Abstract
To create operational criteria for polygraphic assessments of direct transitions from wake to REM sleep (DREM), as a murine analog of human cataplexy, we have analyzed DREM episodes in congenic lines of orexin/ataxin-3 transgenic [TG] mice and wild-type littermates. The sleep stage of each 10-second epoch was visually scored using our standard criteria. Specificity of DREM for narcoleptic TG mice and sensitivity to detect DREM was evaluated using different DREM criteria. We found that DREM transitions by 10-second epoch scoring are not specific for narcoleptic TG mice and also occur in WT mice during light period. These wake-to-REM transitions in WT mice (also seen in TG mice during light period) were characteristically different from DREM transitions in TG mice during dark period; they tended to occur as brief bouts of wakefulness interrupting extended episodes of REM sleep, suggesting that these transitions do not represent abnormal manifestations of REM sleep. We therefore defined the DREM transitions by requiring a minimum number of preceding wake epochs. Requiring no fewer than four consecutive epochs of wakefulness produced the best combination of specificity (95.9%) and sensitivity (66.0%). By definition, DREM in dark-period is 100% specific to narcolepsy and was 95.9% specific overall. In addition, we found that desipramine, a trycyclic anticataplectic, potently reduces DREM, while two wake-promoting compounds have moderate (d-amphetamine) and no (modafinil) effect on DREM; the effects mirror the anticataplectic effects of these compounds reported in canine and human narcolepsy. Our definition of DREM in murine narcolepsy may provide good electrophysiological measure for cataplexy-equivalent episodes.
Keywords: Narcolepsy, Hypocretin/Orexin, Cataplexy, REM sleep, Mice
INTRODUCTION
Narcolepsy, a chronic sleep disorder, is characterized by a tetrad of symptoms that include excessive daytime sleepiness (EDS), cataplexy (a sudden loss of bilateral muscle tone provoked by strong emotions), hypnagogic hallucinations, and sleep paralysis (Nishino and Mignot, 1997; Scammell, 2003). Narcoleptic patients also exhibit sleep onset rapid eye movement (REM) sleep ([SOREMPs] if it occurs within 15 minutes of sleep onset, and the direct transition from wakefulness is not required in this case) (Nishino and Mignot, 1997). Occurrences of REM sleep are controlled by a 90–120 minute ultradian cyclicity in humans; REM sleep usually does not occur until 90 minutes after sleep onset and follows a 90-minute cycle in normal controls (Zepelin, 1994). Along with cataplexy, hypnagogic hallucinations, and sleep paralysis, SOREMPs are often classified as abnormal manifestations of REM sleep or the intrusion of REM sleep during active wake or sleep onset (Nishino and Mignot, 1997).
Although cataplexy is pathognomonic for narcolepsy, the specificity of hypnagogic hallucinations, sleep paralysis, and SOREMPs for narcolepsy is in question, especially since sleep apnea became recognized as a common sleep disorder and since polygraphic evaluations are carried out in a large number of patients with sleep apnea. SOREMPs have been shown to occur in up to 25% of patients with obstructive sleep apnea (Aldrich et al., 1997). Moreover, hypnagogic hallucinations, sleep paralysis, and SOREMPs have also been reported to occur in up to 23% of healthy individuals (Aldrich et al., 1997; Fukuda et al., 1987; Dahlitz and Parkes, 1993; Ohayon et al., 1996), especially when their sleep patterns are disturbed. This leaves EDS and cataplexy as the axial symptoms of narcolepsy. EDS and cataplexy are also the most debilitating symptoms in many patients and as such constitute prime targets for symptom-based treatments.
In addition to the diagnosis based on this tetrad of symptoms, major findings on the pathophysiology of narcolepsy were recently revealed based on animal experiments. Two groups independently identified the hypocretins (orexins), a pair of neuropeptides expressed in the lateral hypothalamus (LH), as playing a major role in the pathogenesis of narcolepsy (Lin et al., 1999; Chemelli et al., 1999). Naturally occurring genetic narcolepsy in Doberman Pinschers and Labradors is caused by a mutation in the gene for hypocretin-receptor-2 (Lin et al., 1999). Studies on mice also support the role of hypocretin in narcolepsy, as both preprohypocretin (preproorexin) knockout mice and orexin/ataxin-3 transgenic (hypocretin-neuron ablated) mice exhibit narcolepsy-like phenotypes (i.e. fragmented sleep and cataplexy-like behavior) (Chemelli et al., 1999; Hara et al., 2001). Soon after the above discovery, two notable findings were reported in human narcolepsy: 1) The level of hypocretin/orexin in the cerebrospinal fluid is generally undetectably low in narcolepsy patients (Nishino et al., 2000a), and 2) the transcription of prepro-hypocretin and hypocretin peptides was not observed in post mortem brains of narcolepsy patients (Peyron et al., 2000; Thannickal et al., 2000). These findings suggested that hypocretin/orexin neurotransmission is also impaired in human narcolepsy, possibly due to the acquired death of hypocretin/orexin neurons. Interestingly, the occurrence of cataplexy is tightly associated with hypocretin deficiency (Mignot et al., 2002), and narcolepsy without cataplexy (generally associated with normal CSF hypocretin-1 levels) has been separated from narcolepsy with cataplexy under the 2nd edition of the International Classification of Sleep Disorders (ICSD) (ICSD-2, 2005).
