Abstract
An αβ T cell response depends on the recognition of antigen plus major histocompatibility complex proteins (MHC)1 by its antigen receptor (TCR). The ability of peripheral αβ T cells to recognize MHC is at least partly determined by MHC dependent thymic selection, by which an immature T cell survives only if its TCR can recognize self MHC2–7. This process may allow MHC reactive TCRs to be selected from a repertoire with completely random and unbiased specificities. However, analysis of thymocytes prior to positive selection, suggested that TCR proteins might have a predetermined ability to bind MHC8–11. Here we show that specific germline-encoded amino acids in the TCR promote “generic” MHC recognition and control thymic selection. In mice expressing single, rearranged TCRβ chains, individual mutation of amino acids in CDR2β to Ala reduced development of the entire TCR repertoire. Altogether, these results show that thymic selection is controlled by germline-encoded MHC-contact points in the αβ TCR and suggest that the diversity of the peripheral T cell repertoire is enhanced by this “built-in” specificity.
The idea that TCRs might have a germline-encoded ability to bind MHC8 is supported by crystallographic analyses of TCR-MHC complexes12, 13. In general, TCRs bind MHC in a diagonal orientation, where the germline-encoded CDR1 and CDR2 loops of the TCR Vα and Vβ segments interact primarily with MHC, while the highly diverse CDR3α and CDR3β loops bind the peptide13. Recently, several TCR-MHC/peptide crystal structures showed that three amino acids (βY46, βY48 and βE54) in the germline-encoded CDR2 regions of mouse Vβ8 bound the same regions on different MHC molecules, suggesting a site for consistent interaction between TCRs and MHC12, 14–16.
Mutational studies of Vβ8.2-expressing TCRs showed that these three Vβ8.2 amino acids were usually required for MHC/peptide binding15, 17, 18. For a very cross-reactive TCR, we showed that the recognition of several ligands was dependent on βY48 and, to a lesser extent, βE5414. To extend such analyses, we examined the contribution of these CDR2β amino acids to MHC/peptide recognition by other Vβ8.2-expressing TCRs. Mutation of βY46, βY48, and βE54 to Ala in the DO-11.10 TCRβ (DOβ) reduced its ability to react with its antigenic targets, IAb or IAd plus an ovalbumin peptide (OVA323-339, Figure 1a, Supplementary Figure 1). These mutations did not affect the TCR conformation, measured by binding of anti-TCR antibodies (Supplementary Figure 2a), or the response of hybridomas expressing them to anti-TCR crosslinking (Supplementary Figure 2b). Additionally, the wild-type (WT) and mutant DOβ chains were similarly surface-expressed even when in competition with another TCRβ chain (Supplementary Figure 2c,d).
Figure 1.
Vβ8.2 amino acids Y46, Y48 and E54 are required for TCR recognition of specific MHC/peptide and allo-MHC complexes.a. IL-2 response of 5KCα-β-hybridomas transduced with retroviruses encoding WT DO-11.10 TCRα plus WT DOβ or Y46A, Y48A, and E54A mutant DOβ after stimulation with 0.4 µg/ml OVA323-339 peptide plus IAb-expressing Chb-2.4.4 cells or IAd-expressing A20.2J cells, no peptide with IAb-expressing Chb-2.4.4 cells, or 1.5 µg/ml plate bound anti-TCRβ (H57-597).b,c. IL-2 response of 5KCα-β- hybridomas transduced with retroviruses encoding WT 2W1S-20.4 TCRα chain (b) or WT 75-55 TCRα chain (c), plus appropriate partner WT, Y46A, Y48A, and E54A TCRβ chain after stimulation with fibroblasts expressing IAb with linked 3K peptide, or splenocytes expressing H2-b (C57BL/6), bm12 (B6.C-H2bm12), d (BALB/c), f (B10.M), q (B10.D1), r (B10.RIII), s (B10.S), u (B10.PL), left unstimulated (No APC), or activated with 10 µg/ml plate-bound anti-TCRβ (H57-597). Data are mean of three independent experiments (a) or two independent experiments (b, c).
The βY46A, βY48A, and βE54A mutations also reduced recognition of IAb/3K and allo-MHC by two other cross-reactive TCRs(Figure 1b,c, Supplementary Figure 3a,b). Thus, the near obligate requirement for βY46, βY48, and βE54 for MHC recognition by seven TCRs (Figure 1 and refs.14–18) suggests that these amino acids might confer “generic” MHC-reactivity on TCRs.
