 | -0.10 -0.03 |
-0.07 | -0.11 -0.03 |
| 2000 | -0.12 | -0.14 -0.09 |
-0.11 | -0.14 -0.08 |
Table 6.
Spline regression analysis, Duodenal bleeding ulcers
| Males | Females | |||
|---|---|---|---|---|
| Year | Coef. | 95% Conf. interval | Coef. | 95% Conf. interval |
| 1975 | -0.23 | -0.52 0.06 |
-0.23 | -0.53 0.08 |
| 1980 | 0.01 | -0.03 0.06 |
0.05 | -0.004 0.10 |
| 1985 | 0.04 | -0.004 0.08 |
0.06 | 0.02 0.11 |
| 1990 | 0.05 | 0.003 0.09 |
0.06 | 0.01 0.11 |
| 1995 | -0.10 | -0.14 -0.05 |
-0.07 | -0.11 -0.02 |
| 2000 | -0.09 | -0.12 -0.05 |
-0.09 | -0.13 -0.06 |
Figure 3.
Incidence of gastric bleeding peptic ulcers in Sweden from 1974 to 2002. Note that the y-axis has been truncated.
Figure 4.
Incidence of duodenal bleeding peptic ulcers in Sweden from 1974 to 2002. Note that the y-axis has been truncated.
Perforated Peptic Ulcer in Females
The incidence of gastric perforation varied between 2.7 to 5.8 and for duodenal perforation from 1.5 to 4.0 per 100 000 inhabitants per year. There were relatively more gastric than duodenal perforations during the whole period. Between 1974 and 1988 there was an increase in incidence (p < 0.001) and from 1988–2002 a fall in incidence (p < 0.001) according to the linear regression analysis. The spline regression analyses regarding perforations in females are presented in tables 3 and 4 as well as in figures 1 and 2. The results are similar to the linear regression analysis
Bleeding Peptic Ulcers in Females
The incidence of bleeding gastric ulcers varied between 9.6 and 24.2 and for bleeding duodenal ulcers from 5.2 to13.6 per100 000 inhabitants per year in females. There were relatively more gastric than duodenal bleeding ulcers during the whole period. Between 1974 and 1988 there was an increase in incidence (p < 0.001) and from 1988 to 2002 a decrease in incidence (p < 0.001) according to the linear regression analysis. The spline regression analyses regarding bleeding in males are presented in tables 5 and 6 as well as in figures 3 and 4. The results are similar to the linear regression analysis
Sales of NSAID and ASA
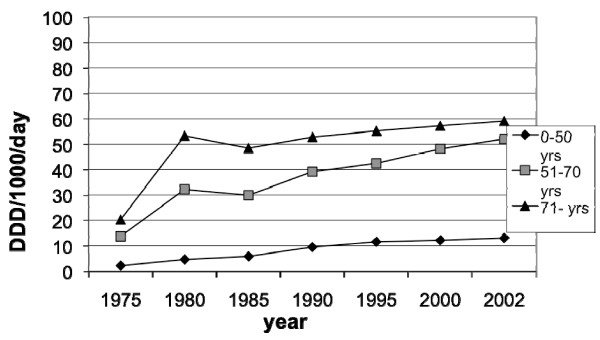
The sales of prescribed NSAID and ASA in Sweden for the years 1975 through 2002 are presented in figures 5, 6, 7, 8 and 9. In males the sales of NSAID and ASA described as DDD per1000 inhabitants per day rose from 2.3 to13.1 in the age group 0–50 years, from 13.7 to 52.1 in the age group 51–70 years and from 20.3–59.1 in the age group 71–99 years.
Figure 5.
Sales of prescribed NSAID and ASA between 1975–2002 in Sweden among males.
Figure 6.
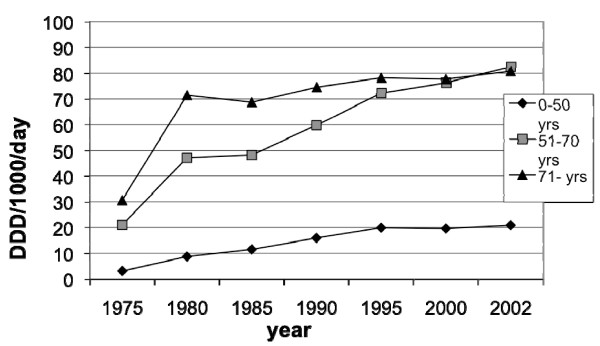
Sales of prescribed NSAID and ASA between 1975–2002 in Sweden among females.
