Abstract
Objectives. We evaluated the use of social networks to reach persons with undiagnosed HIV infection in ethnic minority communities and link them to medical care and HIV prevention services.
Methods. Nine community-based organizations in 7 cities received funding from the Centers for Disease Control and Prevention to enlist HIV-positive persons to refer others from their social, sexual, or drug-using networks for HIV testing; to provide HIV counseling, testing, and referral services; and to link HIV-positive and high-risk HIV-negative persons to appropriate medical care and prevention services.
Results. From October 1, 2003, to December 31, 2005, 422 recruiters referred 3172 of their peers for HIV services, of whom 177 were determined to be HIV positive; 63% of those who were HIV-positive were successfully linked to medical care and prevention services. The HIV prevalence of 5.6% among those recruited in this project was significantly higher than the approximately 1% identified in other counseling, testing, and referral sites funded by the Centers for Disease Control and Prevention.
Conclusions. This peer-driven approach is highly effective and can help programs identify persons with undiagnosed HIV infection in high-risk networks.
Approximately 1.0 to 1.2 million persons are living with HIV in the United States, of whom approximately one quarter (252 000–312 000) are unaware of their HIV infection and are at risk for transmitting it to others.1 HIV-positive persons who are unaware of their infection are believed to be the source of more than half of all new infections.2 The prevalence of high-risk sexual behavior is markedly higher among HIV-positive persons who are unaware of their infection than among those who know their status.3 Furthermore, many persons are diagnosed late in the course of their infection. Of all persons diagnosed with HIV infections in 2004 in the 33 states that had name-based HIV reporting, 40% developed AIDS within 12 months after being diagnosed with HIV.4 The Centers for Disease Control and Prevention (CDC) estimates that only approximately 50% of persons with newly diagnosed HIV are linked to care within 12 months of their initial diagnosis.5 Therefore, identifying HIV-positive persons who are unaware of their infection as early as possible and linking them to medical care and prevention services are national HIV prevention priorities.6,7
Surveillance data show that HIV/AIDS diagnoses are increasing in some groups, such as African American men who have sex with men (MSM),8 and new HIV incidence estimates show that gay and bisexual men of all races and ethnicities and African American men and women are disproportionately affected by HIV.9 Reaching these high-risk groups is essential to stemming the HIV epidemic.
The CDC funds health departments and community-based organizations (CBOs) to conduct HIV counseling, testing, and referral services in sexually transmitted disease clinics; free-standing counseling, testing, and referral sites; community health centers; family planning clinics; correctional facilities; and outreach settings (e.g., mobile vans). Of the approximately 2 million HIV tests performed at these sites each year, an average of 1.3% are positive.10 It is critical that effective strategies be identified and implemented to reach more persons at high risk for HIV.
One such strategy is HIV partner counseling and referral services. Partner counseling and referral services incorporates a partner notification process in which HIV-infected persons are asked for information about their sexual and drug-injection partners, who are then notified of their potential exposure to HIV and provided HIV counseling, testing, and referral services.11 Partner counseling and referral services has been proven effective for reaching persons at very high risk; in studies of HIV partner counseling and referral services, 14% to 26% of tested partners of HIV-infected persons were found to have undiagnosed HIV infections.12 However, a recent survey of health departments in areas with high rates of HIV (and HIV reporting) found that only 52% of HIV-infected persons were interviewed for partner counseling and referral services,4 indicating significant missed opportunities for HIV prevention.
Another strategy that has been explored for reaching individuals at risk for HIV infection is social networks. Persons who are HIV infected or at high risk for infection are asked to identify others in their social networks who they believe may be at risk for infection for referral to HIV counseling, testing, and referral services. These network members, whether they test positive or negative for HIV, are in turn asked to identify other persons they believe to be at risk. This process typically continues for several cycles beyond the original person, facilitating access to the social networks of persons at high risk for infection.