To find out whether positive selection of a broad repertoire of TCRs depends on similar TCR/MHC interactions, we evaluated the effects of these mutations on thymic development in vivo. If these amino acids contribute to “generic” MHC recognition by TCRs, thymocytes with mutant Vβs should be inefficiently positively selected, resulting in a reduction in the development of mature thymocytes and peripheral T cells. To test this, bone marrow stem cells isolated from TCRβ−/− TCRδ−/− mice were transduced with retroviruses encoding the WT, Y46A, Y48A, or E54A DOβ chains and transferred into RAG deficient mice (Figure 2a). These stem cells could generate thymocytes expressing only the WT or mutated DOβ chains plus the entire repertoire of TCRα chains.
Figure 2.
Vβ8.2 amino acids Y46, Y48, and E54 promote efficient thymic selection.a. Schematic illustrating the generation of retroviral bone marrow chimeras.b. Thymic cellularity of bone marrow chimeras expressing WT, Y46A, Y48A, or E54A DOβ analyzed between d29 and d34 post reconstitution. Data are mean plus s.e.m. and are cumulative from 3 independent experiments for WT (n=10) and 2 independent experiments for Y46A (n=5), Y48A (n=4), and E54A (n=6) with TCRβ−/−TCRδ−/− donor bone marrow.c,d,e. FACS analysis of CD4 and CD8 staining (c), CD5 and CD69 staining (d), and HSA (CD24) and TCRβ staining (e) of GFP+ thymocytes from chimeras expressing WT, Y46A, Y48A, or E54A DOβ analyzed between d29 and d34 post reconstitution. Representative FACS plots are shown from 3 independent experiments for WT and 2 independent experiments for Y46A, Y48A, and E54A.f. Absolute number of total mature (HSAloTCRβhi) thymocytes (left panel), CD4SP mature thymocytes (middle panel), and CD8SP mature thymocytes (right panel) for chimeras expressing WT, Y46A, Y48A, or E54A DOβ analyzed between d29 and d34 post reconstitution. Data are mean plus s.e.m. and are cumulative from 3 independent experiments for WT (n=10) and 2 independent experiments for Y46A (n=5), Y48A (n=4), and E54A (n=6); (*, p<0.05;**, p<0.01; ***,P<0.001; NS, not significant).
After reconstitution, chimeras expressing WT and Ala-mutant DOβ chains had similar total thymic cellularity (Figure 2b) and had similar TCRβ expression profiles (Supplementary Figure 4a). For all chimeras, the thymuses contained all CD4-CD8-(DN) subsets (Supplementary Figure 4b) and most thymocytes were immature, unselected CD4+CD8+ (DP) cells (Figure 2c). Upon recognition of MHC, DPs are activated and express CD5 and CD69 before becoming mature thymocytes which are HSAlo/TCRhi and express either CD4 (CD4SP) or CD8 (CD8SP)7. Consistent with a reduced ability to bind MHC and induce positive selection, the frequency of CD4SP cells was reduced in chimeras expressing Y46A, Y48A, and E54A DOβ, while the frequency of CD8SP cells was reduced in chimeras expressing Y46A and Y48A DOβ (Figure 2c). Likewise, we observed a substantial reduction in the frequency of activated CD5+ CD69+ cells and mature, HSAlo/TCRhi, thymocytes (Figure 2d, e and Supplementary Figure 4c, d) in chimeras expressing the mutant DOβ.
To estimate thymic output, we calculated the total number of mature thymocytes. The numbers of mature cells and mature CD4SP cells were significantly reduced in chimeras expressing any of the mutant DOβ chains, compared to WT controls (Figure 2f). The number of mature CD8SP cells was reduced in chimeras expressing βY46A and βY48A, but not in those expressing βE54A (Figure 2f). These results suggest that βY46, βY48, and βE54 are involved in MHCII recognition. In prior mutational and crystallographic studies, βY46 and βY48 were implicated in interactions with MHCI, while βE54 was not17, 18. Thus, βE54 may be primarily involved in TCR binding MHCII, via a salt bridge with a conserved Lys residue at position 39 in the MHCII α chain12, 14–16, which is absent in MHCI proteins12.
The total cellularity of the peripheral lymphoid organs was comparable for all chimeras, except for a ~2-3-fold reduction in the number of splenocytes in chimeras expressing βY48A (Supplementary Figure 5a, b). Similarly, the frequency (Supplementary Figure 5c, d) and total number (Supplementary Figure 5e, f) of peripheral T cells was slightly reduced in chimeras expressing βY48A compared to those expressing WT, βY46A, and βE54A. Thus, the effects of the TCRβ mutations on cell number were much less profound in the periphery than in the thymus. CD44 expression, a marker of activation or homeostatic expansion19, was increased among LN T cells expressing mutant TCRβ chains and the extent of activation inversely correlated with the effect of the mutation on positive selection (Supplementary Figure 5g). Together these experiments suggested that although the Vβ mutations inhibited thymocyte selection, a corresponding influence on peripheral T cell numbers was muted by homeostatic expansion of the few cells capable of maturing.