Figure 7.
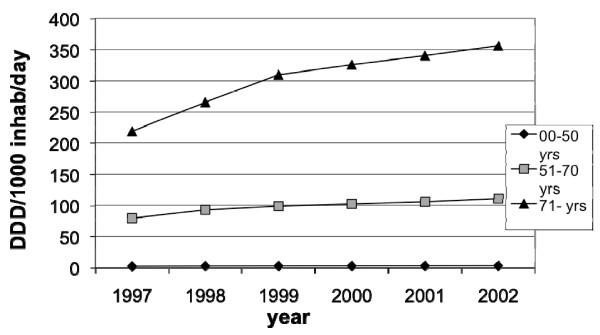
Sales of prescribed low-dose ASA between 1997–2002 in Sweden among males.
Figure 8.
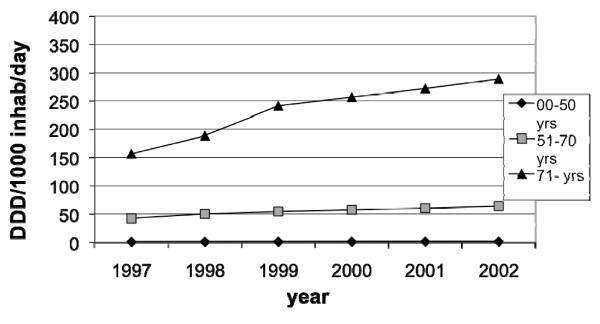
Sales of prescribed low-dose ASA between 1997–2002 in Sweden among females.
Figure 9.
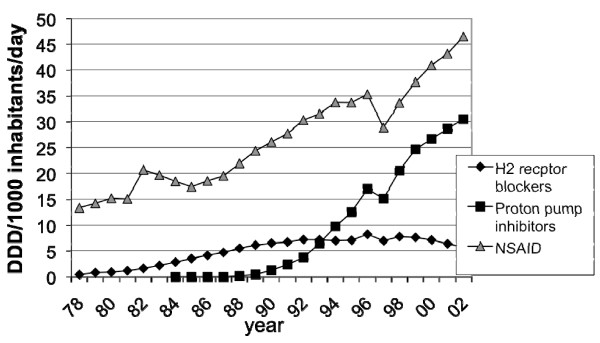
Sales of gastric acid inhibitors and NSAID:s in Sweden 1978–2002.
The corresponding figures for females were from 3.2 to 20.9 in the age group 0–50 years, from 21.0 to 82.5 in the age group 51–70 years and from 30.6 to 80.8 in the age group 71–99 years.
The sales to females compared to males were higher in all age groups and the difference increased towards the end of the study period.
The total sales of prescribed NSAID/ASA (DDD per1000 inhabitants per day) increased from 13.3 in 1978 to 46.5 in 2002.
The total volume of NSAID and ASA (DDD per1000 inhabitants per day) sold both "over the counter" and prescription sales, increased from 45.0 in 1978 to 57.6 in 2002 according to the statistics of Apoteksbolaget AB, Sweden. This means that sales increased 28% during this period.
The sales of low-dose ASA (DDD per1000 inhabitants per day) in males increased from 2.75 to 3.55 in the age group 0–50 years, from 79.2 to 110.9 in the age group 51–70 years and from 219.0–356.2 in the age group 71–99 years (fig 7).
The sales of low-dose ASA (DDD per1000 inhabitants per day) in females increased from 1.5 to 2.0 in the age group 0–50 years, from 42.3 to 64.2 in the age group 51–70 years and from 156.4–288.9 in the age group 71–99 years (fig 8).
Sales of acid inhibitors
H2 receptor blockers were introduced in Sweden in 1978. In 2002 total sales were 5.7 DDD per1000 inhabitants per day (figure 9).
The first PPI were introduced in Sweden in 1988. In 2002 total sales were 30.5 DDD per1000 inhabitants per day (figure 9).
Mean age
In the whole material mean age rose from 59.0 years to 67.9 years from 1974 to 2002. The mean age in males with gastric complications rose from 59 years in 1974 to 66.5 years in 2002 The corresponding figures for females were 63.2 and 70.2 years.
