Abstract
Objectives. We examined the efficacy of the Healthy Living Program in reducing risky sexual behavior and substance use among adults with HIV infection who were marginally housed (i.e., homeless at some point over a 37-month period).
Methods. We had previously conducted a randomized controlled trial with 936 adults living with HIV infection. In that study, 3 intervention modules of 5 sessions each addressed different goals: reducing risky sexual acts and drug use, improving the quality of life, and adhering to healthful behaviors. Participants were interviewed at baseline and at 5, 10, 15, 20, and 25 months; 746 completed 4 or more assessments. In this study, we analyzed sexual behavior and drug use outcomes for the 35% (n = 270 of 767) of participants who were considered marginally housed.
Results. Among the marginally housed participants, there were significantly greater reductions in unprotected risky sexual acts, the number of sexual partners of HIV negative or unknown serostatus, alcohol or marijuana use, and hard drug use among the intervention group than among the control group.
Conclusions. Intensive, skill-focused intervention programs may improve the lives of marginally housed adults living with HIV infection.
The rate of HIV infection among homeless adults in the United States ranges between 10.5% and 21%,1–3 a rate 20 to 40 times higher than that among nonhomeless adults. Risky sexual acts are often associated with alcohol and drug use among the homeless, as they are among housed adults.4–7 Injection drug users who are marginally housed (i.e., homeless at some time during the past 37 months) use shooting galleries and perform sex work twice as often as stably housed injection drug users.7 Given these heightened risks, it is critical that HIV-positive homeless and marginally housed adults receive interventions to reduce HIV transmission.
Efficacious HIV prevention programs are available for homeless persons of unknown serostatus8–12; however, no program has targeted adults living with HIV. Building on other prevention programs for HIV-positive persons13–16 and informed by extensive preliminary qualitative work,17 the National Institute of Mental Health's Healthy Living Project was designed for adults living with HIV infection who continued to engage in risky sexual acts after learning their serostatus.18 In previous analyses of this intervention, exposure to the Healthy Living Project helped adults living with HIV to significantly reduce their numbers of HIV-negative partners or partners of unknown serostatus, increase their condom use, and reduce substance abuse.19,20 We analyzed the impact of the Healthy Living Project on a subsample of marginally housed adults living with HIV.
Surviving while homeless is an all-consuming process, and the resources available to alter habitual sexual and drug behaviors are scarce. Under these stressful conditions, it was unclear whether a behavioral intervention would influence the daily routines of those without stable housing.
We defined study participants who were homeless at any point over a period of 37 months as being marginally housed. Longitudinal studies have recently demonstrated that homelessness is usually sporadic.7,21 In our study, the participants moved in and out of homelessness; only 4 of them remained homeless over the course of the entire study. The primary transmission behaviors examined were risky sexual behavior and drug use.
METHODS
Identifying Marginally Housed Adults Living With HIV
The Healthy Living Project trial recruited participants from April 2000 to January 2002. It was conducted with 936 adults living with HIV who in the previous 3 months had engaged in unprotected sexual acts that increased the risk of transmission of HIV.19 They were recruited from 4 US cities (Milwaukee, WI; San Francisco, CA; New York, NY; and Los Angeles, CA) and were randomized to either the Healthy Living Project intervention or a control group. Participants were interviewed at baseline and at 5, 10, 15, 20, and 25 months.
The participants reported their current living situation at each assessment. In addition, at baseline and at the 15-, 20-, and 25-month assessments, they indicated whether they had been homeless at any time in the previous 12 months. It was difficult to determine exactly when an individual was homeless, because questions about current living situation did not request information about housing status between assessments. The 12-month recall period meant that reported homelessness could have occurred very recently or during a period covered by much earlier assessments (e.g., homelessness indicated at the 15-month assessment could have occurred in the previous week or 4 months after baseline), and that 1 bout of homelessness might be reported at multiple assessments (e.g., homelessness that occurred at month 14 might be reported at assessments 15, 20, and 25).
Because there was no annual retrospective question between the baseline and 15-month assessments, there was a 3-month gap in our ability to document homelessness (from baseline to 3 months later). To ensure adequate data to document homelessness, we excluded from the study any participants who had taken part in fewer than 4 of the 6 assessments. We defined an individual as marginally housed who (1) reported being homeless or living in a shelter or welfare hotel (hotel-like buildings that house people in single rooms but generally have shared bathrooms, kitchens, and living areas; welfare hotels are often frequented by homeless persons seeking marginal rehabilitation) at any assessment interview or (2) lived in a welfare hotel or reported being homeless during the past 12 months at the baseline, 15-month, 20-month, or 25-month assessment.
Study Design
All protocols, as well as a $50 incentive and $10 child care cost reimbursement per assessment, were approved by each site's institutional review board. Between April 2000 and January 2002, adults living with HIV were recruited for a baseline interview from community agencies, medical clinics, and advertisements targeting adults living with HIV. Eligible persons who agreed to participate were assigned to the intervention or control group according to a predetermined sequence of simple random-treatment assignment. Participants randomized to the control group did not receive the intervention modules but were assessed on the same timeline as the intervention group. At the completion of the study, individuals in the control group were given the opportunity to participate in a condensed version of the intervention.