Y46, Y48, and E54 are present in many mammalian Vβs and this conservation suggests these amino acids may often contribute to TCR recognition of MHC12, 20. Among mouse Vβs, Vβ6 has a Tyr at position 46 of its CDR2 loop12, 20. The TEa TCR uses Vβ6 and recognizes IAb plus a peptide from IEα (Eα52–68)21. We compared the ability of hybridomas expressing the WT TEa TCR, and cells expressing WT TEaα plus Y46A TEaβ, to respond to IAb/Eα52–68. The βY46A mutation reduced the sensitivity to antigen 10–15 fold, but did not affect the response to anti-TCR stimulation (Figure 3a).
Figure 3.
Vβ6 Y46 promotes MHC recognition and thymic selection.a. IL-2 response of 5KCα-β- hybridomas transduced with retroviruses encoding TEa TCRα plus WT TEaβ (filled symbols) or Y46A TEaβ (open symbols) after stimulation with the indicated concentration of Eα52–68 peptide plus IAb-expressing Chb-2.4.4 cells (left panel) or 10 µg/ml plate bound anti-TCRβ (right panel). Data are representative of two independent experiments.b. Thymic cellularity of bone marrow chimeras expressing WT or Y46A TCliβ analyzed between d25 and d33 post reconstitution.c. FACS analysis of CD4 and CD8 staining, CD5 and CD69 staining and HSA (CD24) and TCRβ staining of GFP+ thymocytes from chimeras expressing WT or Y46A TCliβ analyzed between d25 and d33 post reconstitution. Representative FACS plots are shown from 3 independent experiments.d. Absolute number of total mature (HSAloTCRβhi) thymocytes (left panel), CD4SP mature thymocytes (middle panel), and CD8SP mature thymocytes (right panel) for chimeras expressing WT or Y46A Tcliβ analyzed between d25 and d33 post reconstitution. For (b) and (d), data points are shown for each mouse, with mean plus s.e.m., from 2 independent experiments for WT (n=3) and Y46A (n=2) with TCRβ−/− donor bone marrow (squares) and 1 experiment for WT (n=3) and Y46A (n=3) with TCRβ−/−TCRδ−/− donor bone marrow (circles); (**, p<0.01; NS, not significant).
We assessed the role of Vβ6 Y46 in thymic selection in experiments similar to those described for Vβ8.2 using the TCRβ from a Vβ6-expressing, IAb/human invariant chain specific TCR, TCli22. We generated bone marrow chimeras using stem cells transduced with retroviruses expressing the WT or Y46A TCli TCRβ chain (TCliβ). This mutation did not affect TCR expression in hybridomas (Supplementary Figure 6a). Likewise, in vivo, mice reconstituted with stem cells expressing WT or Y46A TCliβ had comparable numbers of thymocytes (Figure 3b) and TCR expression profiles (Supplementary Figure 6b). Consistent with impaired thymic selection, however, the frequencies of CD5+CD69+ cells and HSAloTCRhi cells, and numbers of mature thymocytes and CD4SP cells were reduced in mice expressing βY46A TCliβ, compared to WT controls (Figure 3c) while the maturation of CD8SP cells was unaffected (Figure 3d).