Social network strategies have been used successfully by health departments to identify undiagnosed cases of syphilis and HIV. A pilot study in Atlanta, Georgia, that used social network methods to focus on syphilis also identified 24 persons with HIV, of whom 7 were unaware of their HIV infection and 9 were coinfected with syphilis.13 In 1998, Jordan et al. used a social network strategy in a community-based setting to recruit HIV-positive gay men to identify persons in their social networks who might be HIV positive and not receiving care (including those unaware of their infection).14 The high rate of positive tests among the referred network members (38%) supports the value of this strategy for accessing persons at very high risk.
In 2003, the CDC funded 9 CBOs to implement social network strategies as a method for recruiting high-risk persons for HIV counseling, testing, and referral services. Evaluation of this program provided an opportunity to assess the effectiveness of social network strategies in multiple settings among populations with high rates of undiagnosed HIV infection. Preliminary results of the evaluation have been published elsewhere.15 We present a more detailed analysis of the data collected for monitoring and evaluating the program.
METHODS
Nine CBOs were funded in 7 US cities: San Francisco, California; Washington, DC; Orlando, Florida; Lafayette, Louisiana; Boston, Massachusetts; New York, New York; and Philadelphia, Pennsylvania. The CBOs ranged in size from small to large and were located in urban and rural areas. Each CBO selected 1 or more specific populations for this program, including, but not limited to, African American and Hispanic men and women, homeless persons, MSM, high-risk heterosexuals, transgender persons, and injection drug users.
Program Design
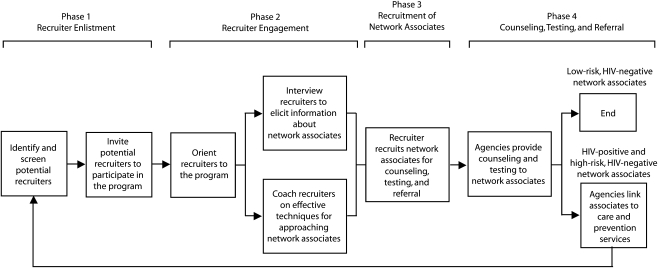
The CBOs started with a common protocol16 that included 4 core program phases (Figure 1), which they adapted to their specific target populations and circumstances.
FIGURE 1.
Social networks strategy flow diagram: Social Networks Demonstration Project, October 2003–December 2005.
Phase 1: recruiter enlistment.
CBO staff invited HIV-positive persons (recruiters) to contact persons they personally knew (network associates) and believed to be at high risk for HIV infection from their social, sexual, or drug-using networks and encourage them to be tested for HIV. The recruiters were receiving other services (e.g., case management) from the participating organizations or were referred by other agencies providing services to HIV-positive persons. Subsequently, persons who were recruited as network associates and were found to be either HIV positive or HIV negative but at high risk of infection were invited to participate as recruiters. HIV-negative persons were considered to be at high risk for HIV infection if within the preceding 12 months they (1) had unprotected sexual intercourse or shared drug injection equipment with a person who was HIV positive or of unknown HIV status, (2) had exchanged sexual intercourse for drugs or money, or (3) were diagnosed with another sexually transmitted disease.
Recruiters were 18 years or older; HIV positive or high-risk HIV negative; largely African American, Hispanic, Native Hawaiian/Pacific Islander, Asian, or American Indian/Alaska Native; and willing and able to refer high-risk persons from their social, sexual, or drug-using networks to HIV counseling, testing, and referral services. Potential recruiters were excluded if they were not deemed mentally capable or were under the influence of alcohol or other drugs at the time they were considered for participation. CBOs used other inclusion or exclusion criteria appropriate for their particular circumstances.
Phase 2: recruiter engagement.
Recruiters were given a brief orientation to the program, provided additional information about their role, and informed that they could decline further participation at any time. Some CBOs obtained written acknowledgment from the recruiters about the scope and limits of their participation. Recruiters were then interviewed by CBO staff about network associates they believed to be at high risk for HIV infection. They were also coached, through role-play techniques, on how to approach network associates and refer them for HIV counseling, testing, and referral services.
Phase 3: recruitment of network associates.
