Abstract
Concurrent sexual partnerships may help to explain the disproportionately high prevalence of HIV and other sexually transmitted infections among African Americans. The persistence of such disparities would also require strong assortative mixing by race. We examined descriptive evidence from 4 nationally representative US surveys and found consistent support for both elements of this hypothesis. Using a data-driven network simulation model, we found that the levels of concurrency and assortative mixing observed produced a 2.6-fold racial disparity in the epidemic potential among young African American adults.
Racial disparities in HIV/AIDS are large and growing, with an increasingly disproportionate burden borne by African Americans. Over time, disparities have emerged in every defined risk group, and some of the largest gaps exist among heterosexuals, particularly women. As noted in a recent Centers for Disease Control and Prevention (CDC) report focusing on cumulative diagnoses of HIV/AIDS from 2001 to 2005, the number of cases and diagnosis rates among Blacks were higher than those among all other racial/ethnic populations combined; Blacks also accounted for the largest percentage of HIV/AIDS diagnoses in every age group and almost every risk group.1
The level of incidence of new HIV/AIDS diagnoses is greater among African American men, but racial disparities in incidence are greatest among African American women. Incidence rates among African American women not only exceed those of every other racial/ethnic group of women by 4- to 21-fold, they also exceed incidence rates among men in those groups.
To understand what might be driving these disparities, it is helpful to consider the patterns observed in prevalence rates of other sexually transmitted infections (STIs). Table 1 shows estimates for a wide range of STIs derived from 3 national data sources; 1 is surveillance reports from the CDC,2 and the other 2 are population based surveys: the National Health and Nutrition Examination Survey3 and the National Longitudinal Survey of Adolescent Health (Add Health).4 In the case of every STI, the disparity between African Americans and other groups is evident. The magnitude varies across the different pathogens, but the prevalence ratio between African Americans and Whites ranges from approximately 4 to more than 20. The only exception is human papillomavirus, which has a high prevalence in all instances and a prevalence ratio of 1.4.
TABLE 1.
Estimated Prevalence Rates of and Disparities in HIV and Other Sexually Transmitted Infections: Add Health, NHANES, and CDC Surveillance Data
| Prevalence (per 100 population) |
|||||
| Survey (Age Range of Respondents) | Non-Hispanic Blacka | Non-Hispanic Whiteb | Hispanicc | Surveyd Prevalence Ratioe | CDC Prevalence Ratioe |
| Add Health 2000 (19–24 y) | |||||
| Chlamydia | 12.5 | 1.9 | 5.9 | 6.5 | 6.0 |
| Gonorrhea | 2.1 | 0.1 | 0.2 | 21.8 | 24.9 |
| Trichomoniasis | 6.9 | 1.2 | 2.1 | 5.8 | … |
| HIV | 0.5 | …f | …f | 22.4 | 6.7 |
| NHANES 2003–2004 | |||||
| Chlamydia (18–39 y) | 5.2 | 1.3 | 2.6 | 4.2 | 6.5 |
| Gonorrhea (19–39 y) | 0.8 | 1.0 | 0.1 | 0.9 | 16.2 |
| Human papillomavirus (14–59 y) | 36.6 | 23.3 | 26.9 | 1.6 | … |
| Herpes simplex virus type 2 (14–49 y) | 44.3 | 13.8 | 14.5 | 3.2 | … |
| Syphilis (18–49 y) | 1.3 | 0.9 | 0.8 | 1.4 | 4.9 |
| Trichomoniasis (14–49 y) | 13.4 | 1.4 | 2.1 | 9.6 | … |
| HIV (18–49 y) | 1.9 | 0.2 | 0.5 | 8.9 | 7.2 |
Note. Add Health = National Longitudinal Survey of Adolescent Health; NHANES = National Health and Nutrition Examination Survey; CDC = Centers for Disease Control and Prevention. CDC surveillance data are based on the authors' tabulations, matched to the year of the survey,2,50 and the closest age range possible from published tables in surveillance reports and bridged-race population estimates.51
Add Health n = 3042; NHANES n = 503–735.
Add Health n = 7741; NHANES n = 705–1256.
Add Health n = 2340; NHANES n = 482–768.
Add Health (top) or NHANES (bottom).
Ratio of non-Hispanic Blacks to non-Hispanic Whites.
There were too few cases in the survey to estimate prevalence by race for these groups. The combined prevalence of HIV for non-Hispanic Whites and Hispanics was 0.02.
These disparities in STIs are also longstanding. CDC data on historical prevalence rates of gonorrhea and syphilis among people aged 15 to 19 years show strikingly large disparities dating back to 1981.5 Equally striking, however, is how fast these disparities can change: rates of syphilis among non-Hispanic Blacks have dropped by more than 90% since 1990, and the prevalence ratio has decreased from more than 60 to approximately 5.6
POTENTIAL EXPLANATIONS
A combination of biological, behavioral, and network factors are responsible for the transmission of HIV. Genetics and coinfection, for example, can influence host susceptibility and infectivity. Researchers have identified several genetic polymorphisms that appear to confer both protection against and risk for HIV infection. Their population distribution, however, suggests little net effect in populations of African descent7,8 and population-attributable effects among those of European descent that are well below observed disparities.9 The wide range of sexually transmitted pathogens that show the same qualitative disparities—bacterial, viral, protozoan—do not suggest a simple genetic difference. By contrast, the rapid, dramatic declines in the disparities for curable STIs are clearly due to changes in behavior or the environment, and they show how powerful these effects can be.