Although hypocretin signaling is impaired in both the canine genetic model (i.e. null mutation of hypocretin-2 receptors) and the preprohypocretin knockout mouse, both do still exhibit hypocretin or hypocretin-containing cells (and normal hypocretin production in the canine genetic model), so these models differ from the pathophyiology of human narcolepsy (Ripley et al., 2001; Crocker et al., 2003). In contrast to genetically narcoleptic dogs in Dobermans and Labradors, the pathophysiology of sporadic narcoleptic dogs resemble that of human narcolepsy more since acquired death of hypocretin/orexin neurons is suggested (Ripley et al., 2001). However, only limited number of sporadic narcoleptic dogs have been found and used for experiments (Nishino and Mignot, 1997). In this regard, the orexin/ataxin-3 model (mice/rats) is a more practical model to perform physiological and/or pharmacological experiments to evaluate treatments for human narcolepsy (Hara et al., 2001). This animal model presents acquired hypocretin neuron loss due to specific expression of the ataxin-3 toxic transgene in hypocretin-containing neurons, and thus closely resembles the pathogenesis of narcolepsy in humans.
Cataplexy-like behavior in orexin/ataxin-3 transgenic mice has been evaluated by several authors (Hara et al., 2001; Mieda et al., 2004). In these original articles, cataplexy-like behavior in narcoleptic mice is characterized by an abrupt cessation of purposeful motor activity associated with a sustained change in posture, and thus, the emotional component was not considered. Several authors reported that emotional excitation such as shifting in food availability and group housing have shown to increase the incidence of cataplexy-like behavior in mice (Scammell et al., 2008). However, quantitative behavioral bioassays using these manipulations for inducible cataplexy have not been standardized. Assessment of cataplexy-like episodes in mice generally depends on subjective assessment of spontaneous cataplectic attacks observed through infrared video (Hara et al., 2001; Mieda et al., 2004). These attacks tend to occur infrequently, at about one to five attacks per hour, and each attack often lasts for less than a few minutes (Hara et al., 2001; Mieda et al., 2004). In contrast, there are several quantitative behavioral bioassays for inducible cataplexy in narcoleptic dogs (i.e., food or play elicited cataplexy testing), and these tests have been successfully used for various physiological and pharmacological studies (Nishino and Mignot, 1997).
In addition to cataplexy-like behavior, orexin/ataxin-3 transgenic mice exhibit direct transitions from wake to REM sleep (Hara et al., 2001; Mieda et al., 2004), a phenomenon that may be associated with cataplexy in humans. Because operational criteria for identifying the three murine vigilance states (wake, non-REM sleep [NR], REM sleep) from EEG and EMG recordings have been well established, the presence of DREM transitions can be objectively quantified polygraphically. Furthermore, previous studies have shown that emotionally excited cataplectic episodes in canines are exclusively linked to DREM transitions (Nishino et al., 2000b; Nishino et al., 1998), suggesting a physiological relationship between the two phenomena.
In the current study, we therefore created and evaluated our operational criteria for the use of DREM transitions as a murine analog of human cataplexy. The evaluation includes analysis of sensitivity and specificity of defined DREM episodes in orexin/ataxin-3 narcoleptic mice and of effects of pharmacological compounds used in the treatment of human narcolepsy on the defined DREM episodes.
MATERIALS AND METHODS
Animals
Eight orexin/ataxin-3 transgenic [TG] mice from the congenic line (N9, backcrossed to C57BL/6) and eight respective wild-type littermates were used. Each group consisted of four males and four females, 219–361 days old (Mean age ± SEM, 267 ± 14 days). The mice were surgically prepared for EEG and EMG recordings with a headstage attached to a cable recorder. Under 3% isoflurane anesthesia, two of four electrodes for the EEG (stainless steel screws) were screwed into the skull 1.5 mm lateral and 1.5 mm anterior to the bregma (over the motor cortex) and the other two were screwed 3 mm lateral and 1 mm anterior to the lambda (over the visual cortex), and two EMG electrodes (multistranded stainless steel wires) were inserted into the neck extensor muscle. The six leads for these electrodes were attached to one 2×3 pin header that was secured to the skull using dental acrylic. After surgery, the mice were given an analgesic (carprofen, 3mg/kg s.c.) and an antibiotic (enrofloxacin. 3mg/kg s.c. ), and were allowed to recover for 2 weeks before the experiments.
Polygraphic Data Collection
The EEG–EMG signals were acquired using Grass Instruments (West Warwick, RI) model 12 amplifiers. The EEG and EMG signals, digitally filtered (30 Hz Low Pass Filter for EEG; 10 – 100 Hz Band Pass Filter for EMG), were captured at 128 Hz using a sleep recording system (Vital Recorder; Kissei Comtec, Matsumoto, Japan). EEG signals collected with ipsilateral bipolar EEG electrodes placed over motor and visual cortices together with the bipolar EMG signals were used for sleep scoring. Sixteen 24 h polygraph recordings from eight TG (mean age ± SEM, 277 ± 19 days) and eight WT (259 ± 9 days) were analyzed. Each mouse was housed in its own individual recording cage equipped with a swivel-commutator and a running wheel. Room temperature was maintained at 24 ± 1° C throughout experimentation. The cages were housed in custom-designed stainless steel cabinets with individual ventilated compartments. Food and water were available ad libitum. A 24-hour light-dark cycle (12 hours lights on, 12 hours off) was maintained throughout the study (lights on at zeitgeber time [ZT] = 0 at 7:00 am).