Recruiters contacted their individual network associates to refer them for testing. The dialogue between recruiters and network associates varied by the nature of their relationships, but all included discussions about the importance of HIV testing. When network associates agreed to be tested, their recruiter gave them a coded referral card for counseling, testing, and referral services or personally escorted them to the HIV counseling, testing, and referral sites, allowing CBO staff to link the network associates to their recruiters. CBO staff followed up with recruiters periodically, in person or by telephone, to review their progress in contacting network associates and discuss any issues that concerned them. All recruiters were enlisted and all network associates were tested between October 1, 2003, and December 31, 2005.
Phase 4: counseling, testing, and referral.
Network associates were offered HIV testing either at the agency facility or in community settings. Sometimes, a collaborating agency provided these services. Written consent was obtained from network associates who chose to be tested. Tests were performed on oral fluid or blood specimens with either standard or rapid HIV tests. Reactive rapid tests were confirmed by Western blot. Network associates were encouraged by CBO staff to return for follow-up testing if they tested HIV negative but were assessed as high risk.
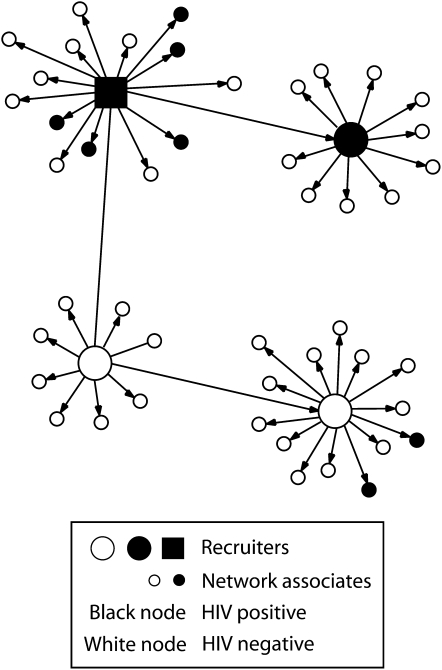
Network associates who tested HIV positive were provided posttest counseling and linked or referred to appropriate medical care and prevention services, including partner counseling and referral services. Linkage to care was considered successfully completed if the network associate attended at least 1 medical appointment. CBOs established tracking systems to verify completion of medical referrals or accompanied individuals to these services. Network associates found to be HIV negative but at high risk were referred to HIV prevention services. HIV-positive and high-risk HIV-negative network associates were assessed for eligibility to become recruiters and invited to participate. This process allowed CBO staff to continue testing and penetrating high-risk networks (Figure 2).
FIGURE 2.
Example of a social network of an HIV-positive recruiter and his network associates: Social Networks Demonstration Project, October 2003–December 2005.
Decisions about the use of incentives were left to the discretion of the individual CBOs. Ultimately, each agency elected to give recruiters and network associates small incentives (e.g., gift cards, certificates of appreciation, or transportation passes) for their participation. One CBO provided cash incentives drawn from other sources of funding. Typically, recruiters received incentives valued at approximately $10.00 for every network associate they successfully recruited for counseling, testing, and referral services, and network associates received incentives valued at approximately $5.00 if they were tested and returned for their results.
CDC staff trained CBO staff and provided intensive technical assistance on-site and by telephone. Staff from all CBOs participated in periodic conference calls to share information about their programs, discuss issues they were encountering, and receive peer support.
Program Evaluation
Data collection and measures.
Data collected to monitor and evaluate the program included demographic information (e.g., age, race/ethnicity, gender), behavioral risk factors (e.g., needle sharing, unprotected sexual intercourse), HIV testing history, and current HIV status. After all personal identifiers were removed, data were electronically submitted to the CDC for analysis via a secure data network in encrypted form. In the event an individual tested more than once during the course of the project, the most recent test result was used in analysis.
Network size was calculated as the number of network associates tested in a recruiter's network. A network index (number of network associates tested divided by number of recruiters) was computed to measure the productivity of recruiters with different demographic and behavioral risk characteristics. The prevalence of newly identified HIV infection among all network associates (the number of newly identified HIV-positive network associates divided by the number of network associates tested) and the number of HIV-positive network associates who accessed medical care following their diagnosis were also calculated.