Other STIs are themselves invoked as a possible biological explanation for disparities in HIV prevalence rates, although clinical trial results regarding the impact of STI treatment on HIV incidence have been disappointing.10,11 At the population level, however, this simply pushes the question back one step: if higher STI rates are leading to faster spread of HIV, what is causing the higher STI rates? In the case of treatable or curable STIs, the answer may be access to screening and care. For STIs that are not treatable or curable, the prevalence disparities must be related to some other component of the transmission system.
Traditional behavioral risk factors—high number of partners, lack of condom use, alcohol and drug abuse—have also repeatedly failed to explain racial disparities in HIV and other STIs. The most comprehensive analysis in this area is the recently published study of Hallfors et al.12 Using Add Health wave 3 data (which include biomarkers for 4 STIs), that study found that although behavior influenced likelihood of infection, the odds of infection among non-Hispanic Blacks were higher in every behavioral context and highest (28 times higher) in the lowest risk context.
The fact that racial disparities in HIV and other STIs cannot be explained by differences in behavior does not mean that there is no behavioral basis for these disparities. It simply means that the behavioral basis is not adequately captured at the individual level. People contract STIs not from their behavior but from their partners (who contract them from their partners, and so forth). At the population level, epidemic potential is determined by underlying network connectivity. Connectivity is influenced by many factors, including the dynamics of partnership formation, dissolution, and sequencing; population mixing by demographic and behavioral attributes; and geographic clustering and access. The goal of network epidemiology is to gain an understanding of the features of this structure that influence disease spread.
It is often assumed that network connectivity requires “high activity” groups or “super spreader” hubs. Although this is an efficient way to link different groups, it is not the only means of establishing connectivity. Another way is through concurrent partnerships.13–15 In such partnerships, a large number of individuals are linked in a sparse, low-degree web (“degree” is the network term for a person's number of partners). Low-degree concurrency can rapidly connect the population, generating overall connectivity that is similar in magnitude to but more robust than that generated by super spreaders or core groups.16,17
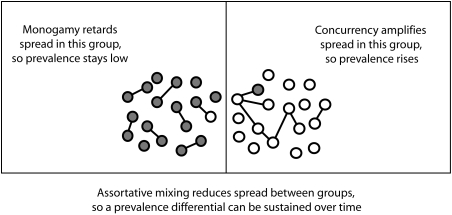
The importance of concurrency among African Americans has received growing attention.18,19 For prevalence differentials to persist, however, there must also be network segregation. In the absence of group segregation, prevalence tends to equalize over time. This leads to the network hypothesis for the emergence of persistent disparities illustrated in Figure 1. In one group, serial monogamy is the norm, and thus connectivity is low and the spread of infection is reduced. In the other group, there is a certain level of concurrency, so connectivity rises and the spread of infection is increased. With assortative mixing (more partnerships within than between groups), the network is segregated according to group, and transmission between groups is rare. Such a network mechanism could provide the foundation for persistent differentials in all STIs, although the magnitudes of these differentials would still be influenced by infectivity and duration of infection. This is consistent with the pattern shown in Table 1.
FIGURE 1.
Network hypothesis for the emergence of persistent disparities in HIV and other sexually transmitted infections.
In the case of racial disparities, the network hypothesis translates into a prediction that rates of concurrency are higher among African Americans, and these rates are reinforced by race-specific patterns of assortative mixing. Note that this is slightly different from the mechanism posited by Laumann and Youm,20 according to which assortative racial mixing creates greater contact between highly active “core” and less active “peripheral” African Americans. It is also different from the type of assortative mixing often examined in transmission modeling studies, wherein groups are defined according to “high” and “low” activity levels21 rather than observable attributes. Social science suggests that partner selection and norm reinforcing sanctions are more likely to be based on observable attributes such as perceived racial/ethnic group and age.
METHODS
Two types of information are needed to test the network hypothesis for racial disparities: the cross-sectional distribution of the number of concurrent partners at a given moment in time and the race of the respondent and each of her or his current partners. Several US-based nationally representative data sets contain the information necessary to test this hypothesis: the National Health and Social Life Survey22; the National Survey of Men23 and its companion, the National Survey of Women24; cycle 6 of the National Survey of Family Growth25; and wave 3 of Add Health.
Although the response rates of the studies just mentioned were similar (ranging from 70%–79%), the age ranges and questionnaire wording were somewhat different. The studies provide comparable data on concurrency and assortative mixing that allow comparisons of 2 groups: adults aged 20 to 38 years (all of the studies other than Add Health) and young adults aged 19 to 25 years (all of the studies).
Descriptive Measures
Concurrent partnerships were defined by the “momentary degree,” which was the number of active sexual partnerships reported by the respondent on the day of the study interview. A momentary degree greater than 1 indicates concurrency. We used 3 summary measures of concurrency: percentage of the sample reporting a momentary degree greater than 1, mean momentary degree, and mean momentary degree for nonisolates (those with at least 1 partner). The first measure indicates the fraction of the sample with concurrent partnerships, the second indicates the overall average partnership rate (partnerships per person), and the third indicates the overall average partnership rate among individuals with at least 1 partner.
Assortative mixing was defined at the partnership level. All current partnerships were cross classified according to the race of the respondent and the reported race of his or her partner, forming a “mixing matrix.” Race/ethnicity was grouped into 3 categories: non-Hispanic Black, non-Hispanic White, and all other racial/ethnic groups. The coarseness of the classification was dictated by the relatively small numbers of survey respondents and partners in the third category.