The Healthy Living Project
The Healthy Living Project intervention comprised 3 modules, each consisting of five 90-minute individual counseling sessions. Module 1 (“Coping”), which was delivered between the baseline and 5-month assessments, addressed ways to cope with problems in health behaviors, access of health services, and negotiation of challenging interpersonal situations. Module 2 (“Act Safe”) took place between the 5- and 10-month assessments and focused on reducing risky sexual acts and substance use. Module 3 (“Stay Healthy”), delivered between the 10- and 15-month assessments, focused on maintaining healthful behaviors and improving the quality of life.
We used the following prevention case management approach to implement the intervention: screening, assessing, and developing a plan to improve health, reduce risky sexual acts and substance use, and improve the quality of life over time; monitoring progress over time; and teaching problem solving to implement consistent safe sexual practices and abstinence from drug use. Each session examined successes and problems that emerged in the past week regarding coping with one's HIV status, initiating assessment and problem-solving in a new domain, practicing implementation skills, and setting a goal for the next week.
Intervention sessions, which were audiotaped and rated for quality assurance, demonstrated high fidelity to the protocol over time.18,19 Supervisors and case managers from the 4 sites were centrally trained at 1-week training sessions for each of the 3 intervention modules. Modeling, practice sessions, certification, and ongoing monitoring for quality assurance were provided centrally and supplemented by on-site supervision.
Measures
A highly trained assessment team of diverse ethnicity, gender, and sexual orientation conducted interviews. Computerized interviews were used for data collection, with audio computer-assisted self-interviewing for measures of a sensitive or personal nature. Audio computer-assisted self-interviewing has been shown to enhance the veracity of self-reports of sensitive behaviors.22,23 Quality assurance ratings of 20% of all interviews indicated satisfactory (≥ 90%) adherence to assessment protocols.
Self-Reports
The participants reported recent sexual acts (i.e., in the previous 3 months) that involved risky sexual behavior and substance use.
Risky sexual behavior.
We defined unprotected sex as any insertive or receptive anal or vaginal intercourse with an HIV-negative partner or partner of unknown serostatus unprotected by a condom. A count of the number of such acts with all partners was summed. Recent sexual encounters were reported in detail for the first 5 partners of each gender; these reports included the partner's serostatus and substance use.
Information on additional partners (reported in 4.3% of assessments) was reported as the total number of unprotected vaginal or anal sexual acts and the total number of HIV-negative partners or partners of unknown serostatus. Estimates for the number of risky acts with partners beyond the first 5 were imputed from the collective information and patterns of behavior reported with the first 5 partners. Multiple imputations were performed with PROC MI procedure in SAS 9.1 (SAS Institute Inc, Cary, NC). The final analyses incorporate results from all imputations.
Substance use indices.
Participants self-reported the use of alcohol, marijuana, barbiturates, cocaine, crack, gamma hydroxybutyrate (GHB), hallucinogens, heroin, inhalants, ketamine, 3,4-methylenedioxymethamphetamine (MDMA, ecstasy), methadone, opiates, sedatives, speedball, steroids, and stimulants (i.e., methamphetamine, amphetamine). They reported the frequency of substance use in the previous 3 months using categories that were translated into counts as follows: “never” (count = 0), “less than once a month” (count = 1.5), “once a month” (count = 3), “2 to 3 times a month” (count = 7.5), “once a week” (count = 12), “2 to 3 times a week” (count = 30), “4 to 6 times a week” (count = 60), “once a day” or “more than once a day” (count = 90).
From these self-reports, we calculated 2 indices of substance use: the number of days of alcohol or marijuana use and the number of days of hard drug use (all substances other than alcohol and marijuana). We calculated the indices by summing the number of days that each substance included in the index was used. Of the 270 marginally housed participants, 19% (n = 50) had recently injected drugs and only 8% (n = 22) had shared used drug paraphernalia. Because of the low numbers of injection drug users and those sharing needles, we did not analyze injection risk.
Statistical Analysis
Using an intention-to-treat analysis, we compared the outcomes of marginally housed adults living with HIV in the intervention group and in the control group. We used a random-intercept zero-inflated Poisson (ZIP) model,24,25 which allowed the simultaneous modeling of having a zero in the response variable because of not doing that behavior at all (a binomial response) versus having a zero value consistent with a count (or Poisson) distribution. The assumptions underlying the standard Poisson model for count or frequency data inflated the estimated variance, reducing its power to identify intervention effects.
The intervention might have worked through inducing the participants to abstain from targeted acts (i.e., risky sexual acts or drug use) or it might have worked by reducing the frequency of acts. The ZIP model allowed both mechanisms of the intervention to be estimated.