The mean age in males with duodenal complications rose from 52.8 years in 1974 to 62.1 years in 2002. In females the corresponding figures were 61.1 and 72.6.
Discussion
In the mid-eighties two events took place that changed the treatment of peptic ulcer patients. First the discovery of the connection between Helicobacter pylori and peptic ulcer disease and second the introduction of the first PPI in 1988. However, despite new and efficient treatment regimes several authors report that the incidence of complications of peptic ulcer disease, perforation and bleeding, has been relatively unchanged during the last thirty years of the 20-th century [1,11-14,17-26]. In contrast we found a significant fall in incidence of both bleeding and perforated ulcers in the Swedish population after 1988 despite increasing sales of ASA and NSAID, drugs known to be ulcerogenic.
Incidence of peptic ulcer complications
According to the literature, the incidence of peptic ulcer complications appears to be relatively stable over the last three decades with approximately 5 to10 episodes per 100 000 inhabitants/year. Some relevant references are listed in table 1. In elderly women, several authors even report an increasing incidence [11-14,27]. At least three studies have compared the incidences of peptic ulcer perforation before and after the introduction of the H2-receptor antagonists but found the incidence to be unchanged [1,22,23]. In contrast to these studies, we report a significant decrease in peptic ulcer complications in the Swedish population over the last two decades. Recently, a Danish group report similar results from 1996 to 2004 regarding perforated peptic ulcer but not for bleeding ulcers [15]. Like us they had a relatively large sample size, the Danish study involved a population of 1, 2 million inhabitants. A plausible explanation for the fact that several studies fail to detect any differences might be that the studied populations have often been too small for a disease that has a relatively low incidence to begin with. Differences in the prevalence of the different risk factors for peptic ulcer complications between populations, which will be discussed below, are other possible explanations.
The significant drop in incidence of peptic ulcer complications reported in this study, can possibly be explained by a generational (cohort) influence, for example less exposure to Helicobacter pylori infections among younger generations compared with older birth cohorts. Another possibility is a secular (periodical) phenomenon, for example the influence of extensive exposure of acid-suppressing drugs or changing trends in the use of factors known to cause ulcer or counteract ulcer healing, such as tobacco, alcohol or ulcerogenic drugs. Further, the use of gastroscopy has probably increased during the study period in Sweden enabling the detection and treatment of symptomatic peptic ulcers before complications occur. In our opinion, the explanation for this uniform decline in complications after 1988 is most likely multifactorial.
In the sections below we will discuss some important risk factors for complicated peptic ulcer disease and their possible influence on our results. When interpreting the results one must be aware that this is an ecological study with risk of "ecological fallacy" [28]. The connections discussed below can, of course, be caused by other factors and should therefore be adopted with caution.
NSAID as a risk factor and influence of PPI
We found an increase in the sales of prescribed NSAID during the study period. The number of prescriptions increased with age and was highest among middle-aged and elderly women. As mentioned earlier, elderly women are also the group in which the incidences of peptic ulcer complications are reported to be increasing relative to other groups [1,11-14]. However, in conjunction with an increase of prescribed sales there was a fall in non-prescribed sales, resulting in a total increase in NSAID sales of 28% during this period.
Several studies support the notion that NSAID is a risk factor not only in uncomplicated peptic ulcer disease, but also in regard to perforated and bleeding ulcers [2,3,5-7,10,29,30]. A systematic review of 18 case-control studies from 1990 to 1999 reported a pooled RR of 3.6 [2]. The higher risk was maintained during treatment and disappeared after treatment termination. The added risk is dose-dependent and also includes low-dose ASA. Studies report higher risk with all doses, with a RR = 2 for bleeding ulcer with daily intake of 75 mg ASA [8,9]. According to a study by Sorensen et al from 2000 the risk increase further when low-dose ASA is combined with NSAID [10].
Bearing this in mind one would expect a rise in incidence of peptic ulcer complications when the sales of NSAID increases, which is quit contrary to our findings. Since ours is a descriptive study one can only speculate as to the relationship between peptic ulcer complications and NSAID use. In a recent German paper from 2005 Ohmann et. al. found no difference in the incidence of peptic ulcer bleeding when comparing the years 1989 and 1999 despite significantly higher NSAID consumption in the latter year [26]. Hawkey conducted a prospective, controlled study of patients with bleeding ulcers and found that H pylori-positive NSAID users had twice the risk compared to H pylori negative patients [31]. The prevalence of H pylori is declining and thus could have reduced the effect of increased NSAID use [32-34].
