Abstract
Objective
Deletion of the lysophospholipid-sensitive receptor, G2A, in low-density lipoprotein receptor knockout (LDLR-/-) mice elevates plasma high-density lipoprotein (HDL) cholesterol and suppresses atherosclerosis. However, chemotactic action of G2A in monocytes/macrophages, in addition to its modulatory effect on HDL, may contribute to the proatherogenic action of G2A.
Methods and Results
We determined that deletion of G2A in LDLR-/- mice increases the ApoA1, ApoE, and cholesterol content of plasma HDL fractions. Hepatocytes were shown to express G2A and hepatocytes from G2A-deficient LDLR-/- mice secreted more ApoA1 and ApoE in HDL fractions compared to their G2A-sufficient counterparts. The atheroprotective and HDL modulatory effects of G2A deficiency were dependent on the presence of ApoE, as deletion of G2A in ApoE-/- and ApoE-/-LDLR-/- mice failed to raise HDL and did not suppress atherosclerosis. G2A deficiency in bone marrow-derived cells of LDLR-/- mice had no effect on atherosclerosis or HDL, whereas G2A deficiency in resident tissues was sufficient to raise HDL and suppress atherosclerosis.
Conclusion
These data demonstrate that the chemotactic function of G2A in bone marrow-derived monocytes does not modulate atherosclerosis in LDLR-/- mice and suggest an ApoE-dependent function for G2A in the control of hepatic HDL metabolism that might contribute to its proatherogenic action.
Keywords: G2A, atherosclerosis, high-density lipoprotein, ApoE, bone marrow, hepatocytes
G2A is a lysophospholipid-sensitive G protein-coupled receptor (GPCR) expressed by monocytes/macro-phages, lymphocytes, and endothelial cells.1,2 G2A is acti-vated by structurally related lysophospholipids3,4 and oxidized free fatty acids such as 9-S-hydroxyoctadecadienoic acid (9-S-HODE).5 The absence of G2A suppresses aortic atherosclerosis in hypercholesterolemic LDLR-/- mice.6 However, both proatherogenic and antiatherogenic effects have been ascribed to G2A. First, G2A mediates macrophage chemotaxis to lysophosphatidylcholine (LPC),3 a lysophospholipid produced during the oxidation of LDL.7 This chemotactic function has therefore been proposed to contribute to the proatherogenic action of G2A in LDLR-/- mice by facilitating intimal monocyte/macrophage recruitment.6Secondly, elevated concentrations of plasma HDL-cholesterol in hypercholesterolemic G2A-deficient LDLR-/- (LDLR-/-G2A-/-) mice6 suggested that modulation of HDL metabolism might also contribute to the proatherogenic action of G2A. Finally, one study reported proinflammatory effects of G2A deficiency in aortic endothelial cells of normal LDLR+/+ (C57BL/6J) mice that resulted in increased monocyte adhesion to aortic endothelium.2 Although this latter study suggests that G2A expressed in endothelium may be atheroprotective, its consequences in atherogenic mouse models have not been determined.
In the current study, we demonstrate that G2A deficiency increases the ApoA1, ApoE, and cholesterol content of plasma HDL fractions in hypercholesterolemic LDLR-/- mice. Primary mouse hepatocytes were found to express G2A, and hepatocytes from hypercholesterolemic LDLR-/-G2A-/- mice secreted significantly more ApoA1 and ApoE containing HDL compared to their LDLR-/-G2A+/+ counterparts. The abilities of G2A deficiency to raise HDL and to confer atheroprotection were both dependent on the presence of ApoE as deletion of G2A in ApoE-/- and ApoE-/-LDLR-/- mice failed to increase plasma HDL levels and did not suppress atherosclerosis. Consistent with an association between HDL elevation and the atheroprotective effect of G2A deficiency, bone marrow transplantation revealed that deletion of G2A in resident tissues is sufficient to raise plasma HDL and suppress atherosclerosis in LDLR-/- mice, whereas the absence of G2A in bone marrow-derived cells has no effect on HDL levels or atherosclerosis. These data collectively demonstrate that the proposed chemotactic function of G2A in bone marrow-derived monocytes is not a mechanism by which this receptor promotes atherosclerosis in LDLR-/- mice and suggest that modulation of hepatic HDL secretion might contribute to an ApoE-dependent proatherogenic action of G2A.
Materials and Methods
Detailed methods can be found in the supplemental materials (available online at http://atvb.ahajournals.org). N10 C57BL/6J G2A-/- mice were bred with C57BL/6J LDLR-/- or with C57BL/6J ApoE-/- mice (The Jackson Laboratories Bar Harbor, Maine), and resulting compound heterozygotes were intercrossed to obtain genetically matched LDLR-/-G2A+/+, LDLR-/-G2A-/-, ApoE-/-G2A+/+, and ApoE-/-G2A-/- mice. ApoE-/-LDLR-/-G2A+/+ and ApoE-/-LDLR-/-G2A-/- mice were generated by intercrossing compound heterozygotes derived from breeding LDLR-/-G2A-/- with ApoE-/-G2A+/+ mice. All experimental procedures were performed with approval from the Animal Care Committee of the University of Alabama. En face and aortic root lesion measurements were performed as previously described.6 Hepatocytes were prepared from mice by hepatic collagenase perfusion. Immunoblotting for apolipoproteins was performed on protein extracts isolated from primary hepatocytes and thioglycolate-elicited peritoneal macrophages, and on lipoprotein fractions from plasma or hepatocyte conditioned medium separated by fast-performance liquid chromatography. Quantitative real-time PCR for G2A and apolipoprotein expression was performed on primary hepatocytes or thioglycolate-elicited peritoneal macrophages.
