Abstract
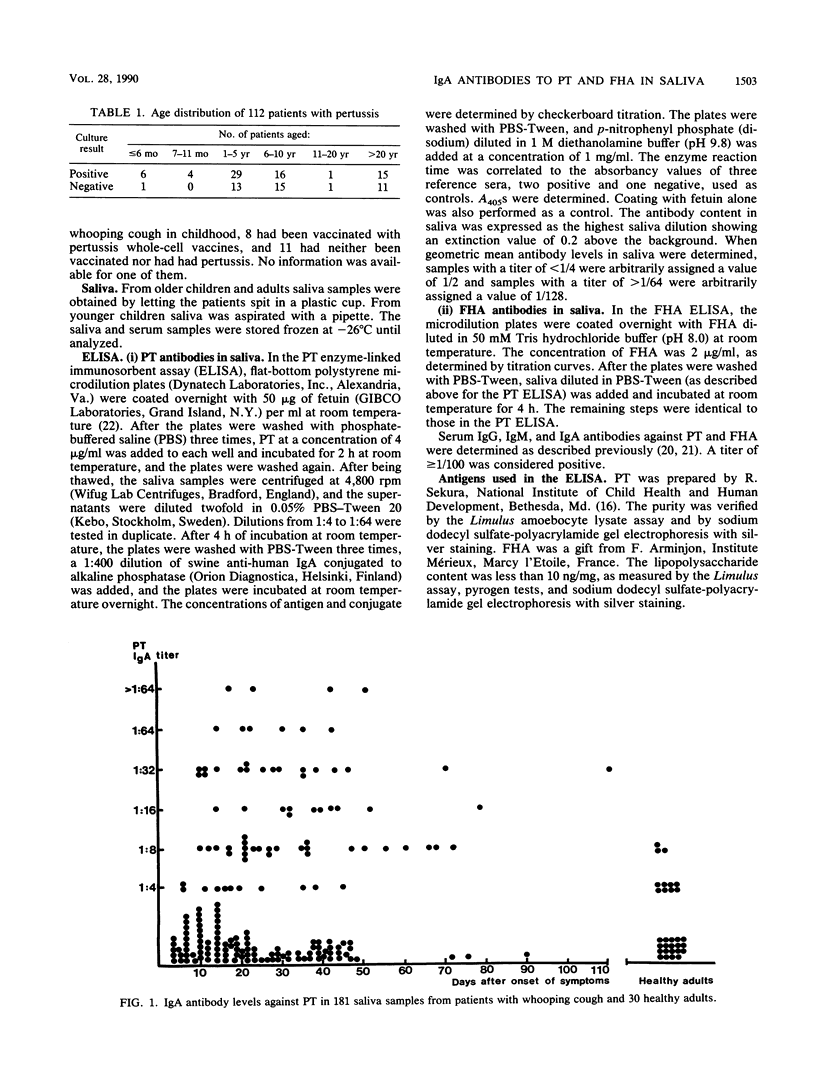
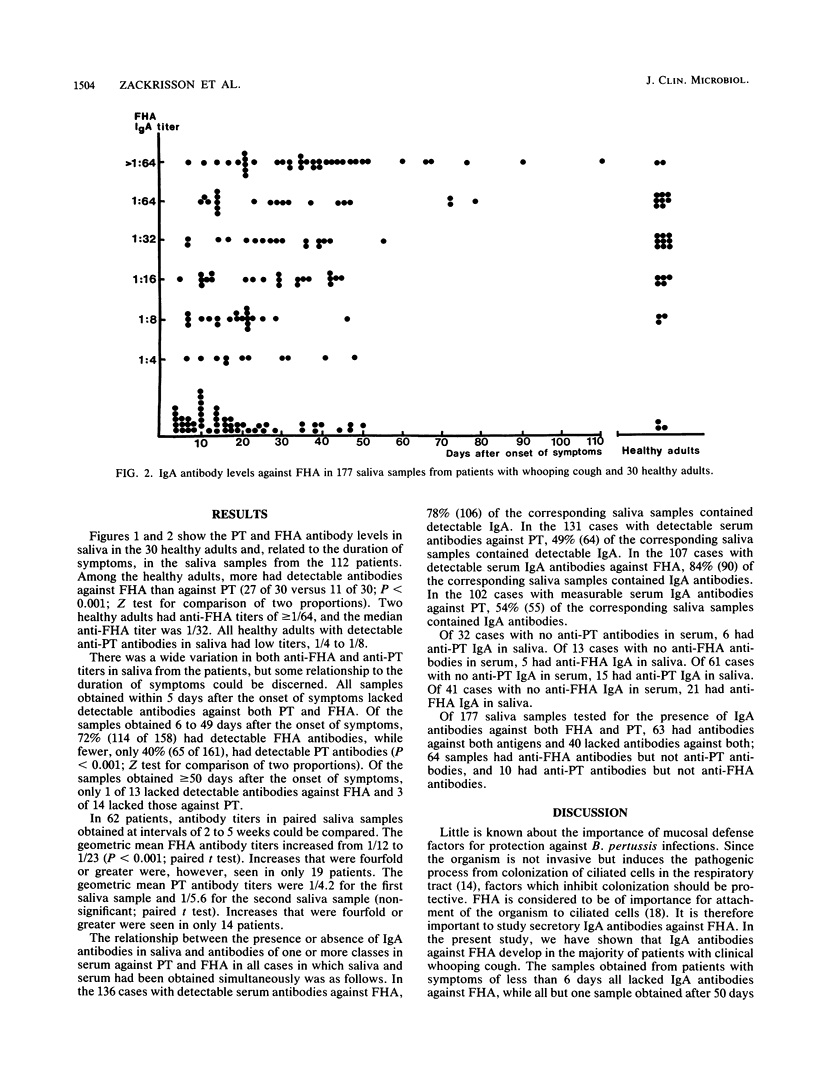
Immunoglobulin A (IgA) antibodies against pertussis toxin (PT) and filamentous hemagglutinin (FHA) in 181 saliva samples obtained during various stages of pertussis from 112 patients were determined. Saliva samples obtained within 5 days after the onset of symptoms did not have detectable IgA antibodies against either of the two antigens. Of the samples obtained between 6 and 50 days after the onset of symptoms, 72% had antibodies against FHA but only 40% had antibodies against PT. With few exceptions, saliva samples obtained more than 50 days after the onset of symptoms contained antibodies against both antigens. In the 59 patients from whom paired saliva samples were obtained at intervals of 2 to 5 weeks, a significant increase in the geometric mean FHA antibody titers but not PT antibody titers occurred. However, increases that were fourfold or greater were observed against FHA in only 19 patients and against PT in 14 patients. Thus, IgA antibodies against FHA and PT in saliva develop during pertussis, and the importance of secretory IgA antibodies for protection against infection and disease should be investigated. Determination of these antibodies in paired saliva samples is, however, of little value for the laboratory diagnosis of pertussis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. L., Belshe R. B., Bartram J., Gurwith M., Hung P., Levner M., Vernon S. K. Clinical and serologic responses to acellular pertussis vaccine in infants and young children. Am J Dis Child. 1987 Sep;141(9):949–953. doi: 10.1001/archpedi.1987.04460090026016. [DOI] [PubMed] [Google Scholar]
- Aoyama T., Hagiwara S., Murase Y., Kato T., Iwata T. Adverse reactions and antibody responses to acellular pertussis vaccine. J Pediatr. 1986 Dec;109(6):925–930. doi: 10.1016/s0022-3476(86)80270-0. [DOI] [PubMed] [Google Scholar]
- Blennow M., Hedenskog S., Granström M. Protective effect of acellular pertussis vaccines. Eur J Clin Microbiol Infect Dis. 1988 Jun;7(3):381–383. doi: 10.1007/BF01962341. [DOI] [PubMed] [Google Scholar]