We used 2 mixing matrix summaries: the proportion of partnerships that were race concordant and “selection coefficients” that measured deviation from proportional mixing.26 The selection coefficients αij were defined as follows:
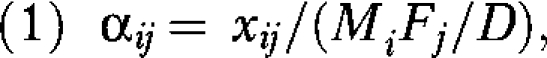 |
where xij is the number of partnerships between men of race i and women of race j; Mi and Fj are the total number of partnerships formed by men of race i and women of race j, respectively; and D is the total number of partnerships (dyads) in the sample. The denominator on the right-hand side is analogous to an expected value under independence. More precisely, it is the expectation under proportional mixing (i.e., given the population composition and partnership rates). Values of αij greater than 1 indicate a positive selection bias, and values less than 1 indicate a negative selection bias.
Simulation Design
We used a stochastic simulation model to evaluate the effects of observed levels of concurrency and mixing on epidemic potential and racial disparities in prevalence. We intended the simulation to serve as a “proof of concept,” with the goal of answering the question of whether observed levels of mixing and concurrency could produce a persistent differential in prevalence rates. The question was not whether these network features do explain racial differentials in prevalence rates of HIV and other STIs, but whether they could. Our objective was to understand how network features influence transmission dynamics rather than to predict the spread of any particular pathogen. We therefore made a number of simplifying assumptions, for both the population and the spread of infection, in representing this process. We note later how these assumptions influenced the quantitative estimates and qualitative findings.
We used an integrated statistical framework—an exponential random graph model implemented in the Statnet software package—to estimate the underlying network parameters and simulate the network. Space limitations preclude a detailed description of the methods, but there are many published and online resources available that provide helpful guides to the theory and implementation of these models for network analysis and simulation (see Handcock et al.27,28). The methods we used here allowed the representation of the network structures of interest to be more precisely controlled, and more faithful to the observed data, than most simulations of this type.
We simulated a sexually active population of 10 000 individuals over 10 years, with no births or deaths. This sample size was selected to avoid artifacts that emerge in small networks, and the duration was a constraint imposed by the absence of demographic dynamics. For simplicity, we used 2 racial groups (rather than the 3 groups used in the descriptive analysis), both genders, and heterosexual partnerships. We used the Add Health data as the basis of the simulation because that study involved the largest number of respondents and, therefore, the most stable estimates.
The key feature of this stylized simulation is that it reproduced the relative group sizes, observed mixing matrix, momentary degree distribution, and average partnership duration from the Add Health data set in a dynamically changing network over time. Thus, the network preserved the population-level cross-sectional statistics observed in the data even though partnerships formed and dissolved over time.
The networks were simulated under 2 scenarios: once with concurrency at the level observed in the data and once with the same number of partnerships but no concurrency. Both preserved the mixing observed in the data. In the simulation without concurrency, the partnerships that would have been concurrent were reallocated to create monogamous partnerships (because more than a quarter of the Add Health respondents reported no partners on the day of their interview, there were isolates who could be paired according to these reallocated partnerships). Thus, the total number of partnerships and their durations were the same in both scenarios. The comparison isolated the unique effect of concurrency and did not confound it with changes in overall partnership levels. The quantitative results reported here were based on 100 runs, 50 for each scenario.
We defined epidemic potential as the “reachable path of infection,” the set of partnerships that occur at the appropriate location and in the appropriate time order to lead to potential transmission from an infected person to her or his uninfected partner. We generated the reachable path by setting infectivity in the simulation to 1; that is, a partnership between an infected and an uninfected individual resulted in an immediate and certain transmission event. This allowed us to ascertain the maximum possible size of an epidemic without regard to the specific biological properties of a pathogen. The simulations began with 10 randomly selected individuals who were infected (the “seeds”), and the reachable paths extended from these individuals.
The reachable path included the seeds of infection and the subsequent time-ordered sequence of partnerships along which transmission was possible. This time-ordered sequence was generated by the dynamics of partnership formation and dissolution; it represented neither the partnerships that were present on any single day nor the cumulative total number of partnerships collapsed over time.
Any real pathogen will be transmitted with a probability below 1 (typically much less) and for a specific duration during which a person is infectious, so it will reach only a subset of the reachable path. There are no conditions under which a pathogen will spread beyond the reachable path, however: the reachable path defines the maximum possible spread of infection. Note that, expressed in terms of the classical formula for the reproductive threshold, R0 = βcD, here β = 1 and D is effectively infinite (although our observation period was only 10 years). Thus, there is no threshold for the reachable path; the infection is guaranteed to spread.
In general, the reachable path will tend to underestimate disparities one might expect to observe for any particular STI. At lower levels of infectivity and duration, a reproductive threshold for spread emerges, and differences in behavior can then lead to qualitatively different results in across groups (e.g., no spread in one group and epidemic spread in another). This would lead to much greater disparities than those estimated here. It is also possible, however, that all groups fall below the reproductive threshold, in which case there would be no spread expected in any group and, thus, no disparities. The observed prevalence and disparities in both HIV and other STIs from the national data in Table 1 make it clear that STI transmission is sustained in each racial group, so we must be above the reproductive threshold. Therefore, our disparity estimates are most likely conservative. It is worth reiterating that, owing to the many simplifying assumptions made here, the results do not provide empirical evidence that concurrency explains racial disparities in rates of HIV and other STIs; rather, the results provide evidence that it could do so, given the mixing and concurrency levels observed in the data.
RESULTS
The findings below are divided into 2 sections: those based on observed data, and those based on stimulations from the observed data.