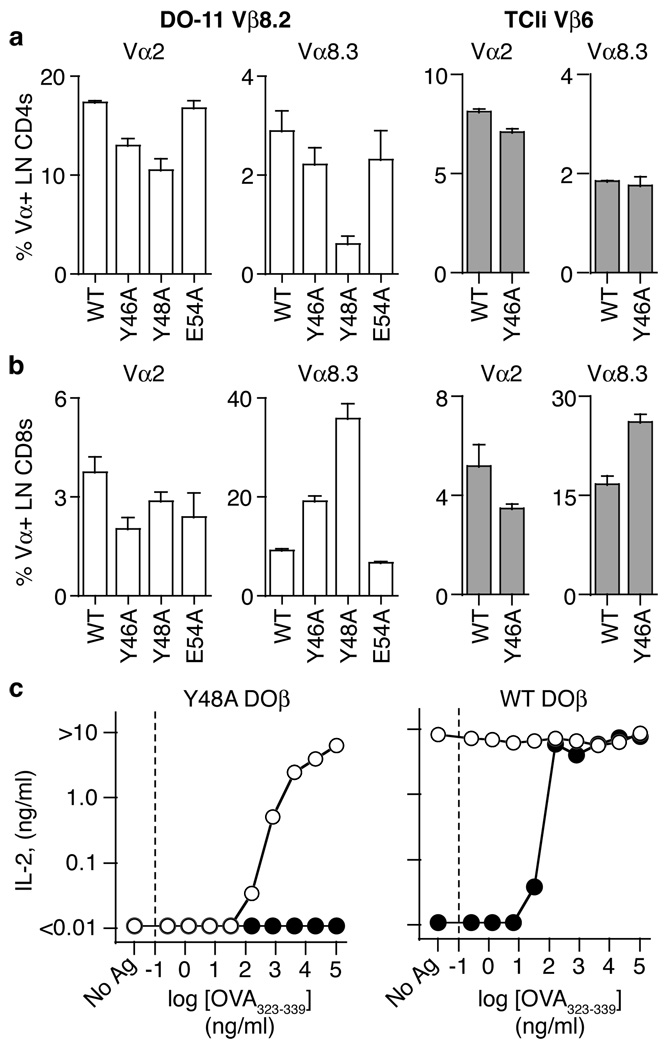
Thymic selection was significantly reduced, but not completely absent, in chimeras expressing the mutant TCRβ chains (Figure 2, Figure 3). This suggested that some thymocytes might have compensated for the reduced MHC reactivity of Ala-mutant TCRβ chains, perhaps by expressing a biased set of TCRα chains. To test this, we analyzed Vα expression among peripheral T cells. In Vβ8.2 chimeras, the frequency of Vα2 and Vα8.3-expressing CD4+ cells was reduced in cells expressing βY46A and βY48A, versus cells expressing βWT (Figure 4a). Among CD8+ cells in Vβ8.2 chimeras, Vα2 expression was similar among all groups, while expression of Vα8.3 was dramatically increased in βY46A and βY48A chimeras compared to WT chimeras (Figure 4b). In Vβ6 chimeras, we observed similar trends in Vα expression, where Vα8.3 expression was increased in CD8+ cells in chimeras expressing βY46 (Figures 4a, b).
Figure 4.
Altered TCRα repertoire in chimeras expressing mutant TCRβ chains. a,b. Frequency of Vα2 and Vα8.3 expression on LN CD4+ T cells (a) or LN CD8+ T cells (b) from chimeras expressing WT, Y46A, Y48A, or E54A DOβ (white bars) and WT or Y46A TCliβ (grey bars) analyzed between d25 and d34 post reconstitution. For Vβ8.2chimeras, data are mean plus s.e.m. from 3 independent experiments for WT (n=10) and 2 independent experiments for Y46A (n=5), Y48A (n=4), and E54A (n=6). For Vβ6 chimeras, data are mean plus s.e.m. from a representative experiment for WT (n=3) and Y46A (n=3) from TCRβ−/− donors.c. IL-2 response of 5KCα-β-hybridomas transduced with retroviruses encoding Y48A DOβ (left panel) or WT DOβ (right panel) plus DO-11.10 TCRα (filled symbols) or S93A TCRα (open symbols) after stimulation with the indicated concentration of OVA323-339 peptide plus IAb-expressing Chb-2.4.4 cells. Data are mean of 2 independent experiments.
The biased TCRα repertoire among chimeras expressing mutant TCRβ chains suggested that positive selection may have “picked out” TCRα sequences that compensate by having, themselves, a higher affinity for MHC. Sequence analysis of CDR3α regions from the Vα8 repertoire among CD8+ cells from mice expressing WT and Y48A DOβ showed that the latter often expressed a “Y” in CDR3α 6 amino acids after the conserved Vα Cys (Supplementary Figure 7). Thus, the TCRβ mutations might have resulted in positive selection of an altered repertoire of TCRα chains.
To compare the specificity of TCRα chains from mice expressing mutant TCRβ chains, we generated an IAb/OVA specific T cell hybridoma from chimeras expressing Y48A DOβ. Surprisingly, the hybridoma expressed a TCRα chain that was identical to the DO-11.10 TCRα (DOα), except for an Ala-to-Ser substitution in CDR3 (αS93A). To compare the MHC reactivity of these TCRα chains, we engineered hybridomas expressing WT or Y48A DOβ, plus either WT DOα or αS93A. In hybridomas expressing Y48A DOβ, the αS93A change improved responses to OVA with IAb (Figure 4c) and IAd (Supplementary Figure 8) compared to WTDOα. When paired with WT DOβ, αS93A slightly reduced responses to OVA plus IAd (Supplementary Figure 8), but created a TCR that was now autoreactive to IAb, even in the absence of OVA (Figure 4c). These results suggest that αS93A may improve MHC recognition compared to the original DOα. This CDR3α change allowed positive selection when paired with the Y48A DOβ chain, but would probably cause negative selection if paired with the WT DOβ because the resultant TCR binds MHC too well.
