Abstract
There is growing evidence to suggest that many disease states are accompanied by chronic elevations in sympathetic nerve activity. The present review will specifically focus on central sympathetic overactivity and highlight three main areas of interest: 1) the pathological consequences of excessive sympathetic nerve activity; 2) the potential role of centrally derived nitric oxide in the genesis of neural dysregulation in disease; and 3) the promise of several novel therapeutic strategies targeting central sympathetic overactivity. The findings from both animal and human studies will be discussed and integrated in an attempt to provide a concise update on current work and ideas in these important areas.
1. Introduction
Since the first suggestion almost 200 years ago that muscle fibres in the blood vessel wall are under neural control, the sympathetic nervous system has become an intense area of research focus (Henle, 1840; Heymans and Folkow, 1982; Stelling, 1867). It is now well established that the tonic rhythmic discharge of the sympathetic nerves contributes importantly to resting vasomotor tone, and through modulation of the arterial baroreflex plays an essential role in blood pressure (BP) homeostasis (Cowley et al., 1973; Ramirez et al., 1985). However, the sympathetic nervous system is not only important for BP control, but is also intimately involved in numerous other physiological processes ranging from metabolism to renal control. Key regulatory sites within the central nervous system that govern sympathetic outflow have been identified and the control of the sympathetic nervous system has been and continues to be an area of intense investigation. Much of the interest in this area stems from the growing evidence that many disease states are accompanied by alterations in central sympathetic regulation and as such, chronically elevated sympathetic nerve activity (SNA). The present review will focus on this central sympathetic overactivity and highlight three main areas of interest: 1) the pathological consequences of excessive SNA; 2) the potential role of centrally derived nitric oxide (NO) in the genesis of neural dysregulation in disease; and 3) the promise of several novel therapeutic strategies targeting central sympathetic overactivity. We have attempted to integrate recent animal and human studies in order to provide a concise update on current work and ideas in each of these areas. Thus, the focus is primarily on more recently published work. Clearly, there is an abundance of research in each of these areas and consequently it would be impossible to cover and reference all of the work within each area. To partially rectify this situation, we have cited a number of reviews in an attempt to direct the reader to research that may have been inadvertently omitted.
2. Assessment of SNA
Experimental quantification of the activity of the sympathetic nervous system can be undertaken using several methodologies (Esler et al., 2003; Grassi, 1998; Grassi and Esler, 1999). Direct recordings of SNA (e.g. renal or lumbar) are commonly obtained in animals by the surgical implantation of recording electrodes onto the appropriate sympathetic fibres. In humans, a comparable direct assessment of central sympathetic activity can be made using the microneurography technique to selectively record from postganglionic muscle or skin sympathetic nerves (Vallbo et al., 1979). Alternatively, global sympathetic activity may also be assessed from analysis of plasma or urine catecholamine concentrations. In addition, the work of Murray Esler and associates has pioneered the use of radiotracer techniques in humans for the determination of global or specific organ spillover rates of noradrenaline, the primary neurotransmitter of the sympathetic nervous system (Esler et al., 2003; Grassi, 1998; Grassi and Esler, 1999). Finally, spectral analysis of heart rate or blood pressure variability has also been used to provide an index of sympathetic drive (Pagani et al., 1997) although the validity of these indices has been questioned (Parati et al., 2006). As the focus of this review is on central regulation of SNA, we will predominantly focus on studies employing direct neural recordings of sympathetic outflow, which is suggested to be strongly correlated with renal, cardiac and whole-body noradrenaline spillover (Wallin et al., 1992; Wallin et al., 1996).