Results
G2A Deficiency Raises Plasma ApoA1-HDL Levels in Hypercholesterolemic LDLR-/-Mice
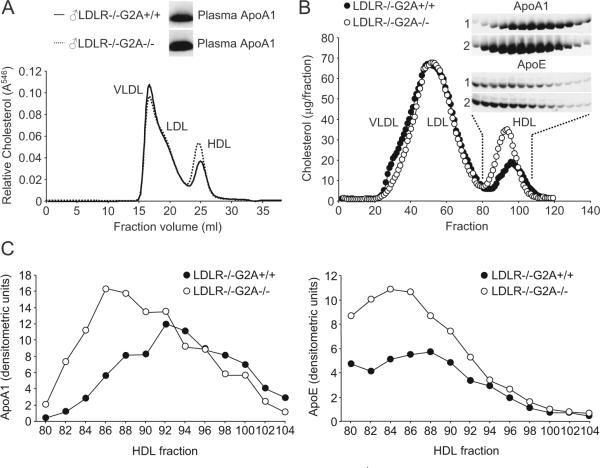
Using fast performance liquid chromatography (FPLC) with online cholesterol analysis, we confirmed significant increases in plasma HDL-cholesterol concentrations in high-cholesterol diet-fed LDLR-/-G2A-/- mice (average 36.4% increase; Figure 1A). Plasma levels of ApoA1 were significantly increased in the same LDLR-/-G2A-/- mice (Figure 1A). Increased ApoA1 content of HDL fractions in LDLR-/-G2A-/- mice was confirmed by fractionation of plasma lipoproteins, subsequent immunoblot analysis, and densitometric scanning (Figure 1B and 1C). Consistent with the high ApoE content of HDL in mice,8 ApoE was also increased in HDL fractions from LDLR-/-G2A-/- mice (Figure 1B and 1C). Therefore, elevated plasma HDL-cholesterol concentrations in hypercholesterolemic LDLR-/-G2A-/- mice are characterized by increases in ApoA1 and ApoE in HDL fractions.
Figure 1.
G2A deficiency increases ApoA1 and ApoE in plasma HDL of LDLR-/- mice. A, Online cholesterol analysis of plasma lipoproteins in high-cholesterol diet-fed (20 weeks) male LDLR-/-G2A+/+ and LDLR-/-G2A-/- mice by FPLC (CLIP). Data shown for individual mice is representative of 3 independent experiments. Inset, Anti-ApoA1 immunoblot of plasma from the same mice. B, FPLC separation of plasma lipoproteins from high-cholesterol diet-fed (20 weeks) LDLR-/-G2A+/+ and LDLR-/-G2A-/- mice. Inset, Immunoblot of pooled consecutive pairs of HDL fractions (indicated by dashed lines) with antibodies specific for ApoA1 and ApoE (1: LDLR-/-G2A+/+, 2: LDLR-/-G2A-/-). C, Densitometric quantification of ApoA1 and ApoE immunoreactivity in the indicated HDL fractions from B.
Although consistent with a greater number of circulating HDL particles in LDLR-/-G2A-/- mice, the increased ApoA1 content of plasma HDL fractions in these animals could also reflect an elevated ApoA1 stoichiometry of individual HDL particles.9 Indeed, this, in addition to increased cholesterol content, could contribute to the slightly larger size of HDL particles in LDLR-/-G2A-/- mice (indicated by a leftward shift of the HDL cholesterol profile in Figure 1).
Increased Secretion of ApoA1 by Hepatocytes in Hypercholesterolemic LDLR-/-G2A-/- Mice
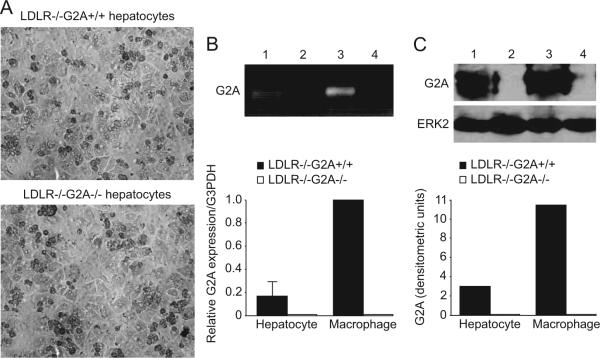
The liver is the major tissue responsible for ApoA1 production and HDL clearance in mice, whereas the small intestine contributes a lesser amount of ApoA1 to the circulating HDL pool.10,11 We therefore isolated primary mouse hepatocytes and performed quantitative real-time PCR (RT-PCR) analysis of G2A expression. Hepatocytes from high-cholesterol diet-fed LDLR-/- mice were found to express G2A (Figure 2B), as were hepatocytes from chow diet-fed LDLR-/- and LDLR+/+ mice (data not shown). Analysis of G2A expression in hepatocytes relative to peritoneal macrophages from the same mice revealed that hepatocytes express approximately 6-fold lower levels of G2A compared to macrophages. Expression of G2A in hepatocytes was confirmed by immunoblot analysis with a G2A-specific antibody (Figure 2C).
Figure 2.
G2A is expressed by primary mouse hepatocytes. A, Morphological characteristics of primary hepatocytes isolated from high-cholesterol diet-fed (20 weeks) LDLR-/-G2A+/+ and LDLR-/-G2A-/- mice. B, Quantitative RT-PCR analysis showing G2A expression by primary hepatocytes (macrophages shown as positive control). The data shown is the average of 3 independent experiments, each performed in triplicate. Inset, Ethidium bromide-stained agarose gel showing the expected size of G2A-specific amplification products. 1: LDLR-/-G2A+/+ hepatocytes, 2: LDLR-/-G2A-/- hepatocytes, 3: LDLR-/-G2A+/+ macrophages, 4: LDLR-/-G2A-/- macrophages. C, Immunoblot analysis of G2A protein levels in hepatocytes and macrophages from LDLR-/-G2A+/+ and LDLR-/-G2A-/- mice. 1: LDLR-/-G2A+/+ hepatocytes, 2: LDLR-/-G2A-/- hepatocytes, 3: LDLR-/-G2A+/+ macrophages, 4: LDLR-/-G2A-/- macrophages. ERK2 immunoblot is shown as a control for protein loading.