Observed Survey Data
Reported rates of concurrency among African American adult men (aged 20–38 years) on the day of their interview ranged from 5% to 10% in the surveys we reviewed; on average, these rates were 3.5 times higher than those among White men and 1.9 times higher than those among men from other racial/ethnic groups. Rates were similar among young African American men (aged 19–25 years), with estimates ranging from 5% to 13%, but the disparities were slightly smaller (2.9 and 2.5 times higher than rates among Whites and young men from other racial/ethnic groups, respectively).
Among women, levels of concurrency were lower, but the race-specific differential was still large. Approximately 4% of African American women reported concurrent partners on the day of their interview. Rates among adult African American women were 2.1 and 4.1 times higher, respectively, than rates among Whites and young women from other racial/ethnic groups, and rates among young African American women were 1.4 and 2.9 times higher than rates among Whites and young women from other racial/ethnic groups.
Across all of the studies reviewed, approximately 80% of reported partnerships were between members of the same racial category. The rate was generally higher among older adults than among younger adults. The largest values for the selection coefficient occurred for the 3 assortative pairings: White (1.4 times more than expected), Black (5.9 times more than expected), and other (3.6 times more than expected). The selection coefficients were well below 1 for White–Black partnerships, indicating little direct contact between members of the 2 groups. Both Whites and Blacks had more contact with individuals in the “other” race/ethnicity category, and thus this group served as somewhat of a bridge. In the case of all 3 groups, there was a remarkable degree of consistency in the selection coefficient estimates across the studies.
These data provide consistent evidence supporting the 2 elements of the network hypothesis: both the concurrency differential and high levels of network segregation were observed across all of the surveys. The question is, are these differences large enough to affect epidemic potential and explain the racial disparity in these survey populations? The assortative mixing was strong, but the rates of concurrency seemed relatively low, especially among women. We used our simulation to investigate potential effects on epidemic potential.
We employed the following Add Health estimates to drive the simulation. The mean momentary degree was 0.8 partners per person, with a 0.06 difference between the races. Concurrency generated a mean momentary degree for nonisolates of 1.09, with a difference of 0.12 between the races; about 5.3% of people were involved in a concurrent partnership on any given day, with a difference of 5.5% between the races. Overall, 95% of partnerships were racially assortative (a rate higher than the 80% observed when the “other” racial category was included, because most cross-group partnerships involved members of that category).
The estimated mean partnership duration, based on a Kaplan–Meier estimate from the Add Health data, was 29 months (SE = 0.29; note that the Add Health sample consisted of participants aged 19–25 years, so this short average duration was plausible). The estimated mean partnership duration for adults in the National Health and Social Life Survey was approximately 128 months (SE = 2.34). Longer partnership durations would probably increase the estimated effects of concurrency, given that monogamy would trap infections for longer spells and reduce infection spread in the parts of the network in which monogamy is more common.
Simulation Data
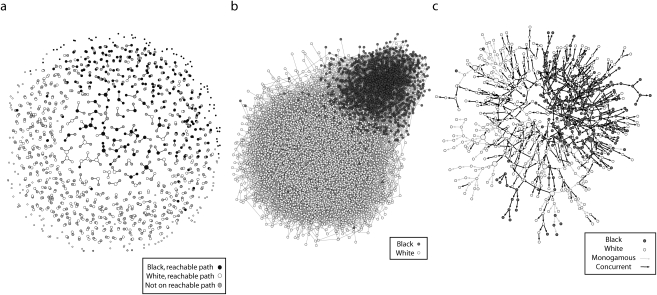
The evolution of the dynamic partnership network and the reachable path is illustrated in Figure 2, which displays results from a typical simulation run. Figure 2a shows a single day from the dynamic contact network for the subset of approximately 600 individuals who will eventually be in the reachable path and the partners they have on that day. This cross-sectional network is quite sparse, in that the momentary degree distribution has a low mean and a small range. Most of the partnership configurations were monogamous dyads (86%), but a few concurrencies can be observed. The largest connected component in the cross section typically contained about 10 nodes.
FIGURE 2.
Simulations based on data from a representative simulation showing (a) contact network from day 1, (b) cumulative contact network (10-year total), and (c) reachable paths from the 10 initially infected seeds.
Note. Data were from the National Longitudinal Survey of Adolescent Health, wave 3.
Figure 2b shows the cumulative contact network at the end of the run (i.e., all partnerships that were formed over the 10 years, collapsed over time). In these simulations, individuals had an average of 3 partners during this period; 95% had 6 or fewer, and the maximum number of partners observed varied from 12 to 15. These cumulative degree distributions were not controlled directly; rather, they were indirectly determined by the combination of the momentary degree distribution and partnership duration. Nonetheless, they reproduced the observed cumulative degree distribution in the Add Health data, suggesting that the partnership dynamics were a reasonably faithful representation of the behavior in this population. Even with these relatively low activity levels, the cumulative connected component by the end of the run included, on average, 99.9% of the population. This cumulative connectivity, however, is not the same as the reachable path. Because infection can be passed only to current or future partners, and not to past partners, the reachable path extending from a person depends on when he or she becomes infected.
Figure 2c shows the reachable paths from the 10 seeds. On average, 3% to 6% of the population was included in the reachable paths. The difference between the sizes of the cumulative connected component and the reachable paths was the effect of partnership timing and sequence.
In the case of monogamous partnerships, the simulation generated reachable paths containing 3% of the population on average. Concurrency doubled the overall epidemic potential to 6%. Recall that the monogamous scenario involved no change in the overall population mean degree but, rather, only a small change in the mean degree for nonisolates (0.09 of a partner). This small change had a remarkably large impact. By comparison, it would have take a hundredfold reduction in infectivity (reducing β from 1 to 0.01) to have the same impact on the reachable path.