- Gahnberg L., Krasse B. Salivary immunoglobulin A antibodies reacting with antigens from oral streptococci: longitudinal study in humans. Infect Immun. 1981 Sep;33(3):697–703. doi: 10.1128/iai.33.3.697-703.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granström G., Askelöf P., Granström M. Specific immunoglobulin A to Bordetella pertussis antigens in mucosal secretion for rapid diagnosis of whooping cough. J Clin Microbiol. 1988 May;26(5):869–874. doi: 10.1128/jcm.26.5.869-874.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granström G., Wretlind B., Salenstedt C. R., Granström M. Evaluation of serologic assays for diagnosis of whooping cough. J Clin Microbiol. 1988 Sep;26(9):1818–1823. doi: 10.1128/jcm.26.9.1818-1823.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granström M., Granström G., Lindfors A., Askelöf P. Serologic diagnosis of whooping cough by an enzyme-linked immunosorbent assay using fimbrial hemagglutinin as antigen. J Infect Dis. 1982 Dec;146(6):741–745. doi: 10.1093/infdis/146.6.741. [DOI] [PubMed] [Google Scholar]
- Granström M., Thorén M., Blennow M., Tiru M., Sato Y. Acellular pertussis vaccine in adults: adverse reactions and immune response. Eur J Clin Microbiol. 1987 Feb;6(1):18–21. doi: 10.1007/BF02097184. [DOI] [PubMed] [Google Scholar]
- Hedenskog S., Granström M., Olin P., Tiru M., Sato Y. A clinical trial of a monocomponent pertussis toxoid vaccine. Am J Dis Child. 1987 Aug;141(8):844–847. doi: 10.1001/archpedi.1987.04460080030021. [DOI] [PubMed] [Google Scholar]
- Håkansson S., Sundin C. G., Granström M., Gästrin B. Diagnosis of whooping cough--a comparison of culture, immunofluorescence and serology with ELISA. Scand J Infect Dis. 1984;16(3):281–284. doi: 10.3109/00365548409070401. [DOI] [PubMed] [Google Scholar]
- Lewis K., Cherry J. D., Holroyd H. J., Baker L. R., Dudenhoeffer F. E., Robinson R. G. A double-blind study comparing an acellular pertussis-component DTP vaccine with a whole-cell pertussis-component DTP vaccine in 18-month-old children. Am J Dis Child. 1986 Sep;140(9):872–876. doi: 10.1001/archpedi.1986.02140230042025. [DOI] [PubMed] [Google Scholar]
- Pichichero M. E., Badgett J. T., Rodgers G. C., Jr, McLinn S., Trevino-Scatterday B., Nelson J. D. Acellular pertussis vaccine: immunogenicity and safety of an acellular pertussis vs. a whole cell pertussis vaccine combined with diphtheria and tetanus toxoids as a booster in 18- to 24-month old children. Pediatr Infect Dis J. 1987 Apr;6(4):352–363. doi: 10.1097/00006454-198704000-00004. [DOI] [PubMed] [Google Scholar]