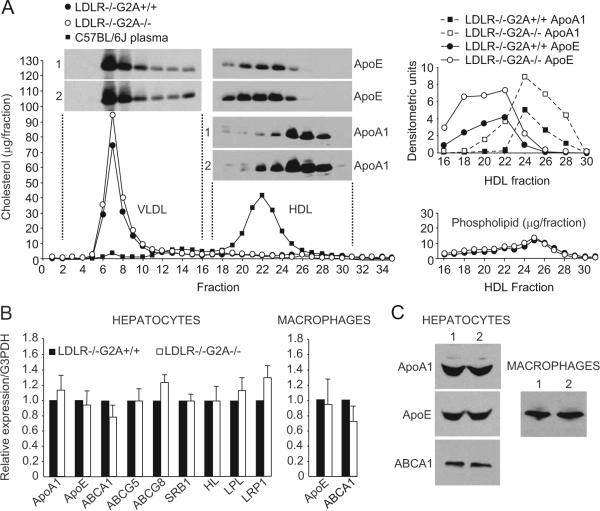
We next investigated whether functional alterations were present in hepatocytes from high-cholesterol diet-fed LDLR-/-G2A-/- mice that may contribute to the elevated plasma ApoA1-HDL levels in these mice. Hepatocytes were isolated from high-cholesterol diet-fed LDLR-/-G2A+/+ and LDLR-/-G2A-/- mice (identical to those in Figure 1), and their ability to secrete ApoA1-HDL was measured in culture. Conditioned medium from hepatocytes cultured for 18 hours was concentrated and fractionated by FPLC. As expected, cholesterol was found predominantly on VLDL particles in conditioned medium from both LDLR-/-G2A+/+ and LDLR-/-G2A-/- hepatocytes, with little detectable in fractions of newly-secreted lipid-poor HDL12 (Figure 3A). Immunoblot analysis revealed significant increases in the amount of ApoA1 and ApoE in HDL fractions secreted by hepatocytes from high-cholesterol diet-fed LDLR-/-G2A-/- mice (Figure 3A), consistent with the apolipoprotein alterations in plasma HDL fractions from the same mice (Figure 1B and 1C). Thus, increased secretion of ApoA1 by hepatocytes might contribute to the elevated plasma HDL levels in hypercholesterolemic LDLR-/-G2A-/- mice.
Figure 3.
Increased ApoA1-HDL secretion by hepatocytes from high-cholesterol diet-fed LDLR-/-G2A-/- mice. A (left), FPLC separation of lipoproteins secreted into culture medium over 18 hours by freshly isolated hepatocytes from high-cholesterol diet-fed (20 weeks) LDLR-/-G2A+/+ and LDLR-/-G2A-/- mice. Inset, Immunoblot of HDL fractions showing significant increases in ApoA1 and ApoE in HDL fractions secreted by LDLR-/-G2A-/- hepatocytes. Anti-ApoE immunoblot of VLDL/LDL fractions shown alongside. Right, Densitometric quantification of ApoA1 and ApoE immunoreactivity and total phospholipid content in the indicated HDL fractions. Data shown is representative of 3 independent experiments. B, Quantitative RT-PCR analysis of the expression of the indicated genes in primary hepatocytes and macrophages from male high-cholesterol diet-fed (20 weeks) LDLR-/-G2A+/+ and LDLR-/-G2A-/- mice. The data shown are the average of 3 independent experiments, each performed in triplicate. C, Immunoblot analysis of ApoA1, ApoE, and ABCA1 levels in primary hepatocytes from high-cholesterol diet-fed (20 weeks) LDLR-/-G2A+/+ and LDLR-/-G2A-/- mice (1: LDLR-/-G2A+/+, 2: LDLR-/-G2A-/-). Macrophage ApoE levels in the same mice shown alongside.
No significant differences in the expression of ApoA1, ApoE, and the principal ATP-binding cassette transporter involved in HDL formation, ABCA1, were detected in freshly isolated hepatocytes from high-cholesterol diet-fed LDLR-/-G2A+/+ and LDLR-/-G2A-/- mice by RT-PCR (Figure 3B). In addition, protein levels of ApoE, ApoA1, and ABCA1 were comparable in these hepatocytes (Figure 3C). The expression of genes encoding other ATP-binding cassette transporters (ABCG5, ABCG8), scavenger receptor class B type 1 (SRB1), LDLR-related protein-1 (LRP1), hepatic lipase (HL), and lipoprotein lipase (LPL) were also unaffected by G2A deficiency in hepatocytes from high-cholesterol diet-fed LDLR-/- mice (Figure 3B). Thus, transcriptional alterations in the hepatic expression of ApoA1, ApoE, and genes involved in HDL biogenesis do not contribute to the HDL elevations in hypercholesterolemic LDLR-/-G2A-/- mice.
G2A Deficiency Does Not Result in Plasma HDL Elevation in the Absence of ApoE
In addition to LDLR-/- mice, ApoE-/- mice are widely used to study atherogenic mechanisms. However, plasma HDL in mice contains considerably larger amounts of ApoE compared to that in humans,8 much of it originating from the hepatic recycling of ApoE from VLDL and remnant lipoproteins.13 It is therefore possible that HDL metabolism may be affected by the absence of ApoE in a manner that influences the modulatory effect of G2A deficiency on HDL. Furthermore, ApoE is a multi-functional protein capable of influencing a broad range of biological processes in different cell types,14 and so its absence could affect processes other than HDL metabolism on which the atheroprotective effect of G2A deficiency is dependent. We therefore examined the consequences of G2A deficiency in the ApoE-/- model. No statistically significant effect of G2A deficiency on plasma HDL-cholesterol or LDL-cholesterol levels was observed in chow diet-fed ApoE-/- mice or high-cholesterol diet-fed ApoE-/- mice (supplemental Figure IA; Table). Furthermore, ApoA1 levels in plasma HDL fractions from ApoE-/-G2A+/+ and ApoE-/-G2A-/- mice were comparable (supplemental Figure IB). Thus, the ApoA1 and cholesterol content of plasma HDL is not affected by G2A deficiency in ApoE-/- mice.
Table.