Data analysis.
We used the Kruskal–Wallis χ2 test (a nonparametric test appropriate for nonnormal distributions) to examine differences in number of network associates tested, by recruiter characteristics. We used the Pearson χ2 test to test associations between the prevalence of newly identified HIV infections among network associates and the characteristics of both recruiters and network associates. When more than 2 groups were compared, we conducted pairwise tests for associations significant at or below the .05 level to determine which groups differed significantly from one another.
RESULTS
Overall, the 9 CBOs enlisted 424 recruiters and tested 3230 network associates. Data from 2 recruiters were excluded because 1 had unknown HIV status and the other was younger than 18 years. A total of 58 network associates were excluded because their recruiter's HIV status was unknown (n = 7), they were recruited erroneously (i.e., they already knew they were HIV positive; n = 50), or their test results were missing (n = 1). Data from 422 recruiters and 3172 network associates were included for analysis.
Recruiter Characteristics and Productivity
Mean recruiter age was 41.7 years (SD = 8.8; range = 18–71). Sixty percent were HIV positive, 63% were male, 82% were 35 years or older, and 61% were African American. Forty-three percent of recruiters initially entered the program as network associates recruited by others. The most frequent self-reported behavioral risk factor was high-risk heterosexual intercourse (46%). The overall network index (number of network associates recruited and tested per recruiter) was 7.4.
The number of network associates tested was significantly associated with recruiter HIV serostatus (P < .01), age group (P = .02), race/ethnicity (P = .02), and risk group (P < .01; Table 1). HIV-negative recruiters recruited significantly more network associates for HIV testing (network index = 9.4) than did HIV-positive recruiters (network index = 6.2). Recruiters aged 25 to 34 years recruited significantly more people on average (network index = 16.9) than did recruiters aged 18 to 24 years (network index = 7.0) or 45 years and older (network index = 5.8). Hispanic recruiters recruited significantly more network associates for testing on average (network index = 13.1) than did African American or White recruiters (network indices = 6.1 and 3.5, respectively). Recruiter gender was not significantly associated with number of network associates tested.
TABLE 1.
Network Index and Prevalence of Newly Identified HIV Infections Among Network Associates in 7 US Cities, by Recruiter Characteristics: Social Networks Demonstration Project, October 2003–December 2005
| Recruiter Characteristic | Recruiters, No. (%) | Network Indexa | Pb | HIV Prevalence,c % (95% CI) | Pd |
| HIV serostatus | <.01 | <.01 | |||
| Positive | 255 (60) | 6.2 | 6.8 (5.6, 8.2) | ||
| Negative | 167 (40) | 9.4 | 4.4 (3.4, 5.5) | ||
| Gender | .63 | .26 | |||
| Men | 265 (63) | 8.4 | 5.8 (4.9, 6.9) | ||
| Women | 128 (30) | 6.1 | 4.7 (3.4, 6.5) | ||
| Transgender | 29 (7) | 4.8 | 7.9 (4.0, 13.7) | ||
| Age, y | .02 | .34 | |||
| 18–24 | 24 (6) | 7.0 | 3.0 (1.0, 6.8) | ||
| 25–34 | 50 (12) | 16.9 | 5.2 (3.8, 6.9) | ||
| 35–44 | 180 (42) | 6.4 | 6.3 (5.0, 7.9) | ||
| ≥ 45 | 168 (40) | 5.8 | 5.7 (4.4, 7.4) | ||
| Race/ethnicity | .02 | .14 | |||
| African American | 257 (61) | 6.1 | 6.4 (5.2, 7.7) | ||
| Hispanic | 99 (23) | 13.1 | 5.2 (4.0, 6.5) | ||
| White | 50 (12) | 3.5 | 3.4 (1.3, 7.3) | ||
| Othere | 16 (4) | 6.4 | 2.9 (0.6, 8.4) | ||
| Risk group | <.01 | <.01 | |||
| High-risk heterosexual | 193 (46) | 7.5 | 5.0 (3.9, 6.2) | ||
| IDU | 55 (13) | 7.7 | 6.3 (4.2, 9.1) | ||
| MSM | 92 (22) | 5.8 | 8.7 (6.4, 11.3) | ||
| MSM and IDU | 58 (14) | 11.6 | 4.0 (2.7, 5.8) | ||
| Risk not specified | 24 (5) | 2.9 | 7.2 (2.4, 15.9) | ||
| All recruiters | 422 (100) | 7.4 | 5.6 (4.8, 6.5) |
Note. CI = confidence interval; MSM = men who have sex with men; IDU = injection drug user. Twenty-six network associates (persons recruited from social networks of recruiters) could not be linked to a specific recruiter. There were no newly identified HIV infections among these 26 network associates.