Altogether, these observations show that specific amino acids in the TCR Vβ promote “generic” recognition of MHC molecules, useful both in recognition of MHC/self peptides during positive selection in the thymus and in recognition of MHC/foreign peptides during specific immune responses. In the absence of these conserved Vβ interactions, thymic development can select for TCRs with affinity for self-MHC, but the resulting TCRα repertoire is biased. These findings confirm the hypothesis that the TCR has a germline-encoded ability to recognize MHC8 and provide an explanation for the high frequency of MHC reactive TCRs in the preselected T cell pool9–11.
Methods Summary
Mutant TCRαβ constructs and retroviral infection
TCR expression constructs were cloned in MSCV-based retroviral plasmids and transfected into Phoenix cells to produce retrovirus-containing supernatants, as described previously23. TCRs were expressed by retroviral transduction in a TCRαβ deficient hybridoma (5KC-73.8.20, referred to as 5KCα-β-)24, and selected on the basis on retroviral reporter and TCRβ expression, as described previously23. For all stimulations, 5×104 hybridoma cells were cultured overnight with the indicated stimuli, and IL-2 production was measured by ELISA, as described previously23.
Generation of retroviral bone marrow chimeras
Bone marrow was harvested from TCRβ−/−TCRδ−/− double deficient or TCRβ−/− deficient mice, 5 days after injection with 5-fluorouracil. Cells were cultured in vitro for four days in complete DMEM supplemented with 15% fetal calf serum, IL-3, IL-6, and SCF, and spin-infected three times with retrovirus-containing supernatant. After transduction, ~5.0×105 GFP+ bone marrow cells were transferred into sublethally irradiated (400 or 600 RAD) RAG1−/− recipient mice.
Thymus, spleen, and LN cell populations were analyzed by flow cytometry from bone marrow chimeras at 25–34 days post reconstitution. Mice that reconstituted poorly (those with less than 3.0×107 total thymocytes) were excluded from the analysis. In all experiments, the two thymic lobes from each mouse were analyzed separately. Data for frequencies of cell populations are shown for each individual lobe. Data for absolute numbers of thymocytes are the sum of both lobes from individual mice.
Statistical Analysis
An unpaired two-tailed Student's t-test was applied using GraphPad software (Prism).
Full Methods
Mutant αβ TCR constructs and retroviral plasmids
Plasmids encoding TCRα and TCRβ chains for the DO-11.10, 2W1S-20.4, and 75-55 TCRs were generated in house. Plasmids encoding TCRα and TCRβ chains for the TEa TCR were generously provided by Dr. Dario Vignali (St. Jude’s Children’s Research Hospital, Memphis, TN, USA). A plasmid encoding the TCli TCRβ chain was generously provided by Dr. Chyi Hsieh (Washington University, St. Louis, MO, USA). TCR mutants were constructed using PCR with overlapping primers and cloned using engineered restriction sites. TCRα constructs were cloned in MSCV-based retroviral plasmids with an IRES plus GFP as a reporter. TCRβ constructs were cloned in MSCV-based retroviral plasmids with an IRES-GFP or IRES-human nerve growth factor receptor as a reporter or PGK-Puror (Clontech) as a selectable marker. All TCR V gene segments are named and their amino acids numbered according to the IUIS/Arden compilation1.
Retroviral packaging
Retroviral plasmids were co-transfected into Phoenix cells with pCLEco accessory plasmid using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Retrovirus-containing supernatants were harvested 48 hr and 72 hr after transfection and 0.45 µm filtered to remove cell debris.
Hybridomas and cell lines
TCR constructs were expressed by retroviral transduction into a hybridoma that lacks TCRα and TCRβ (5KC-73.8.20, referred to as 5KCα-β-)2, as described previously3. For spin-infection of hybridomas, 105 cells were spin-infected with retroviral supernatants containing 8 µg/ml polybrene for 90 min at 37°C in 1.5 ml microcentrifuge tubes at 6500 rpm. After spin-infection, hybridomas were sorted on a MoFlo cell sorter (Dakocytomation) on the basis of retroviral reporter and TCRβ expression. For hybridomas generated with PGK-Puror, cell lines were selected with 2.5 µg/ml puromycin.
For testing surface expression of WT or Ala-mutant DOβ chains in competition with other TCRβ chains, DOβ constructs were transduced into the DO-11.10.3 hybridoma 4. DO-11.10.3 is a subclone of the original DO-11.10 hybridoma that was selected to lose expression of the original DO-11.10 TCRβ chain, but still expresses the DO-11.10 TCRβ chain plus the original TCRβ of the thymoma fusion partner BW5147.