Sleep Scoring
The sleep stage of each 10-second epoch was visually scored using our standard criteria, and 50% or more of a particular state in each epoch is required to score the epoch. Briefly, wakefulness (W) is characterized by a desynchronized, low-amplitude, mixed-frequency (>4 Hz) EEG and high EMG activity. Rhythmic theta/alpha (7–9 Hz) waves with high EMG activities may also appear. NR is characterized by a synchronized, high-amplitude, low-frequency (0.25–4 Hz) EEG and reduced EMG activity compared to wakefulness. EEG activity in REM sleep is similar to that in wakefulness with desynchronized, mixed-frequency, low-amplitude waves. EMG activity during REM sleep is reduced even further than during NR and is completely absent in many cases. Some muscle twitching may be apparent in the EMG trace during REM sleep. During the REM sleep, rhythmic theta/alpha (7–9 Hz) waves with reduced EMG activity may be dominant, but not always, as this has been shown in other species (Kushida et al., 1985; Rechtschaffen and Kales, 1968). Sleep state changes were recorded when at least one 10-second epoch was scored as having a different sleep stage, and state episode length was defined as length of continuous single state episode. All scoring was done by a single investigator (T.C.), blind to the animal information.
Polygraphic data analysis
The total time spent in each vigilance state (W, NR, REM) was assessed for the light period, dark period, and the total 24-hour period. Number of episodes of each vigilance state during the light period, dark period, and the total 24-hour period were also assessed. The mean episode duration in each vigilance state was calculated by dividing the total time spent in each vigilance state by the number of episodes. Finally, total time spent in each vigilance state per hour was also assessed.
The number of direct transitions from wakefulness to REM sleep (DREM) was first determined from the raw recording that was scored using 10-second length epochs. However, we found that DREM also occurs in wild type mice during the light period, and thus questioned whether DREM (defined by the aforementioned criteria) is specific for narcolepsy. In order to more specifically detect REM sleep abnormality specific to narcolepsy, we applied some rules such as requiring a certain length of wakefulness to precede the DREM transitions (more specific for narcolepsy) and a length of DREM (number of epochs). Specificity of the DREM is defined as the percentage of episodes of TG mice having the DREM episodes over the total number of the DREM transitions seen in TG and WT mice. Sensitivity of the DREM is calculated as the percentage of the number of the defined DREM transitions (at a given previous wake requirement or DREM epoch length requirement) over the original number of DREM transitions.
Pharmacological Effects on DREM
In order to evaluate if the effects of pharmacological compounds on DREM are predictive for effects on human cataplexy, we evaluated the effects of desipramine (a tricyclic), d-amphetamine, and modafinil on DREM in orexin/ataxin-3 mice. Desipramine and amphetamine were purchased from Sigma-Aldrich (MO, USA), and modafinil was obtained from Lafon Laboratories (Maisons-Alfort, France). Two drug doses (plus vehicle) of each compound (1mg/kg and 4mg/kg i.p for d-amphetamine; 50 mg/kg and 200 mg/kg p.o. for modafinil; 1.25 mg/kg and 5 mg/kg i,p, for desipramine) were administered at ZT 14, and sleep data for the 6 hours following injections into each animal was scored to detect DREM episodes as described above. The vehicle for d-amphetamine and desipramine injections was saline, while 0.25% methylcellulose in saline was used for the modafinil injections. The injection at ZT 14 was done under the dim red light. The same mice used for the sleep studies were used in all drug studies, and a minimum period of one week was allowed for drug washout for multiple doses/drugs injections.
Statistics
Significant differences (p<0.05) in total time, average duration, and mean duration of each sleep stage between TG and WT mice in the parameters listed were calculated using Student’s t-test. Effects of pharmacological compounds on DREM were analyzed by one-way ANOVA, with the dose as an independent variable. There was no difference in any results between males and females observed, and thus pooled results from both sexes were reported.
RESULTS
Sleep stage analysis
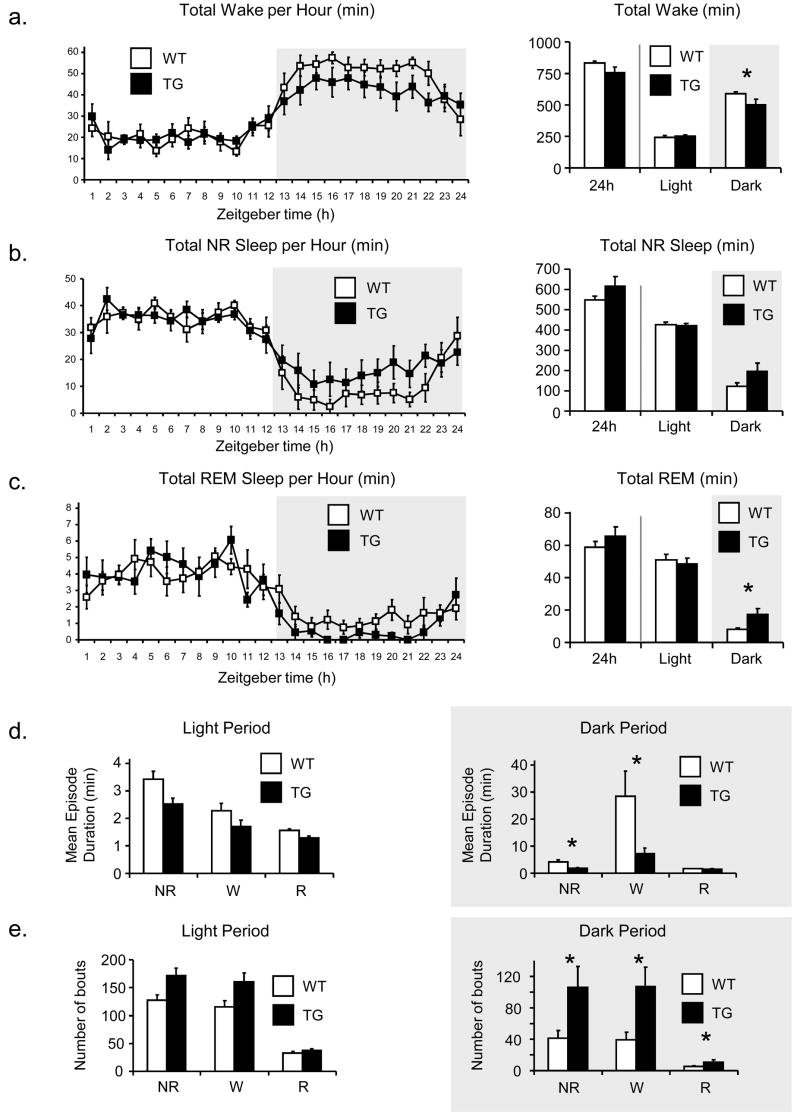
Hourly amounts (in minutes) for each of the three sleep stages (W, NR, REM) and total time spent in each stage during the light period (ZT 1–12), dark period (ZT 13–24), and over the 24 hours are shown in Fig. 1(a-c). During the light (inactive) period, there were no significant differences in the total amounts of any of the sleep stages. During the dark (active) period, however, TG mice showed significantly less wakefulness (p = 0.04); this decrease was consistent across the entire dark period (1a). A similar but opposite trend was observed with NR (p = 0.06) (1b) and REM (p = 0.01) (1c) sleep, both of which were increased in TG mice during the dark period, although the former did not reach statistical significance.