G2A Deficiency Does Not Significantly Alter Plasma Lipid Profiles in Male or Female High-Cholesterol Diet-Fed ApoE-/- Mice
| TC (mg/dl) | EC (mg/dl) | LDL-C (mg/dl) | HDL-C (mg/dl) | TG (mg/dl) | FA (mg/dl) | |
|---|---|---|---|---|---|---|
| ♂ApoE-/-G2A+/+ | 1078±163.5 | 717.4±132.4 | 1059.1±162.3 | 19.3±8.7 | 39.2±27.3 | 48.3±15.7 |
| ♂ApoE-/-G2A-/- | 1280.2±295.4 | 864.2±104.4 | 1257±289.7 | 22.2±10.7 | 42.6±27.6 | 55.7±13.2 |
| ♀ApoE-/-G2A+/+ | 1268.8±365.5 | 874.9±193.4 | 1247.9±362.8 | 20.9±8.7 | 24.4±23.2 | 41.2±8.3 |
| ♀ApoE-/-G2A-/- | 1197.7±215.9 | 868.2±142.6 | 1177±212 | 20.7±7.1 | 19.7±12.5 | 48.2±14.3 |
Plasma concentrations of the indicated lipids/lipoproteins in male and female high-cholesterol diet-fed (20 weeks) ApoE-/-G2A+/+ and ApoE-/-G2A-/- mice. Mean with standard deviation shown. TC indicates total cholesterol; EC, esterified cholesterol; LDL-C, low-density lipoprotein-cholesterol (VLDL+LDL); HDL-C, high-density lipoprotein-cholesterol; TG, triglycerides; FA, free fatty acids.
To determine the impact of ApoE deletion on the ability of G2A deficiency to raise plasma HDL levels in LDLR-/- mice, we generated ApoE-/-LDLR-/-G2A+/+ and ApoE-/-LDLR-/-G2A-/- triple-mutant mice. Although the presence of cholesterol in plasma HDL fractions from ApoE-/-LDLR-/-G2A+/+ and ApoE-/-LDLR-/-G2A-/- mice was very low, immunoblotting revealed no significant differences in the amount of ApoA1 (supplemental Figure IC). Thus, the absence of ApoE in LDLR-/- mice abolishes the effect of G2A on plasma HDL levels.
G2A Deficiency Does Not Suppress Atherosclerosis in the Absence of ApoE
To determine whether the inability of G2A deficiency to raise plasma HDL in the absence of ApoE (supplemental Figure I) coincided with an attenuation of its atheroprotective effect, we examined atherosclerosis in ApoE-/-G2A-/- and ApoE-/-G2A+/+ mice. G2A deficiency in male and female high-cholesterol diet-fed ApoE-/- mice failed to suppress atherosclerosis (supplemental Figure II). Interestingly, male, but not female, high-cholesterol diet-fed ApoE-/-G2A-/- mice developed significantly more aortic atherosclerosis (average 32% increase by en face analysis, P=0.004) compared to their ApoE-/-G2A+/+ counterparts (supplemental Figure IIA). However, no statistically significant difference in lesion size was found in the same male high-cholesterol diet-fed ApoE-/-G2A+/+ and ApoE-/-G2A-/- mice at the aortic root (supplemental Figure IIB). Female high-cholesterol diet-fed ApoE-/-G2A-/- mice, on the other hand, displayed a moderate increase in lesion size at the aortic root (average 11% increase, P=0.048; supplemental Figure IIB).
We also examined atherosclerosis in ApoE-/-G2A+/+ and ApoE-/-G2A-/- mice fed a chow diet. Male and female chow diet-fed ApoE-/-G2A-/- mice did not display statistically significant increases in aortic lesion coverage compared to their ApoE-/-G2A+/+ counterparts (supplemental Figure IID). Male chow diet-fed ApoE-/-G2A-/- mice displayed an increase in lesion size at the aortic root (average 19% increase, P=0.045), whereas female chow diet-fed ApoE-/-G2A+/+ and ApoE-/-G2A-/- mice developed atherosclerosis to comparable extents at this site (supplemental Figure IID).
Finally, we examined atherosclerosis in ApoE-/-LDLR-/- G2A+/+ and ApoE-/-LDLR-/-G2A-/- triple mutant mice. G2A deficiency did not protect against atherosclerosis in ApoE-/-LDLR-/- mice (supplemental Figure III), demonstrating that in addition to abolishing the effect of G2A deficiency on HDL, deletion of ApoE also ameliorates the atheroprotective effect of G2A deficiency in LDLR-/- mice. Thus, irrespective of gender or the type of diet intervention, G2A deficiency does not protect against atherosclerosis in the absence of ApoE. Furthermore, the site-specific increase in atherosclerosis in ApoE-/-G2A-/- mice reveals an atheroprotective effect of G2A in the absence of its HDL modulatory action.