The binomial process (i.e., doing the behavior at all) was modeled with logistic regression. The frequency process was modeled by a Poisson regression conditional on not being in the zero state. Visual inspection of the data suggested that we fit piecewise ZIP models, allowing separate intercepts at the baseline and first follow-up measure and assuming a linear trajectory among the follow-up time points. This was a reasonable model on the basis of longitudinal surveys in which greater changes were seen between the first 2 time points relative to subsequent time points, a phenomenon also known as panel conditioning.24
Separate random intercepts were included for each person in the binomial and Poisson parts of the model, allowing overall mean binomial and Poisson responses across time to be shifted up or down. Model covariates included an intercept term, baseline and follow-up assessment indicators, time (weeks from the baseline interview), an intervention indicator, and interactions of the intervention with the follow-up and time variables. Observed means of outcome measures were plotted and overlaid with predictions from the ZIP model that incorporated the random effects.
The figures include estimates of the significance of the intervention effect in the follow-up period for both the binomial and Poisson portions of the model. The SAS PROC NLMIXED procedure was used to obtain estimates. In calculating estimates for risky sexual acts, we estimated 4 models with 4 multiply imputed values of the outcome. The PROC MIANALYZE procedure was used to compute combined significance levels across the 4 models.
RESULTS
Marginally Housed Adults Living With HIV
Among the 936 adults living with HIV, 82% (n = 767) completed 4 or more assessments. We identified 270 of the 767 (35%) as being marginally housed at some point during the 37 months of assessment (i.e., from 12 months before recruitment to the 25-month follow-up). At every assessment, at least 20% of marginally housed participants were currently homeless (range = 20%–25%). Across the 6 assessments, 1.5% were homeless at all assessments, 38% were currently homeless at 1 or 2 assessments, 18% were currently homeless at 3 to 5 assessments, and 43% were identified as homeless on the basis of their 12-month retrospective reports.
Among the 270 marginally housed participants, 137 were in the intervention group and 133 were in the control group. A total of 72% in the intervention group and 75% in the control group completed all follow-up assessments. The follow-up rates were high at each assessment among participants assigned to the control group: 96% at 5 months, 95% at 10 months, 96% at 15 months, 89% at 20 months, and 92% at 25 months. Intervention group follow-up rates were 93%, 96%, 97%, 88%, and 85%, respectively.
Intervention attendance was also high among the marginally housed adults. Among immediate intervention participants, 92% completed all 5 sessions of module 1 (mean = 4.7), 89% completed module 2 (mean = 4.5 sessions), and 86% completed module 3 (mean = 4.3 sessions). In the control group, 69% attended the delayed intervention, with 74% completing all sessions (mean = 4.9 of 8 possible sessions).
Among those who completed 4 or more assessments, stably housed participants and marginally housed participants were similar regarding gender, years since HIV diagnosis, and the number of partners or risky sexual transmission acts at baseline (P > .05). Compared with stably housed participants, marginally housed participants had more criminal convictions (54% vs 35%; P < .001), were slightly younger (39 vs 41 years; P < .001), were more likely to belong to an ethnic minority (74% vs 64%; P = .001), were less educated (8% vs 23% were college graduates; P < .001), were less likely to have health insurance (73% vs 83%; P < .001), and were less likely to be employed (30% vs 41%; P = .004).
Marginally housed participants were less likely than were stably housed participants to be men having sex with men (48% vs 63%; P < .001), and a greater proportion were injection drug users (19% vs 8%; P < .001). The marginally housed participants self-reported a significantly lower average CD4 count (391 vs 442; P = .016) and were less likely to use antiretroviral therapy (60% vs 77%; P < .001). Beck depression scores26 were higher among the marginally housed participants (14.3 vs 12.3; P = .02) than among the stably housed participants.
The demographic characteristics, health measures, and baseline sexual and drug use behaviors among marginally housed adults in the intervention and control groups are reported in Table 1. Marginally housed participants were typically male (80%), in their late 30s (38.9 years), of ethnic minority heritage (56% African American, 12% Latino, 26% White), and unemployed (70%). More than half had been convicted of a crime, and 27% were uninsured. They had known they were HIV positive for an average of about 11 years and reported a mean CD4 count of 391; 60% were using antiretroviral therapy. Average Beck depression scores were consistent with mild depression.26 As a reflection of our sample selection criteria, 70% had recently engaged in risky sexual acts and 82% had had sexual partners of HIV-negative or unknown serostatus, 78% had used alcohol or marijuana, and 64% had used drugs other than alcohol or marijuana.
TABLE 1.