Fig. 1. Total amount of Wake, NR, and REM (min) in WT and TG mice over dark period, light period, and 24 hours, and sleep and wake bouts over light and dark periods.
Student’s t-test was used to compare the total amount of each sleep stage between TG and WT mice. TG mice exhibited significantly less wakefulness during the dark period (p=0.04), although there is no difference in wakefulness during the light period (a). There is a similar trend with REM sleep (p=0.01) (c) and NR sleep (p=0.06) (b), with TG mice exhibiting more time in of both sleep stages during the dark period but not during the light period, although the latter does not quite reach statistical significance. Over 24 hours, there are no significant differences in the amount of time spent in any of the sleep stages. Similarly there is a significant fragmentation of sleep and wake stages in TG mice. Mean episode durations of wake and NR sleep are significantly shorter in TG mice in the dark period (d), during which TG mice showed significantly more episodes in all three vigilance states (e). *p<0.05.
Similar results were observed for fragmentation of sleep stages. During the dark period, TG mice exhibited significantly more episodes of W (p = 0.02), NR (p = 0.03), and REM (p = 0.02), and the average episode duration of NR (p = 0.03) and W (p = 0.03) was significantly shorter (1d-e). There was no difference observed in the duration of REM episodes (p = 0.4) nor were there differences between any of the sleep stages during the light period (1d-e).
DREM Analysis
Without any adjustments, a total of 131 DREM transitions were observed in WT (25) and TG (106) mice (Table 1). Sixty-five transitions occurred during the light period and 66 during the dark period. Thus, initial overall specificity of DREM in narcolepsy was modest (80.9%) due to the high incidence of light period DREM in WT mice; during the light period, 24 of 65 transitions occurred in WT mice, resulting in 63.1% specificity, while during the dark period, only 1 of 66 transitions occurred in WT mice (98.5% specific).
Table 1.
Sensitivity and specificity of DREM episodes in TG narcoleptic mice with difference in length of (a) preceding wake epochs (b) and DREM episodes.
| (a) Pervious | Light | Dark | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wave length | WT | TG | Spec. | Sens. | WT | TG | Spec. | Sens. | WT | TG | Spec. | Sens. |
| ≥10 sec | 24 | 41 | 63.1% | 100.0% | 1 | 65 | 98.5% | 100.0% | 25 | 106 | 80.9% | 100.0% |
| ≥20 sec | 8 | 29 | 78.4% | 70.7% | 1 | 56 | 98.2% | 86.2% | 9 | 85 | 90.4% | 80.2% |
| ≥30 sec | 5 | 24 | 82.8% | 58.5% | 0 | 50 | 100.0% | 76.9% | 5 | 74 | 93.7% | 69.8% |
| ≥40 sec | 3 | 21 | 87.5% | 51.2% | 0 | 49 | 100.0% | 75.4% | 3 | 70 | 95.9% | 66.0% |
| ≥50 sec | 2 | 19 | 90.5% | 46.3% | 0 | 46 | 100.0% | 70.8% | 2 | 65 | 97.0% | 61.3% |
| ≥100 sec | 1 | 10 | 90.9% | 24.4% | 0 | 41 | 100.0% | 63.1% | 1 | 51 | 98.1% | 48.1% |
| (b) DREM | Light | Dark | Total | |||||||||
| Length | WT | TG | Spec. | Sens. | WT | TG | Spec. | Sens. | WT | TG | Spec. | Sens. |
| ≥10 sec | 24 | 41 | 63.1% | 100.0% | 1 | 65 | 98.5% | 100.0% | 25 | 106 | 80.9% | 100.0% |
| ≥20 sec | 22 | 34 | 60.7% | 82.9% | 1 | 47 | 97.9% | 72.3% | 23 | 81 | 77.9% | 76.4% |
| ≥30 sec | 17 | 21 | 55.3% | 51.2% | 1 | 34 | 97.1% | 52.3% | 18 | 55 | 75.3% | 51.9% |
| ≥40 sec | 14 | 15 | 51.7% | 36.6% | 1 | 24 | 96.0% | 36.9% | 15 | 39 | 72.2% | 36.8% |
| ≥50 sec | 11 | 12 | 52.2% | 29.3% | 1 | 20 | 95.2% | 30.8% | 12 | 32 | 72.7% | 30.2% |
| ≥100 sec | 4 | 2 | 33.3% | 4.9% | 0 | 8 | 100.0% | 12.3% | 4 | 10 | 71.4% | 9.4% |
Specificity (Spec.) of the DREM is defined as the percentage of episodes of TG mice having the DREM episodes over the total number of the DREM transitions seen in TG and WT mice. Sensitivity (Sens.) of the DREM is calculated as the percentage of the number of the defined DREM transitions over the original number of DREM transitions. The lengths of preceding wake episodes significantly influence the sensitivity, and requiring no fewer than four consecutive epochs of wakefulness produced the best combination of specificity (95.9%) and sensitivity (66.0%). In contrast, longer lengths of DREM episodes (in light period) slightly reduced the specificity of DREM for narcolepsy.