Resident Tissues Mediate HDL Elevation and Atheroprotection in LDLR-/-G2A-/- Mice
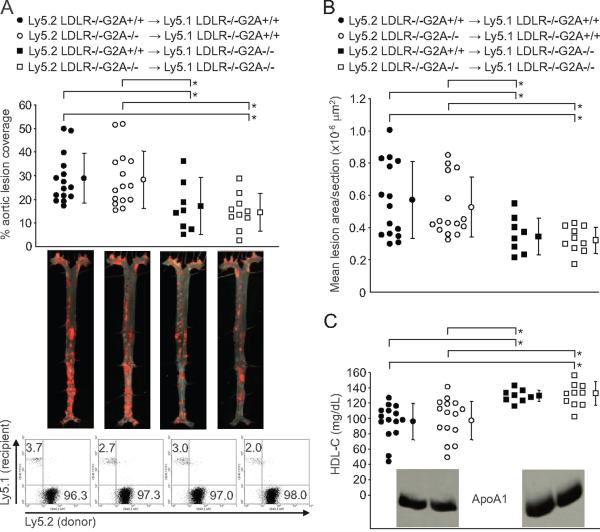
As the chemotactic action of G2A demonstrable in monocytes/macrophages in vitro3 may be expected to be manifested in both ApoE-/- and LDLR-/- atherogenic models, the ability of G2A deficiency to suppress atherosclerosis in LDLR-/- mice6 but not in ApoE-/- or ApoE-/-LDLR-/- mice appeared inconsistent with a significant contribution of this chemotactic function to the proatherogenic action of G2A in LDLR-/- mice. To directly address the role of monocytes/macrophages in mediating the atheroprotective effect of G2A deficiency in LDLR-/- mice, we performed bone marrow (BM) transplantation to generate chimeric LDLR-/- mice in which G2A deficiency was confined to BM-derived cells or resident tissues (Figure 4A). Donor and recipient BM-derived cells were distinguished by differential expression of Ly5.1 and Ly5.2 alloantigens. Hematopoietic reconstitution was confirmed in recipient LDLR-/- mice 8 weeks after transplantation by flow cytometric analysis of Ly5.2 (donor) and Ly5.1 (recipient) expression by major blood cell lineages (CD11b: myeloid, CD19: B lymphocytes, CD3, CD4, and CD8: T lymphocytes). Successfully reconstituted LDLR-/- mice with similarly repopulated peripheral blood CD11b+, CD19+, CD3+, CD4+, and CD8+ lineages were fed a high-cholesterol diet for 20 weeks, effectively excluding any potential effects of varying chimerism on the phenotypes observed in transplanted mice. Deletion of G2A in BM-derived cells did not significantly affect atherosclerosis at the aortic root or throughout the aorta (Figure 4A and 4B), and plasma HDL-cholesterol concentrations were unaffected (Figure 4C). However, deletion of G2A in resident tissues was sufficient to reduce atherosclerosis both at the aortic root and throughout the aorta (average 39.4% decrease in aortic lesion coverage, average 38.8% decrease in aortic root lesion area; Figure 4A and 4B) and also resulted in HDL elevation (average 35.3% increase; Figure 4C). Furthermore, only plasma HDL fractions from chimeric LDLR-/- mice lacking G2A expression in resident tissues had a significantly increased ApoA1 content (supplemental Figure V). Plasma LDL-cholesterol concentrations were not significantly different between the 4 BM transplanted groups (supplemental Figure IV). Finally, we confirmed that lesional macrophages in chimeric LDLR-/- mice were derived from donor BM by coimmunofluorescence staining with anti-CD11b, anti-Ly5.2 (donor-specific), and anti-Ly5.1 (recipient-specific) antibodies (supplemental Figure VI). Thus, resident tissues mediate both the atheroprotective and HDL modulatory effects of G2A deficiency in LDLR-/- mice.
Figure 4.
G2A deficiency in resident tissues is sufficient to raise plasma HDL and suppress atherosclerosis in LDLR-/- mice. BM expressed G2A does not modulate atherosclerosis. A, Aortic lesion coverage in male high-cholesterol diet-fed (20 weeks) Ly5.1 LDLR-/-G2A+/+ and Ly5.1 LDLR-/-G2A-/- mice transplanted with Ly5.2 LDLR-/-G2A+/+ or Ly5.2 LDLR-/-G2A-/- bone marrow (transplantation scheme shown above. ●, ○, ■, □: average, ⊤: standard deviation, for each experimental group). Representative en face aortic specimens shown below. Lower panels, Flow cytometric analysis of peripheral blood reconstitution in representative mice from each of the 4 transplantation groups after high cholesterol diet intervention. Residual host (Ly5.1+) cells (<4% of total WBCs) are exclusively long-lived CD3+ and CD44highCD62Llow T cells (data not shown). B, Average lesion areas at the aortic root in the same chimeric LDLR-/- mice shown in A. C, Plasma HDL-cholesterol concentrations in chimeric LDLR-/- mice. Inset, Immunoblots showing total plasma levels of ApoA1. Differences in aortic lesion coverage, average aortic root lesion area, and plasma HDL-cholesterol concentrations between the 4 groups of chimeric LDLR-/- mice were analyzed by ANOVA (P=0.011). Statistical analysis of reductions in atherosclerosis and elevations in plasma HDL-cholesterol concentrations in pair-wise comparisons were performed by Mann-Whitney Rank Sum test (*P<0.05).
Discussion
In the current study, we demonstrate that plasma HDL-cholesterol elevation in hypercholesterolemic LDLR-/-G2A-/- mice may be attributable, at least in part, to increased ApoA1-HDL secretion by hepatocytes (Figure 3A). Interestingly, the atheroprotective and HDL modulatory effects of G2A deficiency are dependent on the presence of ApoE, as G2A deficiency did not result in HDL elevation (supplemental Figure I; Table) and did not suppress atherosclerosis (supplemental Figures II and III) in ApoE-/- or ApoE-/-LDLR-/- mice. Furthermore, restricting G2A deficiency to BM-derived cells in LDLR-/- mice had no significant effect on atherosclerosis (Figure 4), suggesting that the reported chemotactic function of G2A in monocytes/macrophages3 does not contribute to the proatherogenic action of this receptor.
The collective data in ApoE-/-, ApoE-/-LDLR-/- (supplemental Figures I through III), and LDLR-/-6 models reveals an association between HDL elevation and the atheroprotective effect of G2A deficiency. Furthermore, reciprocal BM transplantation between LDLR-/-G2A+/+ and LDLR-/-G2A-/- mice revealed that atheroprotection and HDL elevation are concomitantly observed and only in mice lacking G2A in resident tissues. However, direct evidence for a cause-effect relationship between HDL elevation and atheroprotection in LDLR-/-G2A-/- mice will require the establishment of mouse models in which G2A deficiency is restricted to specific candidate cell-types. In this regard, resident cell-types in addition to hepatocytes should be considered, despite the potentiating effect of G2A deficiency on hepatocyte ApoA1-HDL secretion in LDLR-/- mice (Figure 3A). For example, small intestinal enterocytes contribute a significant amount of ApoA1 to the circulating HDL pool.11 Although there are no published reports of G2A expression in enterocytes, it is possible that a direct or indirect potentiating effect of G2A deficiency on ApoA1 production by this cell-type could contribute to plasma HDL elevation in LDLR-/-G2A-/- mice. However, such an effect of G2A deficiency would necessarily involve a requirement for exogenously derived ApoE as enterocytes do not synthesize this apolipoprotein.