3. Central sympathetic control
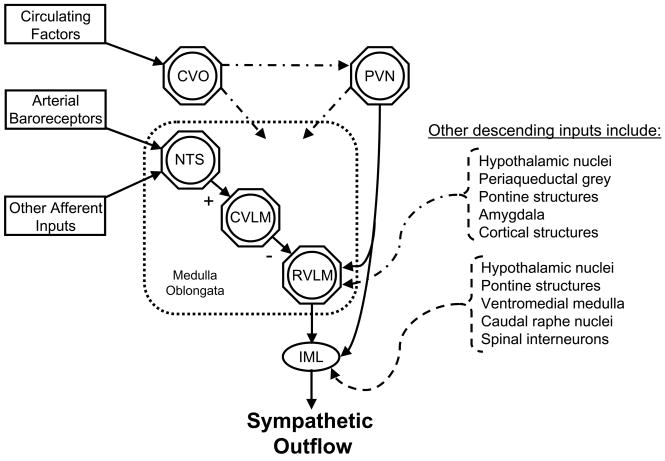
The central regulation of sympathetic outflow primarily occurs within the cardiovascular areas of the brainstem (i.e. medulla oblongata), which is the site of a complex convergence of descending and ascending neural inputs (Dampney, 1994) (Figure 1). Sympathetic preganglionic neurones located in the intermediolateral cell column (IML) of the spinal cord are known to receive strong excitatory drive from neurones of the rostral ventrolateral medulla (RVLM) located in the medulla oblongata. This excitatory drive from the RVLM may be intrinsically generated, chemically mediated (e.g. glutamate, Angiotensin (Ang) II), or attributable to excitatory inputs from other regions of the central nervous system (e.g. pons, hypothalamus and amygdala) (Dampney, 1994). Furthermore, direct excitatory inputs from other regions of the central nervous system may also directly project to sympathetic preganglionic neurones at the IML (Dampney, 1994). For example, using a retrogradely transported tracer Strack et al., (Strack et al., 1989) identified that sympathetic preganglionic neurones also receive inputs from neurons of the ventromedial medulla, caudal raphe nuclei, A5 noradrenergic cell group of the caudal ventrolateral pons, and the paraventricular hypothalamic nucleus (PVN). In addition, the arterial baroreflex provides a powerful modulation of SNA and is critical for the beat-to-beat, and possibly longer term regulation of BP (Joyner et al., 2008; Thrasher, 2006). Baroreceptor afferents terminate at the nucleus tractus solitarius (NTS) in the medulla oblongata, and the NTS exerts a tonic sympathoinhibitory influence on RVLM neurones by its projections relayed via the caudal ventrolateral medulla (Dampney, 1994). Furthermore, afferent inputs from other peripheral reflexes (e.g. arterial chemoreflex) can impinge on the NTS and alter SNA (Loewy, 1990; Schultz et al., 2007). In addition, the circumventricular organs, which lack a blood brain barrier, represent a pathway by which circulating factors can influence key central regions involved in the regulation of SNA and thus, modulate central sympathetic outflow (Simpson, 1981). Although alterations in each of these areas, and/or the reciprocal interactions amongst them, has the potential to modulate central sympathetic outflow, our understanding of the complex neuroanatomical interactions governing the central regulation of SNA is incomplete and remains an intense area of research focus.
Figure 1.
General schematic identifying potential central neural sites involved in the regulation of sympathetic outflow. See text for details. CVO, circumventricular organs; PVN, paraventricular nucleus; NTS, nucleus tractus solitarus; CVLM, caudal ventrolateral medulla; RVLM, rostral ventrolateral medulla; IML, intermediolateral cell column.
4. Consequences of central sympathetic overactivity
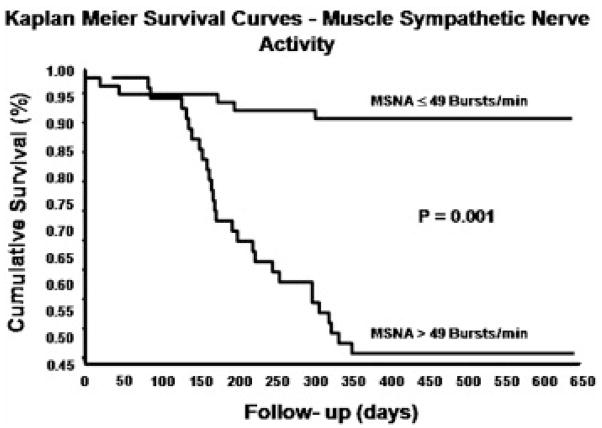
An overactive sympathetic nervous system has become an identified characteristic of several cardiovascular diseases including, ischemic heart disease (Graham et al., 2004), chronic heart failure (Leimbach et al., 1986), and hypertension (Grassi, 1998). However, elevated SNA is not isolated to diseases of the cardiovascular system and has also been reported in a plethora of other conditions including: kidney disease (Converse et al., 1992), type II diabetes mellitus (Huggett et al., 2003), obesity (Grassi et al., 2007), metabolic syndrome (Grassi et al., 2005), obstructive sleep apnea (Narkiewicz and Somers, 1997), pre-eclampsia (Greenwood et al., 2003), depression (Barton et al., 2007), and ulcerative colitis (Furlan et al., 2006). Importantly, sympathetic overactivity is associated with poor prognosis in patients with chronic heart failure (Barretto et al., 2008; Cohn et al., 1984) and end-stage renal disease (Zoccali et al., 2002) as well as in community dwelling elderly individuals (Reuben et al., 2000). For example, a recent study of heart failure patients showed that increased muscle SNA was a significant independent predictor of one-year cardiac mortality (Barretto et al., 2008) (Figure 2). The scale and potential pathological significance of excessive sympathetic nerve activity becomes clear when one considers the prevalence and mortality rates of conditions with which it is associated. These diseases represent some of the major causes of death in industrialised nations (Lloyd-Jones et al., 2008).
Figure 2.