Baseline Characteristics of Marginally Housed Adults Living With HIV Who Completed 4 or More Assessments, by Group: Healthy Living Project, 2000–2002
| Control Group (n = 133) | Intervention (n = 137) | Total (n = 270) | |
| Demographic characteristics | |||
| City of residence, no. (%) | |||
| Los Angeles, CA | 48 (36.1) | 47 (34.3) | 95 (35.2) |
| Milwaukee, WI | 8 (6.0) | 11 (8.0) | 19 (7.0) |
| New York, NY | 30 (22.6) | 35 (25.5) | 65 (24.1) |
| San Francisco, CA | 47 (35.3) | 44 (32.1) | 91 (33.7) |
| Age, y, mean (SD) | 38.9 (6.9) | 38.9 (6.9) | 38.9 (6.9) |
| Age, no. (%) | |||
| 19–40 y | 80 (60.2) | 80 (58.4) | 160 (59.3) |
| 41–50 y | 46 (34.6) | 50 (36.5) | 96 (35.6) |
| ≥ 51 y | 7 (5.3) | 7 (5.1) | 14 (5.2) |
| Race/ethnicity, no. (%) | |||
| White | 34 (25.6) | 35 (25.5) | 69** (25.6) |
| African American | 62 (46.6) | 88 (64.2) | 150 (55.6) |
| Hispanic | 24 (18.0) | 9 (6.6) | 33 (12.2) |
| Other | 13 (9.8) | 5 (3.6) | 18 (6.7) |
| Gender, no. (%) | |||
| Men | 104 (78.2) | 112 (81.8) | 216 (80.0) |
| Women | 29 (21.8) | 25 (18.2) | 54 (20.0) |
| Education, n (%) | |||
| Did not finish high school | 39 (29.3) | 35 (25.5) | 74 (27.4) |
| High school graduate | 36 (27.1) | 38 (27.7) | 74 (27.4) |
| Some college | 49 (36.8) | 52 (38.0) | 101 (37.4) |
| College graduate | 9 (6.8) | 12 (8.8) | 21 (7.8) |
| Employed, no. (%) | 39 (29.5) | 42 (30.7) | 81 (30.1) |
| Ever convicted of a crime, no. (%) | 67 (50.8) | 78 (56.9) | 145 (53.9) |
| Type of medical insurance, no. (%) | |||
| Private | 8 (6.0) | 12 (8.8) | 20 (7.4) |
| Public | 83 (62.4) | 95 (69.3) | 178 (65.9) |
| None | 42 (31.6) | 30 (21.9) | 72 (26.7) |
| Behavioral risk group, no. (%) | |||
| Men who have sex with men | 63 (47.4) | 67 (48.9) | 130 (48.1) |
| Injection drug users | 22 (16.5) | 25 (18.2) | 47 (17.4) |
| Women | 29 (21.8) | 25 (18.2) | 54 (20.0) |
| Heterosexual men | 19 (14.3) | 20 (14.6) | 39 (14.4) |
| Physical health | |||
| Years since HIV positive diagnosis, mean (SD) | 9.9 (5.6) | 11.1 (6.0) | 10.5 (5.8) |
| Self-reported CD4 count, mean (SD) | 421.8 (336) | 361.7 (254) | 391.2 (298) |
| Self-reported viral load < 50 copies/mL, no. (%) | 14 (10.5) | 19 (13.9) | 33 (12.2) |
| ART user, n (%) | 80 (60.2) | 82 (59.9) | 162 (60.0) |
| Among ART users: 100% adherent, n (%) | 42 (53.2) | 41 (51.3) | 83 (52.2) |
| Beck depression inventory score,a mean (SD) | 15.3 (9.6) | 13.4 (9.7) | 14.3 (9.7) |
| MOS physical health summary score, mean (SD) | 43.9 (5.7) | 44.2 (5.1) | 44.0 (5.4) |
| MOS mental health summary score, mean (SD) | 36.7 (12.9) | 35.3 (12.5) | 36.0 (12.7) |
| Recent sexual activity and drug use | |||
| Any risky sexual transmission acts, no. (%) | 95 (72.0) | 92 (67.6) | 187 (69.8) |
| No. of risky sexual transmission acts, mean (SD) | 7.4 (17.0) | 11.9 (46.2) | 9.7 (35.0) |
| Any HIV-positive or unknown-status partners, no. (%) | 115 (86.5) | 106 (77.4) | 221 (81.9) |
| No. of HIV-positive or unknown-status partners, mean (SD) | 4.1 (6.2) | 3.0 (5.0) | 3.6 (5.7) |
| Used alcohol or marijuana, no. (%) | 105 (78.9) | 105 (76.6) | 210 (77.8) |
| No. of days used alcohol or marijuana, mean (SD) | 33.8 (44.8) | 40.0 (49.6) | 37.0 (47.3) |
| Used hard drugs, no. (%) | 88 (66.2) | 85 (62.0) | 173 (64.1) |
| No. of days used hard drugs, mean (SD) | 38.7 (76.3) | 35.2 (58.9) | 36.9 (67.9) |
Note. ART = antiretroviral therapy; MOS = Medical Outcomes Study.27
Scores above 13 indicate the presence of depressive symptoms.
P < .001 (χ2 test of race/ethnicity by treatment).
Marginally housed adults in the intervention and control groups differed only in ethnicity; there were significantly more African Americans in the intervention group and more Hispanic or other non-White adults in the control group. Analyses were conducted with and without control for ethnicity in the models and the results were the same; our results are presented without control for ethnicity.