The mechanistic basis for the ApoE-dependence of G2A-mediated effects on HDL and atherosclerosis may involve cell types other than, or in addition to, hepatocytes. Indeed, both endogenously expressed and exogenous ApoE exert antiatherogenic effects at the level of multiple cell types.14 Determination of the underlying cellular and molecular mechanisms should therefore provide important insight into atherogenic processes that are influenced by G2A. In the meantime, several possible scenarios can be postulated. Firstly, it is possible that the extent to which normal lipoprotein metabolism is perturbed in the absence of ApoE may be sufficient to prevent the modulatory effect of G2A deficiency on plasma HDL levels from being manifested in ApoE-/- mice. For example, modulatory effects of G2A deficiency on HDL may be dependent on the hepatic clearance of plasma VLDL and remnant lipoproteins, a process that requires ApoE binding to hepatic receptors and results in the subsequent recycling of ApoE onto HDL.15 It is also possible that G2A modulates plasma HDL levels in LDLR-/- mice by a mechanism involving direct action of G2A on ApoE. In the absence of transcriptional alterations in hepatocyte ApoE or ApoA1 expression (Figure 3B), it is worthwhile considering how G2A could potentially influence posttranscriptional mechanisms regulating apolipoprotein production. In this respect, G2A activity is regulated in response to extracellular accumulation of LPC and structurally related lysophospholipids by its redistribution from intracellular endosomal receptor pools to the plasma membrane.4,16 This mode of G2A regulation is reminiscent of intracellular recycling mechanisms regulating the endosomal trafficking and subsequent secretion of apolipoproteins by hepatocytes15 and may therefore be mechanistically relevant with respect to the increased ApoA1-HDL secretion by hepatocytes in LDLR-/-G2A-/- mice. However, the selective potentiating effect of G2A deficiency on hepatocyte ApoE-HDL secretion but not ApoE-VLDL secretion in hypercholesterolemic LDLR-/- mice (Figure 3A) suggests that G2A is not broadly regulating hepatocyte ApoE secretion and that a pathway restricted to hepatic ApoA1-HDL biogenesis is involved. Furthermore, the cellular origin of the ApoE on which the HDL modulatory effect of G2A deficiency is dependent may not necessarily be the hepatocyte itself. For example, ApoE derived from macrophages can be recycled by hepatocytes and subsequently secreted on HDL,15 resulting in elevations in HDL ApoA1 and cholesterol content.17 In addition, we cannot exclude a role for ApoE derived from extrahepatic resident cell-types such as adipose tissue, a major tissue mass that could potentially contribute a significant amount of ApoE to lipoproteins.
It is important to consider that HDL elevation may not contribute to the atheroprotective effect of G2A deficiency in LDLR-/- mice, and therefore the dependencies of HDL elevation and atheroprotection on ApoE are unrelated mechanistically. Such a scenario is certainly plausible considering the fact that ApoE is a multi-functional protein capable of influencing a wide range of biological processes other than HDL metabolism that can affect atherogenesis.14 For example, ApoE can attenuate inflammatory processes in the vessel wall at the level of endothelial cells18 and macrophages.19 Furthermore, ApoE is important for the efficient clearance of apoptotic cells and in its absence, the accumulation of necrotic cells contributes to an increase in systemic inflammation in ApoE-/- mice.20 The presence of ApoE can also inhibit proliferative responses of T lymphocytes to antigen stimulation21 and plays an important role in the delivery of lipid antigens to antigen presenting cells mediating activation of CD1 d+ NK T cells.22 Although the results of our BM transplantation study do not support a role for hematopoietic immune cells in mediating the atheroprotective effect of G2A deficiency in LDLR-/- mice (Figure 4), it is possible that the absence of ApoE alters the functional consequences of G2A deficiency in certain hematopoietic cell types compared to their counterparts in LDLR-/- mice and instead conferred a proatherogenic phenotype, as evidenced by the site-specific increase in atherosclerosis in ApoE-/-G2A-/- mice (supplemental Figure II). For example, recent studies demonstrating that LPC produced by cells undergoing apoptosis attracts macrophages through G2A suggest that this receptor may contribute to the efficient clearance of apoptotic cells.23 Considering the fact that phagocytic engulfment of apoptotic cells by macrophages from ApoE-/- mice is significantly impaired,20 it is possible that the absence of both a G2A-mediated attraction signal and an ApoE-dependent engulfment mechanism in ApoE-/-G2A-/- mice may result in an excessive accumulation of postapoptotic/necrotic cells within developing lesions resulting in a proatherogenic augmentation of local inflammation. On the other hand, it is possible that loss of a proapoptotic action of G2A in lesional macrophages may have contributed to the proatherogenic effect of G2A deficiency in ApoE-/- mice.1 If so, it is noteworthy that impaired apoptotic clearance and reduced lesional macrophage apoptosis may be more influential on lesion size at an early rather than advanced stage of atherogenesis.24 It is therefore possible that the proatherogenic effect of G2A deficiency may be more robust in ApoE-/- mice subjected to a shorter period of diet intervention than that used in our study to examine advanced lesions.
Gender exerted a significant influence on the penetrance of the site-specific proatherogenic effect of G2A deficiency in ApoE-/- mice. Although male high-cholesterol diet-fed ApoE-/-G2A-/- mice exhibited a significant increase in aortic atherosclerosis (P=0.004), their female counterparts did not. The increases in atherosclerosis at the aortic root of female high-cholesterol diet-fed and male chow diet-fed ApoE-/-G2A-/- mice, on the other hand, were less significant (P=0.048 and P=0.045 respectively). Although we do not have an explanation for the gender-dependence of this effect of G2A deficiency, it is possible that recognized atheroprotective properties of endogenous estrogens may be involved.25 Studies in ApoE-/- and LDLR-/- mice suggest that antiatherogenic effects of estrogens are mediated to a large extent at the level of inflammatory processes in the vessel wall rather than lipoprotein metabolism,25 raising the possibility that a suppressive effect of endogenous estrogens on local inflammation may have counterbalanced the proatherogenic influence of G2A deficiency in female ApoE-/- mice. This could be addressed by examining whether a similar proatherogenic effect of G2A deficiency to that in male ApoE-/-G2A-/- mice is observed in ovariectomized female ApoE-/-G2A-/- mice and determining whether it can be reversed by estrogen supplementation.