DREM transitions were detected both in WT and TG mice during the light period, at similar frequencies (Fig. 2). Many of these DREMs were characterized by brief bouts of wakefulness interrupting an extended period of normal REM sleep (Fig. 3a). In contrast, DREM in TG mice during the dark period typically interrupted an extended bout of wakefulness and ended with a return to wakefulness (Fig. 3b). In order to minimize these false positives (by means of specificity to narcolepsy), the effect of having requirements for defined DREM transition (a minimum number of preceding wake epochs and a minimum length of the DREM episode for a true DREM transition) on specificity and sensitivity was assessed for the light and dark periods separately.
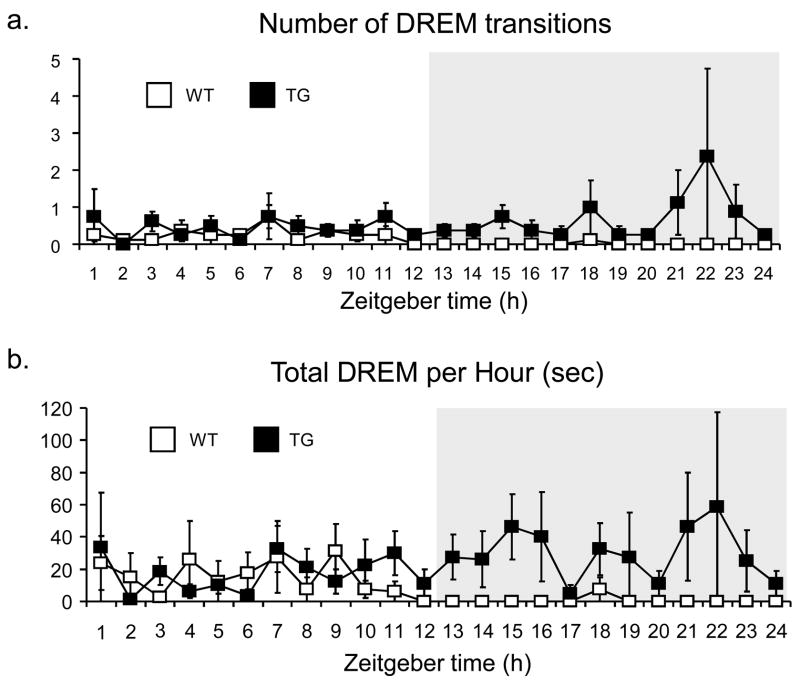
Fig. 2. Hourly DREM transitions over 24hours – Raw Data.
In the raw EEG and EMG data, there is a clear difference in DREM episode frequency (2a) and the total time spent in DREM (2b) during the dark period. However, during the light period, DREM was observed both in WT and TG mice with similar frequency (2a), and the same was true when the total time spent in DREM is evaluated (2b).
Fig. 3. Examples of direct transitions from Wake to REM sleep and wake-interrupted REM sleep.
In this TG mouse, the DREM transition characteristically followed an extended episode of wakefulness, ending with a transition back to wakefulness and occurred during the dark period (ZT 23) (3a). In contrast, the REM-to-Wake transition in the WT mouse interrupted an extended bout of REM sleep and occurred during the light period (ZT 8 and ZT 10.5) (3b, c).
We found that a requiring a minimum preceding wake of 4 epochs resulted in the best balance of sensitivity and specificity. With this DREM definition, overall specificity was 95.9% (and 66.0% sensitivity) of the original 131 DREM transitions detected. For light period transitions, specificity was 87.5% and sensitivity was 51.2%, and for dark period transitions, specificity was 100.0% with 75.4% sensitivity (Table 1a). The mean (±SEM) number of defined DREM in TG mice during light, dark, and 24 hour periods were 2.0 (±0.7), 4.8 (±3.2) and 6.8 (±3.4) respectively. The mean (±SEM) duration of these was 41.3 (±23.7) sec, 53.7 (±13.0) sec, and 50.0 (±29.7) sec, respectively.
The number of DREM transitions exhibited by individual mice for the various wake requirements was also analyzed. Since the requirement for the number of preceding wake epochs was increased, a drop in the number of DREM transitions was detected uniformly across all mice (data not shown), suggesting the increases in specificity by application of the rule was not due to the results of specific mice.
The effect of requiring a minimum length for the actual DREM episode was explored (Table 1b). Interestingly, the specificity decreased when we selected longer DREM episodes, suggesting that the DREM (specific for narcolepsy) are often brief.