Kaplan-Meier analysis of the cumulative rates of survival in patients with heart failure stratified in two groups on the basis of resting muscle SNA (MSNA, bursts/min). Rates of survival were significantly poorer in patients with resting muscle SNA values above the median value of 49 bursts/min. From Barretto et al., (2008), with permission.
One notable deleterious consequence of an increase in SNA is an increase in arterial BP, which is also a common co-morbidity in a number of pathophysiological conditions, including end stage renal disease and obesity (Grassi et al., 1996; Kotanko, 2006). Indeed, there is compelling evidence that sympathetic activation plays a pathogenic role in triggering the essential hypertensive state (Abboud, 1982; Grassi, 1998; Grassi, 2004a; Grassi, 2004b; Smith et al., 2004), although this concept is not universally accepted (Joyner et al., 2008). Muscle SNA (Grassi, 1998), plasma noradrenaline concentrations (Esler et al., 1977; Goldstein, 1983) and noradrenaline spillover from the heart and kidneys (Esler et al., 1990) have been reported to be elevated in patients with essential hypertension. In addition, muscle SNA is elevated in both white coat hypertensives and borderline hypertensives (Anderson et al., 1989; Smith et al., 2004), supporting the role of the sympathetic nervous system in the pathogenesis of hypertension. Furthermore, the degree of sympathetic overactivity has been found to be related to the magnitude of hypertension in cross-sectional patient studies (Grassi et al., 1998) and in longitudinal studies of spontaneously hypertensive rats (Judy et al., 1979). Thus, it appears that the relationship between central sympathetic overactivity and the hypertensive state is not simply associative, but the increase in SNA likely plays a significant role in both the initiation and the development of the hypertension (Abboud, 1982; Grassi, 1998; Grassi, 2004a; Grassi, 2004b; Smith et al., 2004).
An important deleterious consequence of the hypertensive state is that it can give rise to both structural and functional abnormalities of the vasculature. Indeed, it has been recognised for some time that chronic elevations in BP increase vascular smooth muscle cell hypertrophy and hyperplasia, along with inducing structural and biochemical changes in endothelial cells (Feihl et al., 2008; Folkow, 1982). This can lead to abnormal wall to lumen ratio, increased resistance to flow and accelerate the progression of atherosclerosis (Failla et al., 1999; Folkow, 1982). However, there is now accumulating evidence to suggest that sympathetic overactivity can have a deleterious effect on the vasculature that is independent of an increase in BP. Indeed, chronic sympathetic activation without an increase in BP has been demonstrated to cause morphological changes in vascular smooth muscle cells such as hypertrophy and proliferation (Bevan, 1984), an effect that is attenuated by sympathetic denervation (Bevan, 1975). Such sympathetically-mediated vascular remodelling can cause increased aortic medial thickening and enhanced blood pressure variability (Dao et al., 2001), which has been implicated in the mediation of end-organ damage (Mancia and Parati, 2003). Along with such changes in vascular structure, sympathetic tone can also profoundly influence vascular function. In this regard, surgical sympathetic denervation and pharmacological blockade have been shown to increase arterial distensibility in both animals as well as healthy and diseased patient populations (Mancia et al., 1998; Mangoni et al., 1997). Conversely, acute increases in sympathetic tone as caused by mental stress, lower body negative pressure or a cold pressor test have been reported to decrease radial artery compliance in healthy subjects (Boutouyrie et al., 1994; Salzer et al., 2008). Furthermore, Grassi et al., (Grassi et al., 1995) have demonstrated that the increase in SNA with heart failure is associated with the reduced radial artery compliance noted in these patients. The clinical significance of sympathetically-mediated increases in arterial stiffness is evident from the results of large epidemiological studies indicating a strong relationship between increased arterial stiffness and increased mortality rates in populations of patients where elevations in SNA are prevalent, such as hypertension (Blacher et al., 1999a), end stage renal disease (Blacher et al., 1999b; London et al., 2001) and type II diabetes (Cruickshank et al., 2002).