- Pittman M. The concept of pertussis as a toxin-mediated disease. Pediatr Infect Dis. 1984 Sep-Oct;3(5):467–486. doi: 10.1097/00006454-198409000-00019. [DOI] [PubMed] [Google Scholar]
- Sato Y., Izumiya K., Sato H., Cowell J. L., Manclark C. R. Role of antibody to leukocytosis-promoting factor hemagglutinin and to filamentous hemagglutinin in immunity to pertussis. Infect Immun. 1981 Mar;31(3):1223–1231. doi: 10.1128/iai.31.3.1223-1231.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekura R. D., Fish F., Manclark C. R., Meade B., Zhang Y. L. Pertussis toxin. Affinity purification of a new ADP-ribosyltransferase. J Biol Chem. 1983 Dec 10;258(23):14647–14651. [PubMed] [Google Scholar]
- Sekura R. D., Zhang Y. L., Roberson R., Acton B., Trollfors B., Tolson N., Shiloach J., Bryla D., Muir-Nash J., Koeller D. Clinical, metabolic, and antibody responses of adult volunteers to an investigational vaccine composed of pertussis toxin inactivated by hydrogen peroxide. J Pediatr. 1988 Nov;113(5):806–813. doi: 10.1016/s0022-3476(88)80005-2. [DOI] [PubMed] [Google Scholar]
- Tuomanen E. I., Hendley J. O. Adherence of Bordetella pertussis to human respiratory epithelial cells. J Infect Dis. 1983 Jul;148(1):125–130. doi: 10.1093/infdis/148.1.125. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Weiss A. Characterization of two adhesins of Bordetella pertussis for human ciliated respiratory-epithelial cells. J Infect Dis. 1985 Jul;152(1):118–125. doi: 10.1093/infdis/152.1.118. [DOI] [PubMed] [Google Scholar]
- Zackrisson G., Arminjon F., Krantz I., Lagergård T., Sigurs N., Taranger J., Trollfors B. Serum antibody response to filamentous hemagglutinin in patients with clinical pertussis measured by an enzyme-linked immunosorbent assay. Eur J Clin Microbiol Infect Dis. 1988 Dec;7(6):764–770. doi: 10.1007/BF01975044. [DOI] [PubMed] [Google Scholar]
- Zackrisson G., Krantz I., Lagergård T., Larsson P., Sekura R., Sigurs N., Taranger J., Trollfors B. Antibody response to pertussis toxin in patients with clinical pertussis measured by enzyme-linked immunosorbent assay. Eur J Clin Microbiol Infect Dis. 1988 Apr;7(2):149–154. doi: 10.1007/BF01963068. [DOI] [PubMed] [Google Scholar]
- Zackrisson G., Lagergård T., Lönnroth I. An enzyme-linked-immunosorbent assay method for detection of immunoglobulins to pertussis toxin. Acta Pathol Microbiol Immunol Scand C. 1986 Dec;94(6):227–231. doi: 10.1111/j.1699-0463.1986.tb02116.x. [DOI] [PubMed] [Google Scholar]