The simulation also allowed us to observe the entire sequence of transmissions and, thus, assess exactly how concurrency influences infection spread. The primary impact of concurrency is to reduce the average time to secondary transmission; the time between a person becoming infected and passing the infection on is cut by about one third (32%). Because infectivity was set to 1, this reduction was not a function of changing the probability of transmission within partnerships but instead was purely a function of changing the timing between partnerships. Once a person in a monogamous partnership becomes infected by her or his partner, she or he cannot pass the infection on until the partnership dissolves and another one begins. Under concurrency, this waiting time is reduced. Either member may have (or acquire) a concurrent partner before the original partnership ends, accelerating transmission.
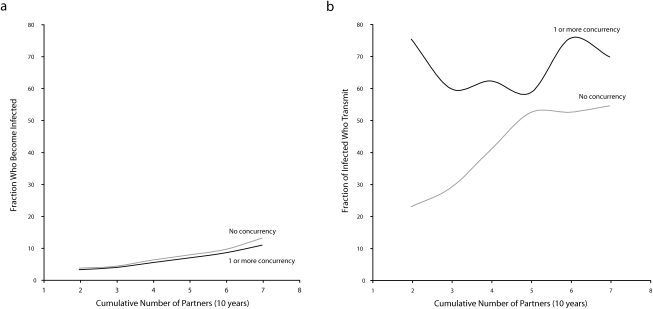
Our ability to observe the entire sequence also makes it possible to verify that, at the individual level, concurrency does not raise the risk of acquiring an infection; rather, it raises the risk of transmitting an infection. This can be seen in Figure 3. In Figure 3a, the probability of a person acquiring infection is shown for 2 groups: those involved in only monogamous partnerships and those with at least one concurrent partner over the 10-year simulation period (thus, concurrency and monogamy were defined as individual attributes). An increase in number of partners increases risk, so both groups are more likely to be in the reachable path as the cumulative number of partners rises. However, there is no difference between the groups once this variable is taken into account. This is why, at the individual level, there is no expected effect of concurrency on the relative risk of acquiring an infection. One's number of partners, not whether one's partners overlap in time, drives one's risk of acquisition.
FIGURE 3.
Simulations showing the effects of concurrency on (a) the probability of infection acquisition and (b) the probability of transmission of infection.
Note. With the cumulative number of partners truncated at 7, this includes 95% or more of all simulation observations. Simulations were based on data from the National Longitudinal Survey of Adolescent Health, wave 3.
Figure 3b shows the probability that a person, once infected, transmits the infection to at least 1 other person. Here there is a clear distinction between the groups. Among those who are monogamous, the probability of transmitting infection is again a function of number of cumulative partners. Among those who have at least one concurrent partner, however, even those with very few cumulative partners have an extremely high probability of transmitting infection.
The primary outcome of interest, however, is whether the network patterns observed in the Add Health study were sufficient to create a large racial disparity in epidemic potential. With the observed levels of concurrency and assortative mixing by race, the average disparity was 2.6: 11.6% of Blacks were in the reachable path of infection, as compared with 4.5% of Whites. When these partnerships were all monogamous, the disparity fell to 1.7, with 4.5% of Blacks and 2.7% of Whites in the reachable path.
It is worth emphasizing what the 2.6-fold disparity represents. Although the assortative mixing bias was very strong in this example, with 95% of all partnerships racially concordant, assortative mixing alone cannot generate persistent differentials. Unless segregation is absolute, infection will eventually spread between groups, and once there it will tend to equalize if the behavior within groups is the same. The differential we observed was therefore due to the way in which assortative mixing amplified the modest difference in partner sequencing between the 2 groups: a 0.06 difference in the momentary mean number of partners. This small difference would be enough to create a 70% disparity in epidemic potential between the groups over 10 years if everyone practiced monogamy. Adding in the observed difference in concurrency, however, leads to a 160% disparity.
In the online supplement to this article, we have included an animated network movie that shows the dynamic growth of the reachable path for a simulation run with concurrency.
DISCUSSION
The sexual network perspective outlined here offers an alternative way to think about concepts such as risk groups and risk behaviors and may lead to a more productive understanding of HIV transmission, disparities, and prevention. It is not only an individual's behavior that defines his or her risk; it is his or her partner's behavior and (ultimately) his or her position in a sexual network. Sexual networks determine levels of individual exposure, the population dynamics of infection spread, and the interactional contexts that constrain behavioral changes. Taking networks seriously necessitates a paradigm shift in HIV prevention as well as research.
Application to Public Health Practice
In the earlier-mentioned CDC report showing that Blacks accounted for 51% of incident HIV cases between 2001 and 2005, despite accounting for only 13% of the US population, it was concluded that “[n]ew interventions and mobilization of the broader community are needed to reduce the disproportionate impact of HIV/AIDS on blacks in the United States.”1(p189) Helping African American communities in particular to understand and mediate the impact of sexual network factors is one such new approach.
Few prevention interventions targeting HIV and other STIs have been designed to address sexual network factors directly, and almost none have addressed the epidemiological benefits of reducing numbers of concurrent partnerships. The initiative undertaken in Uganda, where an understanding of the impact of concurrent sexual partnerships has informed HIV prevention messages, is the single well-known exception.29 The “zero grazing” campaign launched in Uganda in the early 1990s succinctly conveyed that concurrent partnerships fueled that country's HIV epidemic.