The index is the number of network associates tested divided by number of recruiters.
Calculated from the Kruskal–Wallis nonparametric χ2 test for associations between recruiter characteristics and number of network associates tested. Recruiters whose race or risk were not specified were not included in the statistical tests.
This is the prevalence of newly identified HIV infections among network associates (number of network associates with newly identified HIV infection divided by number of network associates tested).
Calculated from the Pearson χ2 test for associations between recruiter characteristics and number of new HIV-positive network associates identified.
Included American Indian/Alaska Native, 8 (2%); Asian, 2 (0.4%); Native Hawaiian/Pacific Islander, 2 (0.5%); and multiple/unknown race, 4 (1%).
Recruiter HIV serostatus (P < .01) and recruiter risk group (P < .01) were significantly associated with HIV prevalence among network associates (Table 1). The prevalence of newly identified HIV infections among network associates recruited by HIV-positive recruiters (6.8%) was significantly greater than among network associates recruited by HIV-negative recruiters (4.4%) and was significantly greater among network associates recruited by MSM recruiters (8.7%) than among those recruited by high-risk heterosexuals (5.0%) or by MSM who were also injection drug users (4.0%). Recruiter gender, age, and race/ethnicity were not significantly associated with prevalence of newly diagnosed HIV infection among network associates.
Almost all of the 3172 network associates (99%) could be linked to a specific recruiter; all of the newly identified HIV-positive network associates were linked. Approximately one third of the recruiters (32%) recruited 5 or more network associates, accounting for 91% of all linkable network associates and 88% of newly identified HIV-positive network associates. Thirty-four percent of recruiters did not recruit any network associates for testing. The average number of network associates tested per recruiter was 7.4; on average, only 2.4 recruiters were needed to test 17.8 network associates and find 1 with newly identified HIV infection (422 recruiters divided by 177 newly identified HIV-positive network associates = 2.4).
Network Associate Characteristics and HIV Prevalence
Mean network associate age was 37.5 years (SD = 11.7; range = 13–77). (Seventy-five individuals aged 13 to 17 years received an HIV test because they were identified as at risk for HIV infection.) Of the 3172 network associates tested, 68% were male, 58% were 35 years or older, 51% were African American, and 57% self-identified as high-risk heterosexual.
Of the 3172 network associates recruited and tested, 177 (5.6%; 95% confidence interval = 4.8%, 6.4%) had newly identified HIV infection (Table 2). The prevalence of newly identified HIV infection among network associates was significantly associated with the associate's age (P = .03), race/ethnicity (P = .05), and risk group (P < .01). Network associates who were aged 35 to 44 years were significantly more likely to test HIV positive (7.4%; P = .005) than were those in all other age groups (4.8%). A significantly greater proportion of African American than White network associates tested HIV positive (6.5% and 3.8%, respectively; P = .005). Significantly greater proportions of network associates who were MSM (12.4%) and MSM injection drug users (14.9%) than who were injection drug users (5.7%) or high-risk heterosexuals (4.4%) tested HIV positive (P < .001). Network associate gender was not significantly associated with prevalence of newly identified HIV infection, but the prevalence was highest among transgendered persons (12.8%).