The hourly amount of time spent in the defined DREM (a) and the number of defined DREM transitions per hour (b) and that after application of the previous wake requirement is shown in Fig. 4. The presence of “putative” DREM transitions in both WT and TG mice during the light period was greatly reduced with application of the rule, and almost all WT DREM was eliminated.
Fig. 4. Hourly defined DREM transitions over 24 hours with previous wake length requirement.
With the requirement of four or more epochs of wakefulness preceding a DREM transition, nearly all wild-type DREM is eliminated (4a), possibly due to the elimination of pseudo-DREM. This is also apparent in the total time spent in DREM (4b). The TG DREM is preserved, and the difference in DREM between TG and TG during the dark cycle is still evident. The amount of time spent in REM sleep during dark period, even after excluding DREM, is still higher in TG mice than in WT (4c, see text).
The total amount of REM sleep excluding time spent in DREM is shown in Fig. 4(c). After adjustment, the difference in dark-period REM between WT and TG mice decreased and was slightly smaller than reported in previous publications (Hara et al., 2001; Mieda et al., 2004), but the difference is still statistically significant (1026±234 vs. 309±64 seconds, p=0.02).
Pharmacological evaluations
We observed that d-amphetamine dose-dependently enhances wakefulness and reduces NR and REM sleep in dark period. Dose effects of d-amphetamine on wake promotion and NR and REM suppressing effects were statistically significant at three hours after the drug administration (Fig. 5). Modafinil at 200mg/kg p.o. also enhanced W and reduced NR and REM sleep, and effects on W and NR were significant at three hours after the drug administration. Modafinil also significantly reduced REM sleep at six hours after the drug administration. In contrast, desipramine had little effects on wakefulness and NR, but significantly reduced REM sleep, and REM sleep was completely suppressed for the four hours following administration of 5mg/kg i.p..
Fig. 5. Effects of d-amphetamine, modafinil, and desipramine on wakefulness, NR, REM sleep (a) and DREM (d) during dark period in TG narcoleptic mice.
Cumulative time spend in each vigilance states (seconds) were plotted. One –way ANOVA was applied to detect significant dose effects on each vigilance state for each compound at 3 hour and 6 hour after the drug administration, and p less than 0.05 and 0.01 is indicated with (*) and (**) respectively.
Effect of each compound on DREM was plotted in the bottom row. We found that desipramine significantly reduced DREM for over six hours. High dose of d-amphetamine moderately, but non-significantly, reduced DREM, while modafinil had no effects.
DISCUSSION
Human cataplexy is characterized by sudden loss of bilateral muscle tone provoked by strong emotions that are usually positive, such as laughter, pride, elation, or surprise (Nishino and Mignot, 1997; Scammell, 2003). Cataplexy is also observed in narcoleptic dogs in forms similar to definitions for the human form, and emotional triggering of cataplexy in these narcoleptic dogs is well documented; multiple cataplectic attacks can be easily elicited by feeding or playing in these animals (Nishino and Mignot, 1997). Cataplexy is pathognomic for narcolepsy, and the occurrence of cataplexy is tightly associated with deficit in hypocretin neurotransmission (Mignot et al., 2002). Cataplexy is disabling and pharmacological treatments are required in most human cases (Nishino and Mignot, 1997). However, mechanisms of induction of cataplexy are not well understood. Better understanding of the mechanisms controlling cataplexy will likely lead to better insights into the pathophysiology and treatments of narcolepsy, and the canine model has been intensively used for this purpose (Nishino and Mignot, 1997; Nishino et al., 2000b).
Several authors reported that narcoleptic mice (i.e. hypocretin null and orexin/ataxin-3 transgenic mice) also exhibit cataplexy-like behavior (i.e. behavioral arrests) (Hara et al., 2001; Mieda et al., 2004). Cataplexy-like behavior in these mice is characterized by “an abrupt cessation of purposeful motor activity associated with a sustained change in posture that was maintained throughout the episode, ending abruptly with complete resumption of purposeful motor activity” (Chemelli et al., 1999; Hara et al., 2001; Mieda et al., 2004). Although attacks in mice were reported to occur often during grooming or excited ambulation, such as food restriction, food availability shifting, new caging and grouping, no standardized cataplexy elicited tests, using these manipulations, have been established (Scammell et al., 2008). These authors usually count the number and length of spontaneously occurring cataplexy-like attacks through continuous infrared video monitoring. Furthermore, these attacks tend to occur infrequently (one to five attacks per hour) and thus long-hour monitoring is required (Hara et al., 2001; Mieda et al., 2004). In contrast, cataplexy in narcoleptic dogs can reliably be elicited by feeding, and most narcoleptic dogs have multiple attacks (up to 20 attacks) within a short evaluation period (within 10 minutes), making the Food Elicited Cataplexy Test a standard behavioral assay for cataplexy in this species. (Nishino and Mignot, 1997; Nishino et al., 2000b). It therefore appears that the current quantitative behavioral bioassays for cataplectic attacks in mice are not only very labor intensive but also subjective. It should also be noted that even severely affected narcoleptic dogs spend only 2.0–3.0% of their time in cataplexy under their regular housing environments (without cataplexy elicitations), making it difficult to standardize the bioassays for spontaneously occurring attacks even in canine narcolepsy.