OVA-specific hybridomas were generated by immunizing bone marrow chimeras expressing the βY48A TCR chain with OVA protein conjugated to the 3K peptide emulsified in complete Freund’s adjuvant. Seven days later, spleen and lymph node cells were isolated and expanded in vitro with OVA protein for 3 days, followed by culture with IL-2 for 5 days. After in vitro culture, activated T cells were fused to BWα-β- and plated at limiting dilutions, as described previously 5. Individual hybridomas were assessed for reactivity to OVA protein plus IAb and IAd expressing antigen presenting cells. The BY48AO-9 hybridoma was isolated from this fusion and responded to OVA plus IAb-expressing CHB-2.4.4 cells and IAd-expressing A20.2J cells. Total RNA was extracted from BY48AO-9 and used to prepare cDNA with oligo-dT primers and Superscript II reverse-transcriptase (Invitrogen) according to manufacturers instructions. cDNA was subjected to a panel of Vα-Cα PCRs, before direct sequencing from the successful PCR reaction. A Vα13.1-Jα20 containing TCRα chain was identified from the BY48AO-9 hybridoma. The sequence of this TCRα chain was identical to that expressed by the DO-11.10 hybridoma, with a single amino acid change at position 93, where a Ser in DO-11.10 TCRα was replaced by Ala in BY48AO-9 hybridoma.
Hybridoma stimulation
For all stimulations, 5×104 hybridoma cells were cultured overnight with different stimuli. For OVA323-339 and Eα52–68 peptide stimulation, hybridomas were cultured with 5×104 IAb-expressing Chb-2.4.4 cells or IAd-expressing A20.2J cells plus the indicated concentration of peptide. For 3K peptide stimulation, hybridomas were stimulated with fibroblasts expressing IAb with linked 3K peptide 6. For stimulation with antibodies, hybridomas were cultured overnight with indicated concentration of plate bound anti-TCRβ (H57-597) or anti-CD3ε (2C11). For stimulation with allotype MHC, hybridomas were cultured overnight with 1×106 total spleen cells from mice expressing different H-2 haplotypes. Hybridoma responses were measured by IL-2 ELISA, using standard protocols.
Generation of retroviral bone marrow chimeras
To isolate donor hematopoetic stem cells, TCRβ −/ − TCRδ −/− double decifient or TCRβ −/− mice were injected intraperitoneally with 5-fluorouracil (150 mg/kg) in balanced salt solution. After 5 days, single cell suspensions of bone marrow cells were prepared from femur, tibia, humerus, pelvis, and sternum and subjected to red blood cell lysis with buffered ammonium chloride solution.
After harvest, cells were cultured at 2×106/ml in DMEM supplemented with 15% fetal calf serum, antibiotics, glucose, and 2-ME plus 10% conditioned-medium containing stem cell cytokines. Stem cell cytokine conditioned medium (generously provided by Dr. Yosef Refaeli, National Jewish Health) was produced by transient transfection of 293 cells with expression plasmids encoding IL-3, IL-6, and SCF. Bone marrow cells were retrovirally infected 3 times on days 1, 2, and 3 post-harvest. For infection, cells were spin-infected with retroviral supernatants containing 8 µg/ml polybrene for 90 min at 37°C in 24 well plates at 670g. During spin-infection, retroviral supernatants were supplemented with IL-3, IL-6, and SCF containing supernatant. After spin-infection, cells were returned to culture conditions described above. On day 4 post-harvest, bone marrow cells were assessed for retroviral reporter expression (GFP) by flow cytometry. For all experiments, GFP expression on total bone marrow cells after 4 days of in vitro culture ranged from 30–60%.
RAG1 −/− recipient mice were irradiated with 400 RAD or 600 RAD on the day of bone marrow transfer. Recipients were injected in the lateral tail vein with transduced bone marrow cells containing ~5.0×105 GFP expressing cells.
Analysis of bone marrow chimeras
Bone marrow chimeras were analyzed at days 25–34 post reconstitution. Single cell suspensions from thymus, spleen, and pooled axillary, brachial and inguinal lymph nodes were subjected to red blood cell lysis with buffered ammonium chloride solution and total cell counts were determined. Mice that reconstituted poorly (those with less than 3.0×107 total thymocytes) were excluded from the analysis. In all experiments, the two thymic lobes from each mouse were analyzed separately. Data for frequencies of cell populations are shown for each individual lobe. Data for absolute numbers of thymocytes are the sum of both lobes from individual mice.