Uganda's epidemiologically sound and culturally contextualized sexual concurrency prevention message engendered understanding and community dialogue and led to a dramatic reduction in the number of extramarital partnerships, with a concomitant decline in HIV incidence that was celebrated worldwide. According to the World Health Organization, the percentage of Ugandan men reporting 3 or more nonmarital sexual partners fell from 15% to 3% from 1989 to 1995,30 and Uganda's HIV prevalence rate declined from 15% in 1991 to 5% in 2001.31 This reduction is the equivalent of a vaccine with 80% effectiveness.32
As our simulation results suggest, achieving small changes in levels of sexual partnership concurrency may also have a dramatic impact on the chain of transmission of HIV and other STIs in African American communities. Thus, an HIV/STI prevention message stressing either “one partner at a time” or condom use with additional partners is as important as messages promoting abstinence or reductions in cumulative numbers of partners over time.33,34 Our simulation also showed that individuals with HIV or other STIs who are involved in concurrent sexual relationships are at risk of transmitting infection, consistent with empirical findings for various STIs (e.g., syphilis and chlamydia)35,36 and with theory.37 This finding has important implications for the partners of such individuals as well as for prevention messages, which may need to target social norms for community protection (rather than self-protection).
Nonsexual social networks can exert important influences on the formation and maintenance of sexual relationships.38 One analysis of Uganda's intervention noted that its success was “distinctively associated with communication … through social networks” and that its “replication will require changes in global HIV/AIDS intervention policies and their evaluation.”32(p714) In the US context, there is also growing evidence on the effectiveness of social network–based interventions among injection drug users39–41 and at-risk, hidden populations.42,43 Many of these interventions involve respondent-driven sampling techniques and are variants on peer-leader models.44,45 As we move forward in the development of interventions, an understanding of the perceived rationale for concurrent partnerships among African Americans will be critical to allow crafting of prevention messages that resonate with this community.
Summary
In a recent article outlining 15 factors that jointly contribute to producing the disproportionate burden of HIV and other STIs among African Americans, Kraut-Becher et al. argued that single-factor approaches to reducing disparities are unlikely to have much success.46 We agree that these disparities have many roots, but we also believe that concurrency warrants a particularly close examination, for 3 reasons.
The first reason is that small differences in the configuration of partnerships have large effects on epidemic potential. This is obscured in the formulation for the reproductive rate of infection, R0 = βcD, which assumes that every contact is made with a randomly chosen member of the population (i.e., there are no partnerships). Once partnerships are introduced, infectivity influences spread within partnerships (the probability of transmitting to a partner with a per contact infectivity of β and c contacts is given by 1 − [1 − β]c), whereas network connectivity is enhanced by concurrency. The first effect means that the population-level impact of reductions in infectivity is mediated through partnerships and declines as the number of contacts per partnership during the infectious period increases. The second effect means that connectivity can be established with very low levels of partnership formation: small differences in concurrency can have a large impact on infection spread.
The second reason is that 44% of the AIDS cases and 40% of the HIV infections reported in 2006 among non-Hispanic Blacks involved individuals who either had HIV-positive partners from no known risk group or had no known risks themselves.47 This was more than double the fraction among non-Hispanic Whites. As the epidemic moves beyond traditional core groups and their partners, it is important that we adjust our focus to understand the different behaviors and relational contexts that facilitate infection spread. The evidence from population-based studies such as Add Health makes it clear that the largest racial disparities in HIV and other STIs occur not in high-risk groups but, rather, in the groups that are at the lowest possible risk (as established through their own behavior).12 Concurrency is among the few factors that can consistently produce patterns such as this one.
The third reason for focusing on concurrency is that the topic resonates with members of the African American and African immigrant communities. From service providers and community activists to engaged members of these communities who are looking for answers, all of the groups to whom we have reached out in our community work find this argument compelling and ask that this message be disseminated. The information alone may be a powerful intervention if it leads to even a little behavior change. The reason is that the same small differences that increase epidemic potential can reduce epidemic potential when they are reversed. We do not need everyone to change their behaviors for the population-level impact to be substantial.
It is clear that a collaborative effort is needed. Partnerships between communities and the public health and academic sectors are essential for translating science into scientifically accurate, culturally relevant, and epidemiologically effective prevention messages. As an example of such a partnership, the Community Action Board of the University of Washington Center for AIDS Research is currently engaged in a local project, funded by the National Institutes of Health, that seeks to use community-based participatory research principles48,49 to translate the science of sexual networks into culturally resonant HIV prevention messages in King County, Washington. Until an effective biomedical prevention strategy is in place, these kinds of collaborative efforts will determine whether we succeed in reducing racial disparities in HIV in the United States.
Acknowledgments
This study was supported by the National Institutes of Health (grants R01DA12831, R01HD41877, R01HD038210, and P30AI27757), the Centers for Disease Control and Prevention (grant K01PS000066), and the Center for Studies in Demography and Ecology (grant R24HD042828).
This study would not have been possible without the work of the statnet development team, led by Mark S. Handcock, who prototyped the dynamic network code and produced the simulations used in this analysis; other team members included David Hunter, Steven M. Goodreau, Carter Butts, and Skye Bender-deMoll. Additional contributions to the code used in this analysis were made by Nicole Bohme-Carnegie, Susan Cassels, and Pavel Krivitsky. We acknowledge the support and partnership provided by the Community Action Board of the University of Washington Center for AIDS Research.
Human Participant Protection
The institutional review board of the University of Washington approved the analyses of the National Longitudinal Survey of Adolescent Health data used in the simulation. All other data sets are publicly available.