In addition to cataplexy-like attacks, orexin/ataxin-3 TG mice exhibit DREM. DREM may be associated with cataplexy in humans, and polygraphic evaluations at the onset of cataplexy in narcoleptic subjects demonstrated a sudden loss of EMG that occurred during active wake (desynchronized fast wave EEG seen during wake or REM sleep) (Guilleminault, 1976). However, cataplexy is not easy to elicit in the clinic or sleep laboratory for most patients, and polygraphic evaluations of cataplexy in humans are still very limited. In contrast, a significant number of polygraphic recordings were carried out at the onset of cataplectic attacks in dogs, and behaviorally-defined catalectic attacks in narcoleptic dogs were exclusively linked to DREM transitions (determined by polygraph) (Nishino et al., 2000b; Nishino et al., 1998), suggesting a physiological relationship between these two phenomena. Since DREM episodes can also be objectively determined by standard sleep recordings in mice, we have evaluated if DREM transitions can be used as murine analogs of cataplexy in human.
In this study, we first evaluated the sleep characteristics of orexin/ataxin-3 TG mouse (N9, congenic C57/BL6 line). As other authors previously reported using mixed background orexin/ataxin-3 TG mice (N1 to N5) (Hara et al., 2001; Mieda et al., 2004), we observed similar electrophysiological sleep abnormalities in our congenic C57/BL6 orexin/ataxin-3 TG mouse; these abnormalities are typically characterized by fragmented wake/sleep (especially inability to maintain wakefulness during the active period), and DREM. It should be noted that the NR (6386 ±936 sec) and REM (308± 54 sec) sleep amounts during dark period, especially in our WT mice, are much smaller than those reported previously (15816±864 sec, and 1626±198 sec, respectively). This is possibly due to the recording environment of two studies, and it is likely that our mice were more active with running wheels during dark period (see also (Espana et al., 2007)). Initially, with the analysis of original raw sleep data divided into 10-second epochs, we were surprised to find that DREM was observed even in WT mice during the light (inactive) period (and one episode during dark period in one mouse). This brought a question about the specificity of DREM episodes for narcoleptic mice; although specificity of DREM for narcoleptic mice during dark (active) period is high at 98.5%, specificity during the light (inactive) period was only 63.1 %.
By examining all DREM transitions in both TG and WT mice, we found that most “putative” DREM transitions in WT animals were characterized as sequences of REM sleep episodes interrupted by a brief wake episode and punctuated by a return to REM sleep. In contrast, the DREM episodes in TG mice during the dark period typically followed long episodes of wakefulness and ended with a return to wakefulness. Since WT mice rarely show this pattern of transitions, these likely reflect the abnormal manifestation of REM sleep that is more closely related to cataplexy in humans. In order to eliminate the light-period pseudo-DREM (more specific for narcolepsy), applying a rule of requiring four epochs of wakefulness in order to identify the DREM transition produced the best sensitivity and specificity. The specificity became 87.5% during light period and 100% during dark period. The length of DREM episode also weakly affected the specificity, and DREM more specific for narcolepsy tended to be brief.
DREM episodes that we defined are specific for TG mice and occur mostly in dark period when mice spend most of time in wakefulness, and therefore the pattern of the occurrences is similar to that of cataplexy-like behavior in these mice. The previous authors reported a significantly large amount of REM sleep during dark period in TG compared to WT mice (more than 2–3 folds) (Hara et al., 2001; Mieda et al., 2004). Some other authors however, pointed out the possibility that REM amounts of narcoleptic mice during dark periods in the earlier studies may be overestimated by including many cataplexy-like attacks. This was the case for preprohypocretin knockout mice showing no significant increase in REM sleep during dark periods; increase was observed in a later study (Mochizuki et al., 2004). In order to compare genuine REM sleep amounts between TG and WT mice, we also subtracted the DREM episodes from the total REM sleep amount (Fig. 5). The result that a significantly high REM sleep amount in TG mice during dark period (1026±234 vs. 309±64 seconds, p=0.02) still exists suggest the higher REM sleep amount in TG mice during dark period is one of the consistent sleep abnormalities in these mice.
It is unknown if the length of the preceding wake length is also important for the scoring of cataplexy-like behavior. As far as we know, none of the authors of previous studies considered this influence. Considering the fact that about 25% of DREM in TG during active period were indeed pseudo DREM, we believe that it is also critical to evaluate the influence of the length of the preceding wake episodes for the scoring of cataplexy-like behaviors.
The second purpose of this study was to evaluate if pharmacological effects of compounds on DREM is predictive of its effects on cataplexy. We therefore tested three compounds currently used for the treatment of human narcolepsy. We found that desipramine, a trycyclic anticataplectic, potently reduces DREM, while two wake promoting compounds have moderate (d-amphetamine) and no effect (modafinil) on DREM. These effects mirror the anticataplectic effects of these compounds previously reported in canine and human narcolepsy (Nishino and Mignot, 1997; Shelton et al., 1995; Billiard et al., 1994) and suggest that DREM evaluations in these animals may also be useful to screen potential anticataplectic compounds for human treatments.
Interestingly, modafinil moderately reduced REM sleep, but had absolutely no effect on DREM in TG mice. Although more compounds need to be included, the results of modafinil may also suggest that pharmacological controls of DREM and REM sleep may be different, as we had previously demonstrated in the canine model of narcolepsy that there was a dissociation in the compounds’ ability to reduce cataplexy and REM sleep (i.e. dopaminergic D2/3 antagonists potently reduce cataplexy, but had no effects on REM sleep) (Okura et al., 2000).
In conclusion, DREM with our definition may be a good electrophysiological measure for cataplexy-equivalent episodes in rodents. This is an objective assessment and can be carried out with regular sleep recordings in this species. The evaluation would be useful for various physiological and pharmacological experiments, for understanding the pathophysiology of narcolepsy, and for developing better treatments for human cataplexy.