In the end of the 1990-ies the less ulcerogenic selective cyclo-oxygenase-2 (COX-2) inhibitors were introduced in Sweden. These drugs were not included in this study since they were only available for use in Sweden during the last years of the study period. Christensen et al report a 44% rise in prescriptions of all NSAID after 1997, mainly due to COX-2 inhibitors [15]. The hospitalization rates of peptic ulcer complications after the introduction of COX-2 inhibitors were, however, stable for bleeding ulcers and decreased from 17 to 12/100000 person-years from 1996 to 2004 for perforations. This indicates that the COX-2 selective drugs might have decreased the risk of peptic ulcer complications for the individual NSAID user.
Proton pump inhibitors (PPI) have become one of the most sold drugs in the world and are nowadays also available over the counter in Sweden. It is a well known fact that PPI protect against peptic ulcer complications in NSAID users [3,35-38]. Interestingly the introduction of PPI was almost simultaneous with the beginning of a falling incidence in peptic ulcer complications (fig 1, 2, 3, 4). We believe that the extensive use of PPI in the population of Sweden has reduced the incidence of peptic ulcer complications. This conclusion is supported by Vonkeman et al (2007) who performed a nested case-control study from a cohort of 52000 NSAID users [39]. They found a significant risk reduction regarding peptic ulcer complications in NSAID users who used PPI concomitantly. Selective COX-2 inhibitors did not reduce the risk in their study.
Is Helicobacter pylori a risk factor in complicated peptic ulcer disease?
In the present study we found a significant decrease in peptic ulcer complications in the mid-eighties that co-inside with the discovery of H pylori as a cause for peptic ulcer disease. H pylori is an important pathogenic factor in uncomplicated peptic ulcer disease, although studies that investigate the connection between H pylori and peptic ulcer complications are somewhat divergent. For example, Gisbert et. al. (2003) calculated a mean prevalence of 68% from 19 studies with a range from 0–100% [40].
We have found five studies comparing patients with peptic ulcer complication patients to patients with non-GI related diseases in the control group. In three of these reports there was no difference in the prevalence of H pylori [5,41-44]. Three studies have compared patients with peptic ulcer complications patients to patients with uncomplicated ulcer disease [43,45,46]. Surprisingly, the prevalence of H pylori was slightly higher in the latter group.
Helicobacter Pylori is, without a doubt, connected to peptic ulcer disease. In the pathogenesis of perforation and bleeding, however, other factors such as NSAID and smoking are of great importance as well. In the time period covered by this study, the prevalence of H pylori infections has decreased in the western world [47] as has the incidence of peptic ulcer complications in our study (fig 1, 2, 3, 4). Since this is a descriptive study one can only speculate as to whether these two phenomena are correlated or not. There is however no doubt in the literature that eradication of H pylori is mandatory after a peptic ulcer complication, if the patient is H pylori positive. Otherwise the risk of recurrent ulcer disease is high [48-50].
What about smoking?
Smoking is a risk factor for peptic ulcer perforation [5,18,51]. A prospective cohort study from Denmark found that smoking > 15 cigarettes daily increased the risk of peptic ulcer perforation 3,5 times [51]. A retrospective study from Norway report that a cohort pattern in prevalence of smoking partly explained the cohort pattern in perforation risk for both sexes [18]. The prevalence of smoking has declined during the last twenty years in Sweden, especially in men, which could perhaps account for fewer ulcer complications [52].
Conclusion
When comparing the periods before and after the introduction of the proton pump inhibitors we found a significant decrease in the incidence of peptic ulcer complications in the Swedish population after 1988 when PPI were introduced on the market. The cause of the decrease is most likely multifactorial, including smoking habits, NSAID consumption, prevalence of Helicobacter pylori and the introduction of the PPI.
Patients with peptic ulcer complications are in average ten years older in 2002 than twenty years earlier. Sales of prescribed NSAID/ASA increased, especially in middle-aged and elderly women. This fact seems to have had little effect on the incidence of peptic ulcer complications, possibly because of a protective role of the PPI.