Outcomes for Risky Sexual Behavior and Substance Use
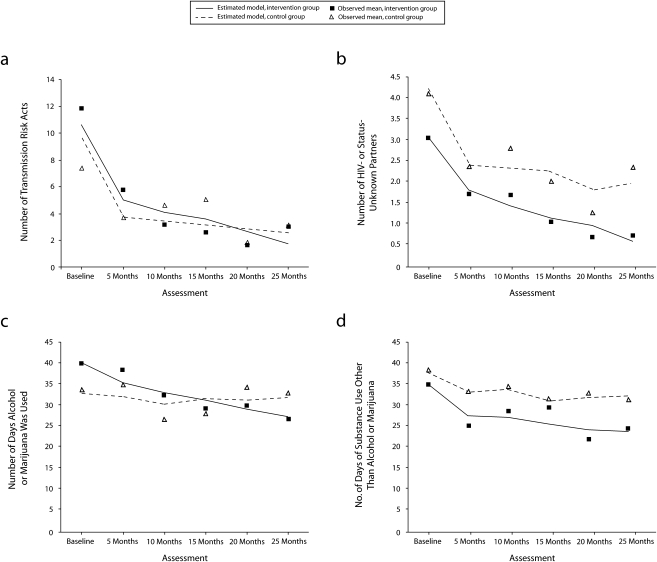
Figure 1 summarizes the observed means of each of the sexual risk and drug use indices at each assessment, as well as predicted values and significance tests from the ZIP models. The binomial estimate from the ZIP analyses answers the question, Was the risk behavior eliminated? There were no statistically significant differences in the estimates from the binomial portions of the ZIP models for intervention effects (P values ranged from .072 for days using alcohol or marijuana to .275 for number of partners who were HIV negative or of unknown serostatus). Thus, the intervention did not significantly influence whether participants engaged in these risky behaviors or not.
FIGURE 1.
Among marginally housed adults living with HIV infection, (a) the number of risky sexual transmission acts, (b) number of partners who were HIV negative or of unknown HIV status, (c) number of days alcohol or marijuana was used during previous 3 months, and (d) number of days hard drugs were used during previous 3 months: Healthy Living Program, 2000–2002.
Note. Observed values and estimates were derived from zero-inflated Poisson regression, by group and assessment.
There were significant intervention effects in the Poisson portion of the model for each outcome, however, indicating significant reductions in the frequency of each outcome measure. The most significant effects were found for the number of partners who were HIV negative or of unknown serostatus (Figure 1b; P < .001) and the number of days of alcohol or marijuana use (Figure 1c; P = .002). The intervention also reduced the number of risky sexual acts (Figure 1a; P = .037) and number of days of hard drug use (Figure 1d; P = .042) compared with the control group.
Table 2 displays mean values of numbers of risky sexual acts, numbers of sexual partners who were HIV negative or of unknown serostatus, and days of drug and alcohol use as estimated by the ZIP model for the control and invention groups. The relative effect sizes in the table can be interpreted as the percentage change in the frequency of each index of risky sexual acts estimated to occur because of the intervention. The effect size estimates show that the impact of the HLP increased over time.
TABLE 2.
Estimated Outcomes for Intervention and Control Groups at Each Assessment: Healthy Living Project, 2000–2002
| Outcome, by Assessment | Control Group | Intervention Group | Relative Effect Size (%)a |
| No. of risky sexual transmission acts | |||
| 5 mo | 3.77 | 5.03 | −33.4 |
| 10 mo | 3.50 | 4.12 | −17.6 |
| 15 mo | 3.19 | 3.63 | −13.6 |
| 20 mo | 2.91 | 2.70 | 7.4 |
| 25 mo | 2.67 | 1.75 | 34.2 |
| No. of HIV-positive or unknown-status partners | |||
| 5 mo | 2.39 | 1.80 | 25.0 |
| 10 mo | 2.35 | 1.41 | 40.0 |
| 15 mo | 2.23 | 1.12 | 49.5 |
| 20 mo | 1.82 | 0.93 | 48.6 |
| 25 mo | 1.97 | 0.56 | 71.5 |
| No. of days used alcohol or marijuana during previous 3 mo | |||
| 5 mo | 32.37 | 35.77 | −10.5 |
| 10 mo | 30.57 | 33.24 | −8.7 |
| 15 mo | 31.92 | 31.57 | 1.1 |
| 20 mo | 31.69 | 29.38 | 7.3 |
| 25 mo | 32.23 | 27.54 | 14.5 |
| No. of days used hard drugs during previous 3 mo | |||
| 5 mo | 33.56 | 27.76 | 17.3 |
| 10 mo | 34.10 | 27.39 | 19.7 |
| 15 mo | 31.43 | 25.76 | 18.0 |
| 20 mo | 32.17 | 24.42 | 24.1 |
| 25 mo | 32.52 | 24.00 | 26.2 |
Note. Estimates were made through a random-intercept zero-inflated Poisson (ZIP) model.