Acknowledgments
This research was supported by a NIH Grant R01MH072525. The authors thank Ms. Mari Matsumura for editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Nishino S, Mignot E. Pharmacological aspects of human and canine narcolepsy. Prog Neurobiol. 1997;52:27–78. doi: 10.1016/s0301-0082(96)00070-6. [DOI] [PubMed] [Google Scholar]
- Scammell TE. The neurobiology, diagnosis, and treatment of narcolepsy. Ann Neurol. 2003;53:154–166. doi: 10.1002/ana.10444. [DOI] [PubMed] [Google Scholar]
- Zepelin H. Mammalian sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. W. B. Saunders Company; Philadelphia: 1994. pp. 81–94. [Google Scholar]
- Aldrich MS, Chervin RD, Malow BA. Value of the multiple sleep latency test (MSLT) for the diagnosis of narcolepsy. Sleep. 1997;20:620–629. [PubMed] [Google Scholar]
- Fukuda K, Miyasita A, Inugami M, Ishihara K. High prevalence of isolated sleep paralysis: Kanashibari phenomenon in Japan. Sleep. 1987;10:279–286. doi: 10.1093/sleep/10.3.279. [DOI] [PubMed] [Google Scholar]
- Dahlitz M, Parkes JD. Sleep paralysis. Lancet. 1993;341:406–407. doi: 10.1016/0140-6736(93)92992-3. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Priest RG, Caulet M, Guilleminault C. Hypnagogic and hypnopompic hallucinations: pathological phenomena? Br J Psychiatry. 1996;169:459–467. doi: 10.1192/bjp.169.4.459. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000a;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignot E, Lammers GJ, Ripley B, Okun M, Nevsimalova S, Overeem S, Vankova J, Black J, Harsh J, Bassetti C, Schrader H, Nishino S. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553–1562. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- ICSD-2. Diagnostic and coding manual. 2. American Academy of Sleep Medicine Westchester; Illinois: 2005. ICSD-2-International classification of sleep disorders. [Google Scholar]
- Ripley B, Fujiki N, Okura M, Mignot E, Nishino S. Hypocretin levels in sporadic and familial cases of canine narcolepsy. Neurobiol of Dis. 2001;8:525–534. doi: 10.1006/nbdi.2001.0389. [DOI] [PubMed] [Google Scholar]
- Crocker AJSLM, Saper CB, Mignot E, Scammell TE. Loss of orexin/dynorphin neurons in human narcolepsy. Soc Neuroscience Abstr. 2003;28 [Google Scholar]
- Mieda M, Willie JT, Hara J, Sinton CM, Sakurai T, Yanagisawa M. Orexin peptides prevent cataplexy and improve wakefulness in an orexin neuron-ablated model of narcolepsy in mice. Proc Natl Acad Sci U S A. 2004;101:4649–4654. doi: 10.1073/pnas.0400590101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell TE, Willie JT, Guilleminault C, Siegel JM. Cataplexy in mouse models of narcolepsy (Commentary for Sleep) Sleep. 2008;31 in press. [PMC free article] [PubMed] [Google Scholar]
- Nishino S, Riehl J, Hong J, Kwan M, Reid M, Mignot E. Is narcolepsy REM sleep disorder? Analysis of sleep abnormalities in narcoleptic Dobermans. Neuroscience Research. 2000b;38:437–446. doi: 10.1016/s0168-0102(00)00195-4. [DOI] [PubMed] [Google Scholar]
- Nishino S, Honda K, Riehl J, Okura M, Mignot E. Neuronal activity in the cholinoceptive basal forebrain of freely moving narcoleptic dobermans. Neuroreport. 1998;9:3653–3661. doi: 10.1097/00001756-199811160-00017. [DOI] [PubMed] [Google Scholar]
- Kushida CA, Baker TL, Dement WC. Electroencephalographic correlates of cataplectic attacks in narcoleptic canines. Electro Clin Neurophysiol. 1985;61:61–70. doi: 10.1016/0013-4694(85)91073-9. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A, editors. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. National Institutes of Health; Washington, D.C.: 1968. [Google Scholar]
- Guilleminault C. Cataplexy. Narcolepsy (Advances in Sleep Research Vol. 3) 1976:125–143. [Google Scholar]
- Espana RA, McCormack SL, Mochizuki T, Scammell TE. Running promotes wakefulness and increases cataplexy in orexin knockout mice. Sleep. 2007;30:1417–1425. doi: 10.1093/sleep/30.11.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki T, Crocker A, McCormack S, Yanagisawa M, Sakurai T, Scammell TE. Behavioral state instability in orexin knock-out mice. J Neurosci. 2004;24:6291–6300. doi: 10.1523/JNEUROSCI.0586-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton J, Nishino S, Vaught J, Dement WC, Mignot E. Comparative effects of modafinil and amphetamine on daytime sleepiness and cataplexy of narcoleptic dogs. Sleep. 1995;18:817–826. [PubMed] [Google Scholar]
- Billiard M, Besset A, Montplaisir J, Laffont F, Goldenberg F, Weill JS, Lubin S. Modafinil: A double-blind multicentric study. Sleep. 1994;17:S107–S112. doi: 10.1093/sleep/17.suppl_8.s107. [DOI] [PubMed] [Google Scholar]
- Okura M, Riehl J, Mignot E, Nishino S. Sulpiride, a D2/D3 Blocker, Reduces Cataplexy but not REM Sleep in Canine Narcolepsy. Neuropsychopharmacology. 2000;23:528–538. doi: 10.1016/S0893-133X(00)00140-8. [DOI] [PubMed] [Google Scholar]