Cell surface staining and flow cytometry
Prior to staining, T cell hybridomas and ex vivo cell suspensions were incubated with anti-CD16/CD32 antibody producing hybridoma supernatant (clone 2.4G2). Cells were stained under saturating conditions with antibodies to mouse TCRβ (clone H57-597), CD3ε (clone 2C11), CD4 (clone GK1.5), CD8 (clone 53-6.7), CD25 (clone PC61), CD44 (clone IM7), CD5 (clone 53-7.3), CD69 (clone H1.2F3), CD24/HSA (clone M1/69), B220 (clone RA3-6B2), Vβ6 (clone RR4–7), Vβ8.1/8.2 (clone MR5-2), Vβ8.1/8.2 (clone KJ16), pan-Vβ8 (clone F23.1), Vβ8.2 (clone F23.2), DO-11.10 TCR (clone KJ1-26), Vα2 (clone B20.1), and Vα8.3 (clone B21.14) purchased from Ebiosciences, BD Pharmingen, or generated in house.
Cells were analyzed by flow cytometry on FACScan (BD Biosciences), FACScalibur (BD Biosciences), LSR II (BD Biosciences), or CyAn (Beckman-Coulter) instruments and data wereanalyzed in FlowJo (Treestar).
Sequencing of CDR3a loops
Sequencing of TCRα chains from hybridomas was performed as described above, for the BY48AO-9 hybridoma. For sequencing of TCRα chains from bulk T cells, CD8+ T cells were isolated by FACS from pooled spleen and LN cells using a MoFlo cell sorter (Dakocytomation). Total RNA was extracted from sorted cells with TRIzol (Invitrogen) and used to prepare cDNA with oligo-dT primers and Superscript II reverse-transcriptase (Invitrogen) according to manufacturer’s instructions. cDNA was subjected to PCR with Vα8 and Cα primers. The PCR reaction was fractionated on an agarose gel and bands of the appropriate length were isolated by gel extraction (QIAgen). Purified DNA fragments were cloned by T/A-overhang ligation (PCR2.1-TOPO, Invitrogen) according to manufacturer’s instructions. After cloning, individual colonies were subjected to a second PCR with Vα8 and Cα primers. Successful PCR reactions were sequenced directly.
Statistical Analysis
An unpaired two-tailed Student's t-test was applied using GraphPad software (Prism).
Supplementary Material
Supplementary Information accompanies the paper on www.nature.com/nature.
Acknowledgements
We thank Drs. Eric Huseby, Kevin Urdahl, Chyi Hsieh, Gabriela Hernández-Hoyos, and members of the Kappler/Marrack and Gapin Labs for thoughtful discussion. We thank the flow cytometry facilities at NJH and UCD for technical assistance with cell sorting. We thank Drs. Rebecca O’Brien and Willi Born (NJH) for providing TCRβ−/−TCRδ−/− mice and Dr. Yosef Refaeli (NJH) for providing stem cell cytokines. This work was supported by grants from the National Institutes of Health (AI18785 and AI22295 to P.M. and J.W.K. and AI057485 to L.G.). P.M. and J.W.K. are investigators of the Howard Hughes Medical Institute. J.P.S.-B. was supported by an NIH training grant (T32 AI07405).
References
- 1.Zinkernagel RM, Doherty PC. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974;248:701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]
- 2.Bevan MJ. In a radiation chimaera, host H-2 antigens determine immune responsiveness of donor cytotoxic cells. Nature. 1977;269:417–418. doi: 10.4049/jimmunol.176.1.677. [DOI] [PubMed] [Google Scholar]
- 3.Fink PJ, Bevan MJ. H-2 antigens of the thymus determine lymphocyte specificity. J Exp Med. 1978;148:766–775. doi: 10.1084/jem.148.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zinkernagel RM, et al. On the thymus in the differentiation of "H-2 self-recognition" by T cells: evidence for dual recognition? J Exp Med. 1978;147:882–896. doi: 10.1084/jem.147.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kisielow P, Teh HS, Bluthmann H, von Boehmer H. Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature. 1988;335:730–733. doi: 10.1038/335730a0. [DOI] [PubMed] [Google Scholar]
- 6.Sha WC, et al. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature. 1988;336:73–76. doi: 10.1038/336073a0. [DOI] [PubMed] [Google Scholar]
- 7.Jameson SC, Hogquist KA, Bevan MJ. Positive selection of thymocytes. Annu Rev Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- 8.Jerne NK. The somatic generation of immune recognition. Eur J Immunol. 1971;1:1–9. doi: 10.1002/eji.1830010102. [DOI] [PubMed] [Google Scholar]
- 9.Zerrahn J, Held W, Raulet DH. The MHC reactivity of the T cell repertoire prior to positive and negative selection. Cell. 1997;88:627–636. doi: 10.1016/s0092-8674(00)81905-4. [DOI] [PubMed] [Google Scholar]