References
- 1.Centers for Disease Control and Prevention Racial/ethnic disparities in diagnoses of HIV/AIDS—33 States, 2001–2005. MMWR Morb Mortal Wkly Rep 2007;56:189–193 [PubMed] [Google Scholar]
- 2.HIV/AIDS Surveillance Report, 2004. Atlanta, GA: Centers for Disease Control and Prevention; 2005 [Google Scholar]
- 3.National Center for Health Statistics National Health and Nutrition Examination Survey. Available at: http://www.cdc.gov/nchs/nhanes.htm. Accessed October 30, 2008
- 4.Udry JR. The National Longitudinal Study of Adolescent Health (Add Health), Wave III, 2001–2002 [machine-readable data file and documentation]. Chapel Hill, NC: Carolina Population Center, University of North Carolina; 2003 [Google Scholar]
- 5.STD Surveillance 2004: STDs in Racial and Ethnic Minorities. Atlanta, GA: Centers for Disease Control and Prevention; 2004 [Google Scholar]
- 6.Sexually Transmitted Disease Surveillance, 1993. Atlanta, GA: Centers for Disease Control and Prevention; 1994 [Google Scholar]
- 7.Gonzalez E, Kulkarni H, Bolivar H, et al. The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility. Science 2005;307:1434–1440 [DOI] [PubMed] [Google Scholar]
- 8.He W, Neil S, Kulkarni H, et al. Duffy antigen receptor for chemokines mediates trans-infection of HIV-1 from red blood cells to target cells and affects HIV-AIDS susceptibility. Cell Host Microbe 2008;4:52–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez E, Dhanda R, Bamshad M, et al. Global survey of genetic variation in CCR5, RANTES, and MIP-1 alpha: impact on the epidemiology of the HIV-1 pandemic. Proc Natl Acad Sci USA 2001;98:5199–5204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wawer MJ, Sewankambo NK, Serwadda D, et al. Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomised community trial. Lancet 1999;353:525–535 [DOI] [PubMed] [Google Scholar]
- 11.Celum C. HPTN 039: a phase III, randomized, double-blind, placebo-controlled trial of acyclovir for the reduction of HIV acquisition among high-risk HSV-2 seropositive, HIV seronegative persons. Paper presented at: 15th Conference on Retrovirology and Opportunistic Infections, February 2008, Boston, MA [Google Scholar]
- 12.Hallfors DD, Iritani BJ, Miller WC, Bauer DJ. Sexual and drug behavior patterns and HIV and STD racial disparities: the need for new directions. Am J Public Health 2007;97:125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris M, Kretzschmar M. Concurrent partnerships and transmission dynamics in networks. Soc Networks 1995;17:299–318 [Google Scholar]
- 14.Hudson C. Concurrent partnerships could cause AIDS epidemics. Int J STD AIDS 1993;4:349–353 [DOI] [PubMed] [Google Scholar]
- 15.Watts CH, May RM. The influence of concurrent partnerships on the dynamics of HIV/AIDS. Math Biosci 1992;108:89–104 [DOI] [PubMed] [Google Scholar]
- 16.Moody J. The importance of relationship timing for diffusion. Soc Forces 2002;81:25–56 [Google Scholar]
- 17.Morris M, Goodreau S, Moody J. Sexual networks, concurrency, and STD/HIV. In: Holmes KK, Sparling PF, Stamm WE, et al.eds Sexually Transmitted Diseases. 4th ed.New York, NY: McGraw-Hill International Book Co; 2007:109–126 [Google Scholar]
- 18.Adimora AA, Schoenbach VJ. Social context, sexual networks, and racial disparities in rates of sexually transmitted infections. J Infect Dis 2005;191:S115–S122 [DOI] [PubMed] [Google Scholar]
- 19.Adimora AA, Schoenbach VJ, Martinson FEA, et al. Heterosexually transmitted HIV infection among African Americans in North Carolina. J Acquir Immune Defic Syndr 2006;41:616–623 [DOI] [PubMed] [Google Scholar]
- 20.Laumann E, Youm Y. Racial/ethnic group differences in the prevalence of sexually transmitted diseases in the United States: a network explanation. Sex Transm Dis 1999;26:250–261 [DOI] [PubMed] [Google Scholar]
- 21.Doherty IA, Shiboski S, Ellen JM, Adimora AA, Padian NS. Sexual bridging socially and over time: a simulation model exploring the relative effects of mixing and concurrency on viral sexually transmitted infection transmission. Sex Transm Dis 2006;33:368–373 [DOI] [PubMed] [Google Scholar]
- 22.Laumann E, Gagnon J, Michael R, Michaels S. The Social Organization of Sexuality. Chicago, IL: University of Chicago Press; 1994 [Google Scholar]
- 23.Tanfer K. National Survey of Men—design and execution. Fam Plann Perspect 1993;25:83–86 [PubMed] [Google Scholar]
- 24.Tanfer K. The 1991 National Survey of Women: A User's Guide to the Machine-Readable Files and Documentation. Los Angeles, CA: Sociometrics Corp; 1995 [Google Scholar]
- 25.National Survey of Family Growth, Cycle 6. Hyattsville, MD: National Center for Health Statistics; 2002 [Google Scholar]
- 26.Morris M. A log-linear modeling framework for selective mixing. Math Biosci 1991;107:349–377 [DOI] [PubMed] [Google Scholar]