TABLE 2.
Network Associate Test Results and Prevalence of Newly Identified HIV Infections Among Network Associates in 7 US Cities, By Network Associate Characteristics: Social Networks Demonstration Project, October 2003–December 2005
| HIV Positive |
||||
| Network Associate Characteristic | HIV Negative No. (%) | No. (%) | Prevalencea (95% CI) | Pb |
| Gender | .09 | |||
| Men | 2028 (68) | 124 (70) | 5.8 (4.8, 6.8) | |
| Women | 929 (31) | 48 (27) | 4.9 (3.6, 6.5) | |
| Transgender | 34 (1) | 5 (3) | 12.8 (4.3, 27.4) | |
| Unknown | 4 (0.1) | 0 | 0 (0, 60.2) | |
| Age, y | .03 | |||
| 13–24 | 526 (18) | 22 (12) | 4.0 (2.5, 6.0) | |
| 25–34 | 709 (24) | 37 (21) | 5.0 (3.5, 6.8) | |
| 35–44 | 850 (28) | 68 (39) | 7.4 (5.8, 9.3) | |
| ≥ 45 | 908 (30) | 50 (28) | 5.2 (3.9, 6.8) | |
| Unknown | 2 (0.1) | 0 | 0 (0, 84.2) | |
| Race/ethnicity | .05 | |||
| African American | 1528 (51) | 107 (61) | 6.5 (5.4, 7.9) | |
| Hispanic | 952 (32) | 50 (28) | 5.0 (3.7, 6.5) | |
| White | 408 (14) | 16 (9) | 3.8 (2.2, 6.1) | |
| Otherc | 107 (3) | 4 (2) | 3.6 (1.0, 9.0) | |
| Risk group | <.01 | |||
| High-risk heterosexual | 1718 (57) | 79 (45) | 4.4 (3.5, 5.5) | |
| IDU | 526 (18) | 32 (18) | 5.7 (4.0, 8.0) | |
| MSM | 269 (9) | 38 (22) | 12.4 (8.9, 16.6) | |
| MSM and IDU | 114 (4) | 20 (11) | 14.9 (9.4, 22.1) | |
| Risk not specified | 368 (12) | 8 (5) | 2.1 (0.9, 4.2) | |
| Total | 2995 (100) | 177 (100) | 5.6 (4.8, 6.4) | |
Note. CI = confidence interval; MSM = men who have sex with men; IDU = injection drug user. Network associates were persons recruited from social networks of recruiters.
This is the prevalence of newly identified HIV infections among network associates (number of network associates with newly identified HIV infection divided by number of network associates tested).
Calculated from the Pearson χ2 test for associations between network associate characteristics and number of new HIV-positive network associates identified. Individuals with unknown gender, unknown age, other race/ethnicity, or risk not specified were not included in the statistical tests.
Included American Indian/Alaska Native, 32 (29%); Asian, 19 (17%); Native Hawaiian/Pacific Islander, 10 (9%); and multiple/unknown race, 50 (45%).
Linkage to Care
Of the 177 network associates identified as HIV positive, 151 (85%) were referred for HIV medical care or case management by program staff. (For the remaining 26 individuals, referrals were not made or the data were missing.) Of network associates who were given such referrals, 111 (74%) successfully accessed those services. Of the 40 individuals who received referrals but did not successfully access services, 29 were lost to follow-up and 11 declined the referrals. Thus, 63% (111 out of 177) of all newly identified HIV-positive individuals reached through this strategy successfully accessed HIV medical care or case management services.
DISCUSSION
Our program evaluation showed that the participating CBOs effectively used a social network strategy to access and provide HIV testing to a population of persons with a high prevalence of undiagnosed HIV infection. More than half of those who were newly identified as HIV positive were subsequently linked to HIV medical care and prevention services. The CBOs were able to use the social network strategy successfully to reach diverse at-risk populations in 7 different geographic areas. These findings support those of earlier reports from Los Angeles, California,14 and Seattle, Washington,17 and show that a peer-driven, network-oriented strategy can be a powerful tool for identifying persons with undiagnosed HIV infection in populations that may be difficult to reach with other methods.