The proatherogenic influence of G2A deficiency in ApoE-/- mice is considerably less pronounced at the aortic root compared to the rest of the aorta (supplemental Figure II). Interestingly, the atheroprotective effect of G2A deficiency in LDLR-/- mice is also less pronounced at the aortic root, becoming manifested in advanced lesions but not at an early stage of atherogenesis.1,6 Such site-specific differences may be related to the higher local hemodynamic forces at sites of predilection to atherosclerosis such as the aortic root that can potentiate endothelial inflammation and cell adhesion molecule expression.26,27 It is possible that the resulting potentiation of inflammatory cell recruitment during lesion development may counterbalance the atheroprotective influence of G2A deficiency in LDLR-/- mice at early stages of atherogenesis. However, other explanations are equally worthy of consideration. For example, LPC levels in aortic tissue increase with atherosclerotic lesion burden.1 It is therefore possible that as local concentrations of LPC or other lipid activators increase with lesion progression, the presence of G2A in cell types of the vessel wall may become more influential at later stages of atherogenesis. In this respect, monocytes/macrophages are unlikely to be a target of such LPC action, as deleting G2A in BM-derived cells does not affect lesion progression at the aortic root or throughout the aorta in LDLR-/- mice (Figure 4). Although one could postulate that G2A influences the chemoattraction or activation of long-lived lymphocyte subsets with a more significant role in the progression of atherogenesis that were not completely replaced by BM transplantation in our study, one would have expected to observe a milder atheroprotective effect at the aortic root in LDLR-/-G2A-/- mice transplanted with LDLR-/-G2A+/+ BM compared to LDLR-/-G2A-/- mice transplanted with LDLR-/-G2A-/- BM if this were so. This was not the case (Figure 4B). Rather, G2A deficiency in resident cell types is sufficient to attenuate lesion progression in LDLR-/- mice (Figure 4), and so a role for resident cell-types in the vessel wall should be considered. For example, G2A is expressed by aortic endothelial cells, but not by aortic smooth muscle cells.1,2 Bolick et al demonstrated a proinflammatory influence of G2A deficiency on the expression of endothelial cell adhesion molecules that contributes to increased monocyte adhesion to aortic endothelium in G2A-/- C57BL/6J (LDLR+/+) mice.2 Although such an effect would be expected to promote, rather than attenuate, atherosclerosis, it is possible that the absence of G2A may have an opposite effect on endothelial cell inflammation under conditions of prolonged hypercholesterolemia in which local concentrations of LPC in the vessel wall are particularly high. On the other hand, assuming that HDL elevation is obligatory for atheroprotection in LDLR-/-G2A-/- mice, we cannot exclude the possibility that a proinflammatory/proatherogenic effect of G2A deficiency in endothelial cells was obscured in LDLR-/- mice by this concomitant atheroprotective influence of HDL elevation. If so, one could postulate that a proatherogenic endothelial phenotype was manifested in ApoE-/-G2A-/- mice because of the absence of HDL elevation and thus contributed to the site-specific increase in atherosclerosis in this model.
Further studies in mice with tissue-specific deficiency or overexpression of G2A will be required to determine whether a cause/effect relationship exists between HDL elevation and athero-protection in LDLR-/-G2A-/- mice and to establish the mechanistic basis for the ApoE-dependence of G2A-mediated proatherogenic action. These animal models will also be important to investigate the role of monocytes/macrophages and other immunomodulatory cell-types in mediating the site-specific proatherogenic effect of G2A deficiency in ApoE-/- mice. Such approaches will be vital, as the cumulative data suggest that G2A may have opposing actions in different cell-types with respect to atherogenesis. Further-more, the phenotypic penetrance of these actions in mice is influenced by the hypercholesterolemic model used as well as the stage of atherogenesis (early versus advanced lesions).1,6 In addition, studies examining the molecular interactions involved in G2A action must address the potential involvement of a broad range of lipid activators generated under hypercholesterolemic conditions (lysophospholipids and oxidized free fatty acids)4,5 and strive to recapitulate in vitro their modes of production in vivo in response to hypercholesterolemia and during atherogenesis. It is hoped that in combination with in vivo studies aimed at further dissecting cell-specific functions of G2A in atherosclerosis, such experimental approaches will identify the target tissues in which selective modulation of G2A function may have the potential to reduce atherosclerosis risk.
Supplementary Material
Acknowledgments
Sources of Funding This work was supported by a grant from the National Heart, Lung, and Blood Institute (NHLBI) (RO1 HL088642) and a Grant-in-aid from the American Heart Association (AHA-0655366B) to J.H.S.K. B.W.P. was supported by a National Institutes of Health Training Grant (T32 HL007918).
We thank Dr David Garber (UAB) for his invaluable advice and assistance with FPLC.
Footnotes
Disclosures None.