Competing interests
This study was supported financially by Astra-Zeneca AB.
Authors' contributions
Michael Hermansson was involved in the design of the study and in the collection of data. He has also written the paper. Anders Ekedahl has collected the information from the National Prescription Survey and helped with the interpretation of the results. Jonas Ranstam has performed the statistical calculations and helped with the interpretation of the results. Thomas Zilling has designed the study and helped with the editing of the paper and the interpretation of the results. All authors have read and approved of the manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Michael Hermansson, Email: michael.hermansson@vgregion.se.
Anders Ekedahl, Email: anders.ekedahl@med.lu.se.
Jonas Ranstam, Email: jonas@ranstam.se.
Thomas Zilling, Email: thomas.zilling@lthallland.se.
Acknowledgements
The authors like to thank Mr Kurt-Lennart Spetz at the National Hospital Discharge register for providing the incidence figures.
References
- Hermansson M, Stael von Holstein C, Zilling T. Peptic ulcer perforation before and after the introduction of H2-receptor blockers and proton pump inhibitors. Scand J Gastroenterol. 1997;32(6):523–529. doi: 10.3109/00365529709025093. [DOI] [PubMed] [Google Scholar]
- Hernandez-Diaz S, Rodriguez LA. Association between nonsteroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding/perforation: an overview of epidemiologic studies published in the 1990s. Arch Intern Med. 2000;160(14):2093–2099. doi: 10.1001/archinte.160.14.2093. [DOI] [PubMed] [Google Scholar]
- Hippisley-Cox J, Coupland C, Logan R. Risk of adverse gastrointestinal outcomes in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis. Bmj. 2005;331(7528):1310–1316. doi: 10.1136/bmj.331.7528.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: a meta-analysis. Lancet. 2002;359(9300):14–22. doi: 10.1016/S0140-6736(02)07273-2. [DOI] [PubMed] [Google Scholar]
- Lanas A, Serrano P, Bajador E, Esteva F, Benito R, Sainz R. Evidence of aspirin use in both upper and lower gastrointestinal perforation. Gastroenterology. 1997;112(3):683–689. doi: 10.1053/gast.1997.v112.pm9041228. [DOI] [PubMed] [Google Scholar]
- Langman MJ. Ulcer complications associated with anti-inflammatory drug use. What is the extent of the disease burden? Pharmacoepidemiol Drug Saf. 2001;10(1):13–19. doi: 10.1002/pds.561. [DOI] [PubMed] [Google Scholar]
- Gutthann SP, Garcia Rodriguez LA, Raiford DS. Individual nonsteroidal antiinflammatory drugs and other risk factors for upper gastrointestinal bleeding and perforation. Epidemiology. 1997;8(1):18–24. doi: 10.1097/00001648-199701000-00003. [DOI] [PubMed] [Google Scholar]
- Slattery J, Warlow CP, Shorrock CJ, Langman MJ. Risks of gastrointestinal bleeding during secondary prevention of vascular events with aspirin–analysis of gastrointestinal bleeding during the UK-TIA trial. Gut. 1995;37(4):509–511. doi: 10.1136/gut.37.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil J, Colin-Jones D, Langman M, Lawson D, Logan R, Murphy M, Rawlins M, Vessey M, Wainwright P. Prophylactic aspirin and risk of peptic ulcer bleeding. Bmj. 1995;310(6983):827–830. doi: 10.1136/bmj.310.6983.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen HT, Mellemkjaer L, Blot WJ, Nielsen GL, Steffensen FH, McLaughlin JK, Olsen JH. Risk of upper gastrointestinal bleeding associated with use of low-dose aspirin. Am J Gastroenterol. 2000;95(9):2218–2224. doi: 10.1111/j.1572-0241.2000.02248.x. [DOI] [PubMed] [Google Scholar]