The proportion of network associates with newly identified HIV infection in the Social Networks Demonstration Project (5.6%) was similar to that reported from Seattle (5.0%).17 However, the case-finding yield in the program we studied was higher because fewer recruiters were needed to identify 1 network associate with previously undiagnosed HIV infection (2.4 in this program compared with 12.9 in the Seattle program). The reason for this difference is not readily apparent. Both programs worked closely with the recruiters, and the incentives offered to recruiters and network associates were similar. However, recruiters in the Seattle program were all MSM and most were White, whereas the majority of recruiters in the program we studied were high-risk heterosexual and African American. Also, this program was conducted entirely by CBOs, whereas the Seattle program was primarily under the direction of the health department.
The case-finding effectiveness of the CBOs in this program also exceeded that of HIV partner counseling and referral services in health departments. A recent systematic review of partner counseling and referral services identified 6 reports that met strict inclusion criteria and provided data regarding number of index cases, number of partners elicited, and number of new HIV cases identified.12 Data from these studies suggest that, on average, approximately 9.1 index patients were interviewed to identify 1 new HIV case. By comparison, in this program, enlistment of just 2.4 recruiters resulted in the detection of 1 person with newly identified HIV infection. Furthermore, recruiters in the program themselves contacted and referred the network associates, whereas in partner counseling and referral services, this work is usually conducted by health department personnel. Therefore, considerably less staff time was needed to bring network associates to testing than is typically needed to bring partners to testing in partner counseling and referral services. This suggests that a social network strategy may be useful in aiding CBOs and health departments to work together to identify populations of persons with undiagnosed HIV infection and may be an efficient adjunct to traditional partner notification methods.
In 2004, nearly 2 million HIV tests were performed in CDC-supported sites. Slightly more than 1% were positive.10 Positive test rates were highest in community health centers and public health clinics (2.4%); medical care settings (2.2%); HIV counseling, testing, and referral sites (1.8%); and field visits (1.8%). The 5.6% prevalence found in this program was 5 times the average prevalence found in CDC-supported sites and more than twice as high as in the settings with the highest prevalence. Furthermore, although the 9 CBOs tested a relatively low number of persons during the 2 years of this program (average of 352 persons each), their yield of persons with newly identified HIV infection was relatively high (average of 20 persons each during the 2 years). Therefore, the use of a social network strategy may be useful for counseling, testing, and referral programs that are not achieving optimal results with more traditional approaches.
Our evaluation had several limitations. It was designed as a program evaluation, not research; there were no control groups, and it is not possible to make direct comparisons with other recruitment strategies. Furthermore, the CBOs received extensive training and technical assistance throughout the course of the program. It is unclear whether their success would be sustained, or replicated by other CBOs, without equally intensive, ongoing support.
Finally, confirming the linkage of HIV-positive clients to medical care was limited by lack of existing infrastructure and systems for tracking referrals. Thus, the proportion of persons with newly identified HIV infection who were successfully linked to such services in this program may be underestimated.
The social network strategy is proving to be an efficient, high-yield approach to accessing and providing HIV counseling, testing, and referral services to high-risk populations that may be difficult to reach with other strategies. This is a significant public health achievement, because persons who learn that they are HIV positive tend to reduce their high-risk behaviors to avoid infecting others and have the opportunity to access medical care and other services to improve their personal health.
Acknowledgments
This study was supported by the Centers for Disease Control and Prevention at 9 project sites (contract 2003-N-00895).