- 27.Handcock MS, Hunter DR, Butts CT, Goodreau SM, Morris M. statnet: Software Tools for the Statistical Modeling of Network Data. Seattle, WA: Statnet Project; 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Handcock MS, Hunter DR, Butts CT, Goodreau SM, Morris M. statnet: software tools for the representation, visualization, analysis and simulation of network data. J Stat Software. 2008;24 Available at: http://www.jstatsoft.org/v24/i01/.42(i01). Accessed October 6, 2008 [DOI] [PMC free article] [PubMed]
- 29.Epstein H. The Invisible Cure: Africa, the West, and the Fight Against AIDS. New York, NY: Farrar, Straus & Giroux; 2007 [Google Scholar]
- 30.Bessinger R, Akwara P, Halperin D. Sexual Behavior, HIV and Fertility Trends: A Comparative Analysis of Six Countries. Phase I of the ABC Study. Washington, DC: US Agency for International Development; 2003 [Google Scholar]
- 31.Murphy EM, Greene ME, Mihailovic A, Olupot-Olupot P. Was the “ABC” approach (abstinence, being faithful, using condoms) responsible for Uganda's decline in HIV? PLoS Med 2006;3:e379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stoneburner RL, Low-Beer D. Population-level HIV declines and behavioral risk avoidance in Uganda. Science 2004;304:714–718 [DOI] [PubMed] [Google Scholar]
- 33.Morris M, Kretzschmar M. Concurrent partnerships and the spread of HIV. AIDS 1997;11:641–648 [DOI] [PubMed] [Google Scholar]
- 34.Halperin DT, Epstein H. Concurrent sexual partnerships help to explain Africa's high HIV prevalence: implications for prevention. Lancet 2004;364:4–6 [DOI] [PubMed] [Google Scholar]
- 35.Koumans E, Farely T, Gibson J. Characteristics of persons with syphilis in areas of persisting syphilis in the United States: sustained transmission associated with concurrent partnerships. Sex Transm Dis 2001;28:497–503 [DOI] [PubMed] [Google Scholar]
- 36.Potterat J, Zimmerman-Rogers H, Muth S, et al. Chlamydia transmission: concurrency, reproduction number, and the epidemic trajectory. Am J Epidemiol 1999;150:1331–1339 [DOI] [PubMed] [Google Scholar]
- 37.Morris M. Concurrent partnerships and syphilis persistence: new thoughts on an old puzzle. Sex Transm Dis 2001;28:504–507 [DOI] [PubMed] [Google Scholar]
- 38.Michaels S. Integrating quantitative and qualitative methods in the study of sexuality. In: Bancroft J, ed Researching Sexual Behavior. Bloomington, IN: Indiana University Press; 1997:299–308 [Google Scholar]
- 39.De P, Cox J, Boivin JF, Platt RW, Jolly AM. Social network-related risk factors for bloodborne virus infections among injection drug users receiving syringes through secondary exchange. J Urban Health 2008;85:77–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Latkin CA, Knowlton AR. Micro-social structural approaches to HIV prevention: a social ecological perspective. AIDS Care 2005;17(suppl 1):S102–S113 [DOI] [PubMed] [Google Scholar]
- 41.Neaigus A. The network approach and interventions to prevent HIV among injection drug users. Public Health Rep 1998;113(suppl 1):140–150 [PMC free article] [PubMed] [Google Scholar]
- 42.Kelly JA, Amirkhanian YA, Kabakchieva E, et al. Prevention of HIV and sexually transmitted diseases in high risk social networks of young Roma (Gypsy) men in Bulgaria: randomised controlled trial. BMJ 2006;333:1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amirkhanian YA, Kelly JA, Kabakchieva E, et al. A randomized social network HIV prevention trial with young men who have sex with men in Russia and Bulgaria. AIDS 2005;19:1897–1905 [DOI] [PubMed] [Google Scholar]
- 44.Doherty IA, Minnis A, Auerswald CL, Adimora AA, Padian NS. Concurrent partnerships among adolescents in a Latino community: the Mission District of San Francisco, California. Sex Transm Dis 2007;34:437–443 [DOI] [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention Use of social networks to identify persons with undiagnosed HIV infection—seven U.S. cities, October 2003–September 2004. MMWR Morb Mortal Wkly Rep 2005;54:601–605 [PubMed] [Google Scholar]
- 46.Kraut-Becher J, Eisenberg M, Voytek C, Brown T, Metzger DS, Aral S. Examining racial disparities in HIV—lessons from sexually transmitted infections research. J Acquir Immune Defic Syndr 2008;47:S20–S27 [DOI] [PubMed] [Google Scholar]
- 47.HIV/AIDS Surveillance Report, 2006. Atlanta, GA: Centers for Disease Control and Prevention; 2007 [Google Scholar]
- 48.Community-Campus Partnerships for Health Principles of good community-campus partnerships. Available at: http://depts.washington.edu/ccph/principles.html. Accessed October 15, 2008
- 49.O'Fallon L, Tyson F, eds Successful Models of Community Based Participatory Research. Research Triangle Park, NC: National Institute of Environmental Health Sciences; 2000 [Google Scholar]
- 50.Sexually Transmitted Disease Surveillance, 2004. Atlanta, GA: Centers for Disease Control and Prevention; 2005 [Google Scholar]
- 51.US Dept of Health and Human Services Bridged-race population estimates, United States July 1 resident population by state, county, age, sex, bridged-race, and Hispanic origin, compiled from 1990–1999 bridged-race intercensal population estimates and 2000–2004 (Vintage 2004) bridged-race postcensal population estimates [CDC WONDER online database]. Available at: http://wonder.cdc.gov. Accessed September 8, 2008