- Higham J, Kang JY, Majeed A. Recent trends in admissions and mortality due to peptic ulcer in England: increasing frequency of haemorrhage among older subjects. Gut. 2002;50(4):460–464. doi: 10.1136/gut.50.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janik J, Chwirot P. Perforated peptic ulcer–time trends and patterns over 20 years. Med Sci Monit. 2000;6(2):369–372. [PubMed] [Google Scholar]
- Paimela H, Tuompo PK, Perakyl T, Saario I, Hockerstedt K, Kivilaakso E. Peptic ulcer surgery during the H2-receptor antagonist era: a population-based epidemiological study of ulcer surgery in Helsinki from 1972 to 1987. Br J Surg. 1991;78(1):28–31. doi: 10.1002/bjs.1800780110. [DOI] [PubMed] [Google Scholar]
- Walt R, Katschinski B, Logan R, Ashley J, Langman M. Rising frequency of ulcer perforation in elderly people in the United Kingdom. Lancet. 1986;1(8479):489–492. doi: 10.1016/S0140-6736(86)92940-5. [DOI] [PubMed] [Google Scholar]
- Christensen S, Riis A, Norgaard M, Thomsen RW, Sorensen HT. Introduction of newer selective cyclo-oxygenase-2 inhibitors and rates of hospitalization with bleeding and perforated peptic ulcer. Aliment Pharmacol Ther. 2007;25(8):907–912. doi: 10.1111/j.1365-2036.2007.03274.x. [DOI] [PubMed] [Google Scholar]
- Nilsson AC, Spetz CL, Carsjo K, Nightingale R, Smedby B. [Reliability of the hospital registry. The diagnostic data are better than their reputation] Lakartidningen. 1994;91(7):603–595. [PubMed] [Google Scholar]
- Gustavsson S, Kelly KA, Melton LJ 3rd, Zinsmeister AR. Trends in peptic ulcer surgery. A population-based study in Rochester, Minnesota, 1956–1985. Gastroenterology. 1988;94(3):688–694. doi: 10.1016/0016-5085(88)90240-5. [DOI] [PubMed] [Google Scholar]
- Svanes C, Lie RT, Kvale G, Svanes K, Soreide O. Incidence of perforated ulcer in western Norway, 1935–1990: cohort- or period-dependent time trends? Am J Epidemiol. 1995;141(9):836–844. doi: 10.1093/oxfordjournals.aje.a117519. [DOI] [PubMed] [Google Scholar]
- Welch CE, Rodkey GV, von Ryll Gryska P. A thousand operations for ulcer disease. Ann Surg. 1986;204(4):454–467. doi: 10.1097/00000658-198610000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SK, Byth K, Ng MM, Hui WM, McIntosh J, Piper DW. Perforated peptic ulcer in Hong Kong and New South Wales. J Gastroenterol Hepatol. 1992;7(5):508–511. doi: 10.1111/j.1440-1746.1992.tb01029.x. [DOI] [PubMed] [Google Scholar]
- Paimela H, Oksala NK, Kivilaakso E. Surgery for peptic ulcer today. A study on the incidence, methods and mortality in surgery for peptic ulcer in Finland between 1987 and 1999. Dig Surg. 2004;21(3):185–191. doi: 10.1159/000079654. [DOI] [PubMed] [Google Scholar]
- Christensen A, Bousfield R, Christiansen J. Incidence of perforated and bleeding peptic ulcers before and after the introduction of H2-receptor antagonists. Ann Surg. 1988;207(1):4–6. doi: 10.1097/00000658-198801000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makela J, Laitinen S, Kairaluoma MI. Complications of peptic ulcer disease before and after the introduction of H2-receptor antagonists. Hepatogastroenterology. 1992;39(2):144–148. [PubMed] [Google Scholar]
- Makela JT, Kiviniemi H, Ohtonen P, Laitinen SO. Factors that predict morbidity and mortality in patients with perforated peptic ulcers. Eur J Surg. 2002;168(8–9):446–451. doi: 10.1080/110241502321116424. [DOI] [PubMed] [Google Scholar]
- Canoy DS, Hart AR, Todd CJ. Epidemiology of duodenal ulcer perforation: a study on hospital admissions in Norfolk, United Kingdom. Dig Liver Dis. 2002;34(5):322–327. doi: 10.1016/S1590-8658(02)80124-4. [DOI] [PubMed] [Google Scholar]