We thank the staff at the Social Networks project sites for successfully implementing the social networks strategy: Cicily Emerson, MSW, Continuum, San Francisco, CA; Ned Howey, Tenderloin AIDS Resource Center, San Francisco, CA; Sean Illemsky, Whitman Walker Clinic, Washington, DC; Laud Jean-Jacques, Center for Multicultural Wellness and Prevention, Orlando, FL; Robin Boyles, Southwest Louisiana Area Health Education Center, Lafayette, LA; Georgia Simpson, MA, Multicultural AIDS Coalition, Boston, MA; Nilda Carrasquillo, Latino Commission on AIDS, New York, NY; Daniel Daltry, MSW, ActionAIDS; and Waleska Maldonado, Congreso de Latino Unidos, Philadelphia, PA. We also thank Terry Wang, MSPH, Division of HIV/AIDS Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, for her expertise in database development for the project.
Human Participant Protection
Plans for monitoring and evaluating these programs were reviewed by human participant protection staff at the Centers for Disease Control and Prevention and were determined to be program evaluation activities but not research. However, all program and evaluation plans were reviewed by local review boards in accordance with local requirements.
References
- 1.Glynn K, Rhodes P. Estimated HIV prevalence in the United States at the end of 2003. Paper presented at: National HIV Prevention Conference; June 12–15, 2005; Atlanta, GA: Abstract T1-B1101 [Google Scholar]
- 2.Marks G, Crepaz N, Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware they are infected with the virus in the United States. AIDS 2006;20(10):1447–1450 [DOI] [PubMed] [Google Scholar]
- 3.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr 2005;39:446–453 [DOI] [PubMed] [Google Scholar]
- 4.Golden MR, Hodgen M, Potterat JJ, Handsfield HH. HIV partner notification in the United States: a national survey of program coverage and outcomes. Sex Transm Dis 2004;31:709–712 [DOI] [PubMed] [Google Scholar]
- 5.Glynn K, Harris N. Estimates of out of care: background and methods for CDC estimate. Presented at: HRSA Engaging in Care Consultation; April 27, 2005Washington, DC [Google Scholar]
- 6.Centers for Disease Control and Prevention Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Morb Mortal Wkly Rep 2006;55:1–17 [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Advancing HIV prevention: new strategies for a changing epidemic—United States, 2003. MMWR Morb Mortal Wkly Rep 2003;52:329–332 [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention Trends in HIV/AIDS diagnoses among men who have sex with men—33 States, 2001—2006. MMWR Morb Mortal Wkly Rep 2008;57(25):681–686 [PubMed] [Google Scholar]
- 9.Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the Unites States. JAMA 2008;300(5):520–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention HIV counseling and testing at CDC-supported sites: 1999–2004. 2006. Available at: http://www.cdc.gov/hiv/topics/testing/reports.htm. Accessed April 16, 2009
- 11.Centers for Disease Control and Prevention HIV Partner Counseling and Referral Services Guidelines. Atlanta, GA: US Dept of Health and Human Services; 1998 [Google Scholar]
- 12.Hogben M, McNally T, McPheeters M, Hutchinson AB. The effectiveness of HIV partner counseling and referral services in increasing identification of HIV-positive individuals: a systematic review. Am J Prev Med 2007;33(2 suppl):S89–S100 [DOI] [PubMed] [Google Scholar]
- 13.Rothenberg R, Kimbrough L, Heath R. Social networks methods for endemic foci of syphilis: a pilot project. Sex Transm Dis 2000;27:12–18 [DOI] [PubMed] [Google Scholar]
- 14.Jordan WC, Tolbert L, Smith R. Partner notification and focused interventions as a means of identifying HIV-positive patients. J Natl Med Assoc 1998;90:542–546 [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention Use of social networks to identify persons with undiagnosed HIV infection—seven US cities, October 2003–September 2004. MMWR Morb Mortal Wkly Rep 2005;54:601–605 [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention Social Networks Testing. A Community-Based Strategy for Identifying Persons with Undiagnosed HIV Infection. Interim Guide for HIV Counseling, Testing, and Referral Programs. Available at http://www.cdc.gov/Hiv/resources/guidelines/snt/index.htm. Accessed November 18, 2008
- 17.Golden MR, Gift TL, Brewer DD, et al. Peer referral for HIV case-finding among men who have sex with men. AIDS 2006;20:1961–1968 [DOI] [PubMed] [Google Scholar]