Relative effect size = 100 × (ec – ei)/ec, where ec and ei are estimated outcomes for the control and intervention groups, respectively.
For the number of risky sexual acts, the relative effect size was negative in the earlier assessments, reflecting greater frequency among those in the intervention group. Over the course of 25 months, however, those in the intervention group decreased the frequency of risky sexual acts significantly more than did those in the control group and were projected to have 34% fewer risky sexual acts at the final assessment.
Similarly, regarding number of partners who were HIV positive or of unknown serostatus, those in the intervention group demonstrated significantly greater decreases over time than did those in the control group; by the end of the study, they were predicted to have 72% fewer such partners than were control participants. By the 25-month assessment, the relative effect size for number of days of alcohol or marijuana use was almost 15%, and for days of hard drug use, it was 26%.
DISCUSSION
In the United States, HIV infection is concentrated among subpopulations coping with significant life stressors. Almost one third (29%) of our sample of adults living with HIV from 4 American cities were marginally housed at some point over a period of 37 months. Compared with consistently housed adults living with HIV, these marginally housed adults were more likely to be African American or Latino, uninsured, and less educated; to have been in jail; and to have a history of injection drug use. Therefore, those coping with housing stressors in particular also had a history of more significant life stressors in general.
Overall, these longitudinal data punctuate the importance of prevention programs for marginally housed adults living with HIV. In particular, it appears that to reduce HIV incidence in the United States, adults living with HIV who are marginally housed need access to prevention case management services.
Although living in difficult circumstances, the participants significantly and substantially reduced the number of their risky sexual acts to prevent transmission to others, particularly partners who were HIV negative or of unknown serostatus. The effect sizes were substantial and maintained for 2 years. Simultaneously, alcohol, marijuana, and hard drug use was significantly reduced among the marginally housed in response to the Healthy Living Project. Drug use was more common among the marginally housed than among housed adults (56% vs 33%), and drug use decreased significantly; these results are similar to those found for the overall sample receiving the Healthy Living Project.20
These results are encouraging, especially in the face of trials that have shown substantial relapse after initial responsiveness to efforts to prevent the transmission of HIV.28 Marginally housed adults living with HIV reduced their numbers of risky sexual acts and their drug use in response to an individually delivered, skill-focused, case-management intervention. The Healthy Living Project is designed to comprehensively address the challenges experienced by all persons living with HIV: adults, adolescents, mothers, gay men, and others. Rather than focusing on 1 outcome that benefits society—reducing transmission acts—the program also targets the health and mental health needs of these adults. These results strongly support the Centers for Disease Control and Prevention's current emphasis on using case-management approaches with adults living with HIV to prevent the transmission of HIV.
It is noteworthy that this 15-session intervention was carried out in the United States with both marginally and stably housed adults living with HIV. In this country, access to prevention services for adults living with HIV are guaranteed by federal legislation (the Ryan White Act).29 It will be critical to examine whether models such as the Healthy Living Project can be adapted and carried out in a cost-efficient manner in the developing world.
Limitations
There are several limitations to our study that must be considered when interpreting these results. First, this is not a representative sample of marginally housed adults living with HIV. The overall sample of adults living with HIV infection is highly similar to the demographic characteristics and risk behaviors of nationally representative samples30; however, it was not recruited to be representative.
Second, most of the marginally housed participants were men, consistent with other studies of homeless populations. Consequently, we could not examine gender differences, and do not know if there were any. Third, the definition of marginally housed creates ambiguities in the relation between homelessness and risk outcomes, because we did not have clear dates of when homelessness began and ended.
Finally, the broad diffusion of the intensive 15-session Healthy Living Project intervention to other settings is questionable. A key issue in its diffusion will be the focus on identifying the key effective features of the intervention and developing the trained skills, strategies, and techniques that allow broad replication in the next generation of programs to prevent the transmission of HIV.
Conclusions
HIV is a lifelong condition, presenting new challenges as one's developmental capacity shifts or medical breakthroughs lead to new treatment regimens. Although a program with 15 sessions sounds intensive, changing daily routines is difficult. The cognitive, affective, and behavioral skills taught in the Healthy Living Project are the same across modules. Participants were taught skills to apply in the situations targeted in the module: maintaining health and interacting with health providers, reducing the numbers of risky sexual acts and the amount of drug use, and improving mental health. Because of the repeated application of problem-solving approaches to diverse content areas, participants were prepared to apply these skills in future situations.
The Healthy Living Project is similar to case-management intervention for young people living with HIV.15 Multiple modules delivered in an individual case-management format focused on health transmission and mental health have sustained benefits for young adults living with HIV. Similar skills and modules are efficacious in multiple family groups.13,31,32 Formats on the Internet and in programs in the developing world are also similar.31,32
The similarity of programs for different populations and with different delivery formats suggests the effectiveness of the program. It will be necessary to mount programs similar to the Healthy Living Project with paraprofessionals for adaptation in the developing world. To broadly diffuse prevention programs for adults living with HIV, community-based facilitators who can develop long-term relationships with these adults and support them through life transitions will be needed. The next generation of programs will identify whether the effective intervention components of newer interventions are similar to those of the Healthy Living Project and whether the program is portable. Our finding suggest that the program helps those with even the highest level of need, the marginally housed. Future research must examine how, in what format, and by whom the next programs will be implemented.