- Ohmann C, Imhof M, Ruppert C, Janzik U, Vogt C, Frieling T, Becker K, Neumann F, Faust S, Heiler K. Time-trends in the epidemiology of peptic ulcer bleeding. Scand J Gastroenterol. 2005;40(8):914–920. doi: 10.1080/00365520510015809. [DOI] [PubMed] [Google Scholar]
- Coggon D, Lambert P, Langman MJ. 20 years of hospital admissions for peptic ulcer in England and Wales. Lancet. 1981;1(8233):1302–1304. doi: 10.1016/S0140-6736(81)92470-3. [DOI] [PubMed] [Google Scholar]
- Morgenstern H. Uses of ecologic analysis in epidemiologic research. Am J Public Health. 1982;72(12):1336–1344. doi: 10.2105/AJPH.72.12.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier DS, Pain JA. Non-steroidal anti-inflammatory drugs and peptic ulcer perforation. Gut. 1985;26(4):359–363. doi: 10.1136/gut.26.4.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen TG. Drug consumption before perforation of peptic ulcer. Br J Surg. 1977;64(4):247–249. doi: 10.1002/bjs.1800640406. [DOI] [PubMed] [Google Scholar]
- Hawkey CJ. Risk of ulcer bleeding in patients infected with Helicobacter pylori taking non-steroidal anti-inflammatory drugs. Gut. 2000;46(3):310–311. doi: 10.1136/gut.46.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banatvala N, Mayo K, Megraud F, Jennings R, Deeks JJ, Feldman RA. The cohort effect and Helicobacter pylori. J Infect Dis. 1993;168(1):219–221. doi: 10.1093/infdis/168.1.219. [DOI] [PubMed] [Google Scholar]
- Haruma K, Okamoto S, Kawaguchi H, Gotoh T, Kamada T, Yoshihara M, Sumii K, Kajiyama G. Reduced incidence of Helicobacter pylori infection in young Japanese persons between the 1970s and the 1990s. J Clin Gastroenterol. 1997;25(4):583–586. doi: 10.1097/00004836-199712000-00006. [DOI] [PubMed] [Google Scholar]
- Roosendaal R, Kuipers EJ, Buitenwerf J, van Uffelen C, Meuwissen SG, van Kamp GJ, Vandenbroucke-Grauls CM. Helicobacter pylori and the birth cohort effect: evidence of a continuous decrease of infection rates in childhood. Am J Gastroenterol. 1997;92(9):1480–1482. [PubMed] [Google Scholar]
- Cullen D, Bardhan KD, Eisner M, Kogut DG, Peacock RA, Thomson JM, Hawkey CJ. Primary gastroduodenal prophylaxis with omeprazole for non-steroidal anti-inflammatory drug users. Aliment Pharmacol Ther. 1998;12(2):135–140. doi: 10.1046/j.1365-2036.1998.00288.x. [DOI] [PubMed] [Google Scholar]
- Ekstrom P, Carling L, Wetterhus S, Wingren PE, Anker-Hansen O, Lundegardh G, Thorhallsson E, Unge P. Prevention of peptic ulcer and dyspeptic symptoms with omeprazole in patients receiving continuous non-steroidal anti-inflammatory drug therapy. A Nordic multicentre study. Scand J Gastroenterol. 1996;31(8):753–758. doi: 10.3109/00365529609010347. [DOI] [PubMed] [Google Scholar]
- Hawkey CJ, Karrasch JA, Szczepanski L, Walker DG, Barkun A, Swannell AJ, Yeomans ND. Omeprazole compared with misoprostol for ulcers associated with nonsteroidal antiinflammatory drugs. Omeprazole versus Misoprostol for NSAID-induced Ulcer Management (OMNIUM) Study Group. N Engl J Med. 1998;338(11):727–734. doi: 10.1056/NEJM199803123381105. [DOI] [PubMed] [Google Scholar]
- Yeomans ND, Tulassay Z, Juhasz L, Racz I, Howard JM, van Rensburg CJ, Swannell AJ, Hawkey CJ. A comparison of omeprazole with ranitidine for ulcers associated with nonsteroidal antiinflammatory drugs. Acid Suppression Trial: Ranitidine versus Omeprazole for NSAID-associated Ulcer Treatment (ASTRONAUT) Study Group. N Engl J Med. 1998;338(11):719–726. doi: 10.1056/NEJM199803123381104. [DOI] [PubMed] [Google Scholar]