References
- 1.Parks BW, Gambill GP, Lusis AJ, Kabarowski JH. Loss of G2A promotes macrophage accumulation in atherosclerotic lesions of lowdensity lipoprotein receptor-deficient mice. J Lipid Res. 2005;46:1405–1415. doi: 10.1194/jlr.M500085-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.Bolick DT, Whetzel AM, Skaflen M, Deem TL, Lee J, Hedrick CC. Absence of the G protein-coupled receptor G2A in mice promotes mono-cyte/endothelial interactions in aorta. Circ Res. 2007;100:572–580. doi: 10.1161/01.RES.0000258877.57836.d2. [DOI] [PubMed] [Google Scholar]
- 3.Yang LV, Radu CG, Wang L, Riedinger M, Witte ON. Gi-independent macrophage chemotaxis to lysophosphatidylcholine via the immunoregulatory GPCR G2A. Blood. 2004;105:1127–1134. doi: 10.1182/blood-2004-05-1916. [DOI] [PubMed] [Google Scholar]
- 4.Frasch SC, Zemski-Berry K, Murphy RC, Borregaard N, Henson PM, Bratton DL. Lysophospholipids of different classes mobilize neutrophil secretory vesicles and induce redundant signaling through G2A. J Immunol. 2007;178:6540–6548. doi: 10.4049/jimmunol.178.10.6540. [DOI] [PubMed] [Google Scholar]
- 5.Obinata H, Izumi T. G2A as a receptor for oxidized free fatty acids. Prostaglandins Other Lipid Mediat. 2008 doi: 10.1016/j.prostaglandins.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Parks BW, Lusis AJ, Kabarowski JH. Loss of the lysophosphatidylcholine effector, G2A, ameliorates aortic atherosclerosis in low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol. 2006;26:2703–2709. doi: 10.1161/01.ATV.0000246774.02426.71. [DOI] [PubMed] [Google Scholar]
- 7.Parthasarathy S, Barnett J. Phospholipase A2 activity of low density lipoprotein: evidence for an intrinsic phospholipase A2 activity of apoprotein B-100. Proc Natl Acad Sci U S A. 1990;87:9741–9745. doi: 10.1073/pnas.87.24.9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L, Thompson PA, Kitchens RL. Infection induces a positive acute phase apolipoprotein E response from a negative acute phase gene: role of hepatic LDL receptors. J Lipid Res. 2008;49:1782–1793. doi: 10.1194/jlr.M800172-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavigiolio G, Shao B, Geier EG, Ren G, Heinecke JW, Oda MN. The interplay between size, morphology, stability, and functionality of high-density lipoprotein subclasses. Biochemistry. 2008;47:4770–4779. doi: 10.1021/bi7023354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A, Gebre AK, Coutinho JM, Colvin PL, Smith TL, et al. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J Clin Invest. 2005;115:1333–1342. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunham LR, Kruit JK, Iqbal J, Fievet C, Timmins JM, Pape TD, Coburn BA, Bissada N, Staels B, Groen AK, et al. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J Clin Invest. 2006;116:1052–1062. doi: 10.1172/JCI27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dietschy JM, Turley SD. Control of cholesterol turnover in the mouse. J Biol Chem. 2002;277:3801–3804. doi: 10.1074/jbc.R100057200. [DOI] [PubMed] [Google Scholar]
- 13.Heeren J, Grewal T, Laatsch A, Rottke D, Rinninger F, Enrich C, Beisiegel U. Recycling of apoprotein E is associated with cholesterol efflux and high density lipoprotein internalization. J Biol Chem. 2003;278:14370–14378. doi: 10.1074/jbc.M209006200. [DOI] [PubMed] [Google Scholar]
- 14.Getz GS, Reardon CA. Apoprotein E as a lipid transport and signaling protein in the blood, liver and artery wall. J Lipid Res. 2008 doi: 10.1194/jlr.R800058-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heeren J, Beisiegel U, Grewal T. Apolipoprotein E recycling: implications for dyslipidemia and atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:442–448. doi: 10.1161/01.ATV.0000201282.64751.47. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Radu CG, Yang LV, Bentolila LA, Riedinger M, Witte ON. Lysophosphatidylcholine-induced surface redistribution regulates signaling of the murine G protein-coupled receptor G2A. Mol Biol Cell. 2005;16:2234–2247. doi: 10.1091/mbc.E04-12-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spangenberg J, Curtiss LK. Influence of macrophage-derived apolipoprotein E on plasma lipoprotein distribution of apolipoprotein A-I in apolipoprotein E-deficient mice. Biochim Biophys Acta. 1997;1349:109–121. doi: 10.1016/s0005-2760(97)00123-9. [DOI] [PubMed] [Google Scholar]
- 18.Stannard AK, Riddell DR, Sacre SM, Tagalakis AD, Langer C, von Eckardstein A, Cullen P, Athanasopoulos T, Dickson G, Owen JS. Cell-derived apolipoprotein E (ApoE) particles inhibit vascular cell adhesion molecule-1 (VCAM-1) expression in human endothelial cells. J Biol Chem. 2001;276:46011–46016. doi: 10.1074/jbc.M104812200. [DOI] [PubMed] [Google Scholar]
- 19.Tenger C, Zhou X. Apolipoprotein E modulates immune activation by acting on the antigen-presenting cell. Immunology. 2003;109:392–397. doi: 10.1046/j.1365-2567.2003.01665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grainger DJ, Reckless J, McKilligin E. Apolipoprotein E modulates clearance of apoptotic bodies in vitro and in vivo, resulting in a systemic proinflammatory state in apolipoprotein E-deficient mice. J Immunol. 2004;173:6366–6375. doi: 10.4049/jimmunol.173.10.6366. [DOI] [PubMed] [Google Scholar]
- 21.Kelly ME, Clay MA, Mistry MJ, Hsieh-Li HM, Harmony JA. Apolipoprotein E inhibition of proliferation of mitogen-activated T lymphocytes: production of interleukin 2 with reduced biological activity. Cell Immunol. 1994;159:124–139. doi: 10.1006/cimm.1994.1302. [DOI] [PubMed] [Google Scholar]
- 22.van den Elzen P, Garg S, Leon L, Brigl M, Leadbetter EA, Gumperz JE, Dascher CC, Cheng TY, Sacks FM, Illarionov PA, et al. Apolipoprotein-mediated pathways of lipid antigen presentation. Nature. 2005;437:906–910. doi: 10.1038/nature04001. [DOI] [PubMed] [Google Scholar]
- 23.Peter C, Waibel M, Radu CG, Yang LV, Witte ON, Schulze-Osthoff K, Wesselborg S, Lauber K. Migration to apoptotic `Find-Me' signals is mediated via the phagocyte receptor G2A. J Biol Chem. 2007 doi: 10.1074/jbc.M706586200. [DOI] [PubMed] [Google Scholar]
- 24.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 25.Hodgin JB, Maeda N. Minireview: estrogen and mouse models of atherosclerosis. Endocrinology. 2002;143:4495–4501. doi: 10.1210/en.2002-220844. [DOI] [PubMed] [Google Scholar]
- 26.VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol. 2004;24:12–22. doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- 27.Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci U S A. 2000;97:9052–9057. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.