Acknowledgments
This research was supported by the National Institute on Mental Health (NIMH; grant MH57615t).
Members of the NIMH's Healthy Living Trial Group are as follows. Research Steering Committee (site principal investigators and NIMH staff collaborators): Margaret A. Chesney (University of California, San Francisco [UCSF]), Anke A. Ehrhardt (New York State Psychiatric Institute/Columbia University, New York, NY [NYSPI/Columbia]), Jeffrey A. Kelly (Medical College of Wisconsin, Milwaukee, WI [MCW]), Willo Pequegnat (NIMH, Bethesda, MD), and Mary Jane Rotheram-Borus (UCLA); collaborating scientists, coprincipal investigators, and investigators: Abdelmonem A. Afifi (UCLA), Eric G. Benotsch (MCW), Michael J. Brondino (MCW), Sheryl L. Catz (MCW), Edwin D. Charlebois (UCSF), W. Scott Comulada (UCLA), William G. Cumberland (UCLA), Don C. Des Jarlais (Beth Israel Medical Center, New York, NY), Naihua Duan (UCLA), Theresa M. Exner (NYSPI/Columbia), Rise B. Goldstein (UCLA), Cheryl Gore-Felton (MCW), A. Elizabeth Hirky (NYSPI/Columbia), Mallory O. Johnson (UCSF), Robert M. Kertzner (NYSPI/Columbia), Sheri B. Kirshenbaum (NYSPI/Columbia), Lauren E. Kittel (NYSPI/Columbia), Robert Klitzman (NYSPI/Columbia), Martha Lee (UCLA), Bruce Levin (NYSPI/Columbia), Marguerita Lightfoot (UCLA), Stephen F. Morin (UCSF), Steven D. Pinkerton (MCW), Robert H. Remien (NYSPI/Columbia), Fen Rhodes (UCLA), Juwon Song (UCLA), Wayne T. Steward (UCSF), Susan Tross (NYSPI/Columbia), Lance S. Weinhardt (MCW), Robert Weiss (UCLA), Hannah Wolfe (St Luke's–Roosevelt Medical Center, New York, NY), Rachel Wolfe (St Luke's–Roosevelt), and F. Lennie Wong (UCLA); data management and analytic support: Philip Batterham (UCLA), W. Scott Comulada (UCLA), Tyson Rogers (UCLA), and Yu Zhao (UCLA); site project coordinators: Jackie Correale (NYSPI/Columbia), Kristin Hackl (MCW), Daniel Hong (UCLA), Karen Huchting (UCLA), Joanne D. Mickalian (UCSF), and Margaret Peterson (MCW).
Human Participant Protection
The overall study protocol was approved by the institutional review board of the University of California, Los Angeles.
References
- 1.Reback CJ, Kamien JB, Amass L. Characteristics and HIV risk behaviors of homeless, substance-using men who have sex with men. Addict Behav 2007;32:647–654 [DOI] [PubMed] [Google Scholar]
- 2.Robertson MJ, Clark RA, Charlebois ED, et al. HIV seroprevalence among homeless and marginally housed adults in San Francisco. Am J Public Health 2004;94:1207–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Communities at risk. Available at: http://www.cdcnpin.org/scripts/population/homeless.asp#2. Accessed June 23, 2007.