- 10.Merkenschlager M, et al. How many thymocytes audition for selection? J Exp Med. 1997;186:1149–1158. doi: 10.1084/jem.186.7.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blackman M, et al. The T cell repertoire may be biased in favor of MHC recognition. Cell. 1986;47:349–357. doi: 10.1016/0092-8674(86)90591-x. [DOI] [PubMed] [Google Scholar]
- 12.Marrack P, Scott-Browne JP, Dai S, Gapin L, Kappler JW. Evolutionarily Conserved Amino Acids That Control TCR-MHC Interaction. Annu Rev Immunol. 2008;26:171–203. doi: 10.1146/annurev.immunol.26.021607.090421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–466. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- 14.Dai S, et al. Crossreactive T Cells spotlight the germline rules for alphabeta T cell-receptor interactions with MHC molecules. Immunity. 2008;28:324–334. doi: 10.1016/j.immuni.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng D, Bond CJ, Ely LK, Maynard J, Garcia KC. Structural evidence for a germline-encoded T cell receptor-major histocompatibility complex interaction 'codon'. Nat Immunol. 2007;8:975–983. doi: 10.1038/ni1502. [DOI] [PubMed] [Google Scholar]
- 16.Maynard J, et al. Structure of an autoimmune T cell receptor complexed with class II peptide-MHC: insights into MHC bias and antigen specificity. Immunity. 2005;22:81–92. doi: 10.1016/j.immuni.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Lee PU, Churchill HR, Daniels M, Jameson SC, Kranz DM. Role of 2CT cell receptor residues in the binding of self- and allo-major histocompatibility complexes. J Exp Med. 2000;191:1355–1364. doi: 10.1084/jem.191.8.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manning TC, et al. Alanine scanning mutagenesis of an alphabeta T cell receptor: mapping the energy of antigen recognition. Immunity. 1998;8:413–425. doi: 10.1016/s1074-7613(00)80547-6. [DOI] [PubMed] [Google Scholar]
- 19.Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 20.Arden B, Clark SP, Kabelitz D, Mak TW. Mouse T-cell receptor variable gene segment families. Immunogenetics. 1995;42:501–530. doi: 10.1007/BF00172177. [DOI] [PubMed] [Google Scholar]
- 21.Grubin CE, Kovats S, deRoos P, Rudensky AY. Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II-bound self-peptides. Immunity. 1997;7:197–208. doi: 10.1016/s1074-7613(00)80523-3. [DOI] [PubMed] [Google Scholar]
- 22.Wong P, Goldrath AW, Rudensky AY. Competition for specific intrathymic ligands limits positive selection in a TCR transgenic model of CD4+ T cell development. J Immunol. 2000;164:6252–6259. doi: 10.4049/jimmunol.164.12.6252. [DOI] [PubMed] [Google Scholar]
- 23.Scott-Browne JP, et al. Germline-encoded recognition of diverse glycolipids by natural killer T cells. Nat Immunol. 2007;8:1105–1113. doi: 10.1038/ni1510. [DOI] [PubMed] [Google Scholar]
- 24.White J, Pullen A, Choi K, Marrack P, Kappler JW. Antigen recognition properties of mutant V beta 3+ T cell receptors are consistent with an immunoglobulin-like structure for the receptor. J Exp Med. 1993;177:119–125. doi: 10.1084/jem.177.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arden B, Clark SP, Kabelitz D, Mak TW. Mouse T-cell receptor variable gene segment families. Immunogenetics. 1995;42:501–530. doi: 10.1007/BF00172177. [DOI] [PubMed] [Google Scholar]
- 26.White J, Pullen A, Choi K, Marrack P, Kappler JW. Antigen recognition properties of mutant V beta 3+ T cell receptors are consistent with an immunoglobulin-like structure for the receptor. J Exp Med. 1993;177:119–125. doi: 10.1084/jem.177.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott-Browne JP, et al. Germline-encoded recognition of diverse glycolipids by natural killer T cells. Nat Immunol. 2007;8:1105–1113. doi: 10.1038/ni1510. [DOI] [PubMed] [Google Scholar]
- 28.Yague J, et al. The T cell receptor: the alpha and beta chains define idiotype, and antigen and MHC specificity. Cell. 1985;42:81–87. doi: 10.1016/s0092-8674(85)80103-3. [DOI] [PubMed] [Google Scholar]
- 29.Kappler JW, Skidmore B, White J, Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981;153:1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huseby ES, et al. How the T cell repertoire becomes peptide and MHC specific. Cell. 2005;122:247–260. doi: 10.1016/j.cell.2005.05.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information accompanies the paper on www.nature.com/nature.