- Vonkeman HE, Fernandes RW, Palen J van der, van Roon EN, Laar MA van de. Proton-pump inhibitors are associated with a reduced risk for bleeding and perforated gastroduodenal ulcers attributable to non-steroidal anti-inflammatory drugs: a nested case-control study. Arthritis Res Ther. 2007;9(3):R52. doi: 10.1186/ar2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisbert JP, Pajares JM. Helicobacter pylori infection and perforated peptic ulcer prevalence of the infection and role of antimicrobial treatment. Helicobacter. 2003;8(3):159–167. doi: 10.1046/j.1523-5378.2003.00139.x. [DOI] [PubMed] [Google Scholar]
- Reinbach DH, Cruickshank G, McColl KE. Acute perforated duodenal ulcer is not associated with Helicobacter pylori infection. Gut. 1993;34(10):1344–1347. doi: 10.1136/gut.34.10.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kate V, Ananthakrishnan N, Badrinath S. Effect of Helicobacter pylori eradication on the ulcer recurrence rate after simple closure of perforated duodenal ulcer: retrospective and prospective randomized controlled studies. Br J Surg. 2001;88(8):1054–1058. doi: 10.1046/j.0007-1323.2001.01831.x. [DOI] [PubMed] [Google Scholar]
- Debongnie JC, Wibin E, Timmermans M, Mairesse J, Dekoninck X. Are perforated gastroduodenal ulcers related to Helicobacter pylori infection? Acta Gastroenterol Belg. 1995;58(2):208–212. [PubMed] [Google Scholar]
- Henriksson AE, Edman AC, Nilsson I, Bergqvist D, Wadstrom T. Helicobacter pylori and the relation to other risk factors in patients with acute bleeding peptic ulcer. Scand J Gastroenterol. 1998;33(10):1030–1033. doi: 10.1080/003655298750026705. [DOI] [PubMed] [Google Scholar]
- Matsukura N, Onda M, Tokunaga A, Kato S, Yoshiyuki T, Hasegawa H, Yamashita K, Tomtitchong P, Hayashi A. Role of Helicobacter pylori infection in perforation of peptic ulcer: an age- and gender-matched case-control study. J Clin Gastroenterol. 1997;25(Suppl 1):S235–239. doi: 10.1097/00004836-199700001-00037. [DOI] [PubMed] [Google Scholar]
- Okan A, Tankurt E, Aslan BU, Akpinar H, Simsek I, Gonen O. Relationship between non-steroidal anti-inflammatory drug use and Helicobacter pylori infection in bleeding or uncomplicated peptic ulcers: A case-control study. J Gastroenterol Hepatol. 2003;18(1):18–25. doi: 10.1046/j.1440-1746.2003.02889.x. [DOI] [PubMed] [Google Scholar]
- Go MF. Review article: natural history and epidemiology of Helicobacter pylori infection. Aliment Pharmacol Ther. 2002;16(Suppl 1):3–15. doi: 10.1046/j.1365-2036.2002.0160s1003.x. [DOI] [PubMed] [Google Scholar]
- Chu KM, Kwok KF, Law SY, Tuen HH, Tung PH, Branicki FJ, Wong J. Helicobacter pylori status and endoscopy follow-up of patients having a history of perforated duodenal ulcer. Gastrointest Endosc. 1999;50(1):58–62. doi: 10.1016/S0016-5107(99)70345-7. [DOI] [PubMed] [Google Scholar]
- Ng EK, Lam YH, Sung JJ, Yung MY, To KF, Chan AC, Lee DW, Law BK, Lau JY, Ling TK. Eradication of Helicobacter pylori prevents recurrence of ulcer after simple closure of duodenal ulcer perforation: randomized controlled trial. Ann Surg. 2000;231(2):153–158. doi: 10.1097/00000658-200002000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian M, Chandran VP, Elashaal YI, Sim AJ. Helicobacter pylori infection in perforated peptic ulcer disease. Br J Surg. 1995;82(3):360–362. doi: 10.1002/bjs.1800820325. [DOI] [PubMed] [Google Scholar]
- Andersen IB, Jorgensen T, Bonnevie O, Gronbaek M, Sorensen TI. Smoking and alcohol intake as risk factors for bleeding and perforated peptic ulcers: a population-based cohort study. Epidemiology. 2000;11(4):434–439. doi: 10.1097/00001648-200007000-00012. [DOI] [PubMed] [Google Scholar]
- Swedish-central-bureau-of-statistics (SCB) Undersökningarna av levnadsförhållanden (ULF) 2006. http://www.scb.se