- 4.Zanis DA, Cohen E, Meyers K, Cnaan RA. HIV risks among homeless men differentiated by cocaine use and psychiatric distress. Addict Behav 1997;22:287–292 [DOI] [PubMed] [Google Scholar]
- 5.Aidala A, Cross JE, Stall R, Harre D, Sumartojo E. Housing status and HIV risk behaviors: implications for prevention and policy. AIDS Behav 2005;9:251–265 [DOI] [PubMed] [Google Scholar]
- 6.Reyes JC, Robles RR, Colón HM, et al. Homelessness and HIV risk behaviors among drug injectors in Puerto Rico. J Urban Health 2005;82:446–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coady MH, Latka MH, Thiede H, et al. Housing status and associated differences in HIV risk behaviors among young injection drug users (IDUs). AIDS Behav 2007;11:854–863 [DOI] [PubMed] [Google Scholar]
- 8.Sears C, Guydish JR, Weltzien EK, Lum PJ. Investigation of a secondary syringe exchange program for homeless young adult injection drug users in San Francisco, California, USA. J Acquir Immune Defic Syndr 2001;27:193–201 [DOI] [PubMed] [Google Scholar]
- 9.Susser E, Valencia E, Berkman A, et al. Human immunodeficiency virus sexual risk reduction in homeless men with mental illness. Arch Gen Psychiatry 1998;55:266–272 [DOI] [PubMed] [Google Scholar]
- 10.Rotheram-Borus MJ, Koopman C, Haignere C, Davies M. Reducing HIV sexual risk behaviors among runaway adolescents. JAMA 1991;266:1237–1241 [PubMed] [Google Scholar]
- 11.Nyamathi A, Flaskerud JH, Leake B, Dixon EL, Lu A. Evaluating the impact of peer, nurse case-managed, and standard HIV risk-reduction programs on psychosocial and health-promoting behavioral outcomes among homeless women. Res Nurs Health 2001;24:410–422 [DOI] [PubMed] [Google Scholar]
- 12.Rotheram-Borus MJ, Song J, Gwadz M, et al. Reductions in HIV risk among runaway youth. Prev Sci 2003;4:173–187 [DOI] [PubMed] [Google Scholar]
- 13.Rotheram-Borus MJ, Lee MB, Gwadz M, et al. An intervention for parents with AIDS and their adolescent children. Am J Public Health 2001;91:1294–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rotheram-Borus MJ, Lee MB, Murphy DA, et al. Efficacy of a preventive intervention for youths living with HIV. Am J Public Health 2001;91:400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rotheram-Borus MJ, Swendeman D, Comulada S, et al. Prevention for substance using HIV+ young people: telephone and in-person delivery. J Acquir Immune Defic Syndr 2004;37(suppl 2):S68–S77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walley AY, Cheng DM, Libman H, et al. Recent drug use, homelessness and increased short-term mortality in HIV-infected persons with alcohol problems. AIDS 2008;22:415–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson MO, Catz SL, Remien RH, et al. Theory-guided, empirically supported avenues for intervention on HIV medication nonadherence: findings from the Healthy Living Project. AIDS Patient Care STDS 2003;17:645–656 [DOI] [PubMed] [Google Scholar]
- 18.Gore-Felton C, Rotheram-Borus MJ, Weinhardt LS, et al. The Healthy Living Project: an individually tailored, multidimensional intervention for HIV-infected persons. AIDS Educ Prev 2005;17(1 suppl A):21–39 [DOI] [PubMed] [Google Scholar]
- 19.The Healthy Living Project Team Effects of a behavioral intervention to reduce risk of transmission among people living with HIV: the Healthy Living Project randomized controlled study. J Acquir Immune Defic Syndr 2007;44(2):213–221 [DOI] [PubMed] [Google Scholar]
- 20.Wong FL, Rotheram-Borus MJ, Lightfoot M, et al. Effects of a behavioral intervention on substance use among people living with HIV: the Healthy Living Project randomized controlled study. Addiction 2008;103:1206–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenthal D, Rotheram-Borus MJ, Batterham P, et al. Housing stability over two years and HIV risk among newly homeless youth. AIDS Behav 2007;11:831–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner CF, Ku L, Rogers SM, et al. Adolescent sexual behavior, drug use, and violence: increased reporting with computer survey technology. Science 1998;280:867–873 [DOI] [PubMed] [Google Scholar]
- 23.Gribble JN, Miller HG, Rogers SM, Turner CF. Interview mode and measurement of sexual behaviors: methodological issues. J Sex Res 1999;36:16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall DB. Zero-inflated Poisson and binomial regression with random effects: a case study. Biometrics 2000;56:1030–1039 [DOI] [PubMed] [Google Scholar]
- 25.Lambert D. Zero-inflated Poisson regression with an application to defects in manufacturing. Technometrics 1992;34:1–14 [Google Scholar]
- 26.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck depression inventory: twenty-five years of evaluation. Clin Psychol Rev 1988;8:77–100 [Google Scholar]
- 27.Ware JE, Jr, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care 1995;33(4 Suppl):AS264–AS279 [PubMed] [Google Scholar]
- 28.Koblin B, Chesney M, Coates T, EXPLORE Study Team Effects of a behavioural intervention to reduce acquisition of HIV infection among men who have sex with men: the EXPLORE randomised controlled study. Lancet 2004;364:41–50 [DOI] [PubMed] [Google Scholar]
- 29.HRSA Care ACTION: HIV/AIDS in Racial and Ethnic Minorities. Rockville, MD: Health Resources and Services Administration; 1999 [Google Scholar]
- 30.Supplement to HIV/AIDS Surveillance (SHAS): Demographics and Behavioral Data From a Supplemental HIV/AIDS Behavioral Surveillance Project, 1997–2000. Atlanta, GA: Centers for Disease Control and Prevention; 2004:1–27 Special Surveillance Report 2 [Google Scholar]
- 31.Kalichman SC, Eaton L, Cain D, et al. Changes in HIV treatment beliefs and sexual risk behaviors among gay and bisexual men, 1997–2005. Health Psychol 2007;26:650–656 [DOI] [PubMed] [Google Scholar]
- 32.Wu Z, Rotheram-Borus MJ, Detels R, et al. Selecting at-risk populations for sexually transmitted disease/HIV prevention studies. AIDS 2007;21(suppl 8):S81–S87 [DOI] [PubMed] [Google Scholar]