Recent evidence suggests that arterial stiffness is not only determined by vascular smooth muscle tone and the passive properties of the structural components of the vessel (i.e. elastin and collagen cross linking), but also by changes in endothelial function (Kinlay et al., 2001). Indeed, removal of the endothelium in rats causes significant changes in the mechanical properties of the carotid artery, indicating the importance of the endothelium to large artery distensibility (Levy et al., 1989). The clinical significance of impairments in endothelial function in the development of atherosclerosis (Neunteufl et al., 1997) and as a predictor of future cardiovascular risk (Fischer et al., 2005; Neunteufl et al., 2000; Perticone et al., 2001; Schachinger et al., 2000) is now well established. However, perhaps less well appreciated is the interaction between endothelial function and the sympathetic nervous system. Acute increases in SNA induced by experimental sympatho-excitatory manoeuvres have been reported to impair endothelial dependent vasodilatation in response to shear stress in groups of healthy subjects (Hijmering et al., 2002; Thijssen et al., 2006) and patients with chronic heart failure (Santos et al., 2005). Although these findings have not been universal (Dyson et al., 2006), these acutely induced impairments in endothelial function support the suggestion that chronic sympathetic activation may contribute to the endothelial dysfunction and the subsequent development of atherosclerosis (Neunteufl et al., 1997). Experimental support of this contention is provided by classic studies of normocholesterolemic cynomolgus monkeys, where social stress mediated increases in sympathetic activity have been found to be directly related with impairments in vascular function and an increased risk of coronary artery atherosclerosis (Kaplan and Manuck, 1997; Kaplan et al., 1983). Notably, this atherogenic effect was observed to occur independently of changes in heart rate or BP, but was decreased following administration of β-blockade (Kaplan et al., 1987), suggesting that sympathetic arousal may make a direct contribution to the pathogenesis of atherosclerosis with chronic stress. Further, mechanistic insight into the association between sympathetic activation and atherosclerosis comes from the work of Pettersson et al., (Pettersson et al., 1990) who demonstrated that chloralose anaesthesia-induced increases in SNA caused endothelial cell damage; an effect that was completely abolished by pre-treatment with β-blockade. Collectively, these studies suggest that pathological elevations of SNA play a significant role in the process of vascular remodelling and endothelial dysfunction, and as such may represent one way in which sympathetic overactivity precipitates and accelerates the development of cardiovascular disease.
Sympathetically-induced increases in peripheral vascular resistance and arterial stiffness also have notable influences on cardiac function, via a process of ventricular-arterial coupling (Frenneaux and Williams, 2007). Increased arterial stiffness leads to increased pulse wave amplification and BP (Kannel, 1976), and the subsequent increase in aortic impedance and afterload necessitate a greater myocardial energy demand for a given stroke volume, thus reducing cardiac reserve (Frenneaux and Williams, 2007). Furthermore, this can increase cardiac wall stress and lead to the development of ventricular stiffness and hypertrophy (Frenneaux and Williams, 2007). However, independent of sympathetically-mediated changes in peripheral vascular function, chronically elevated SNA can have direct effects on myocardial structure and function. Administration of sub-pressor doses of noradrenaline have been reported to have a direct trophic effect on cardiac myocytes and increase left ventricular mass and wall thickness in animals (Sen and Young, 1991; Simpson, 1983). Similarly, it has recently been demonstrated in essential hypertensive patients that left ventricular hypertrophy, as determined using cardiac magnetic resonance imaging, is related to elevations in muscle SNA (Burns et al., 2007). This observation has clinical significance as left ventricular hypertrophy is associated with the progression to diastolic heart failure (Frenneaux and Williams, 2007) and is an independent risk factor for future cardiovascular events and sudden cardiac death (Levy et al., 1990). In fact, experimental increases in SNA raise the incidence of fatal cardiac rhythm disturbances, particularly when combined with decreased parasympathetic nerve activity (Lown and Verrier, 1976). These data suggest that sympathetic overactivity may contribute to the increased risk of sudden cardiac death in several conditions strongly associated with elevated SNA, including heart failure (Packer, 1985) and hypertension (Grassi, 1998).
The pathological consequences of sympathetic activation are not restricted to the cardiovascular system. Indeed, increased SNA can also have profound negative effects on the function of other organs. One such organ is the kidney, where elevated renal SNA increases vascular tone while altering renal sodium and water homeostasis, thus contributing to excessive fluid volume, oedema formation and increases in BP (DiBona, 2002). Notably, renal nerve transection ameliorates excess fluid retention indicative of the direct role of sympathetic overactivity in renovascular hypertension (DiBona, 2002). Moreover, evidence suggests that elevated SNA can impair renal function, independent of increases in BP. Indeed, in spontaneously hypertensive rats the administration of non-hypotensive doses of the central sympatholytic agent moxonidine has been shown to reduce SNA and the development of glomerulosclerosis (Amann et al., 2000). More recently, it has been reported that non-hypotensive doses of moxonidine can also elicit an antialbuminuric effect in type I diabetic patients (Strojek et al., 2001). These observations highlight the potential beneficial effects of reductions in SNA on renal function in humans, independent of any changes in BP.
Pathological sympatho-excitation has also been linked with metabolic diseases. Over two decades ago Landsberg proposed a link between the development of metabolic abnormalities, such as insulin resistance and dyslipidemia, and the activation of the sympathetic nervous system (Landsberg, 1986). According to this hypothesis elevations in circulating insulin levels, resulting from the insulin resistance associated with obesity, cause increases in central SNA which precipitate the development of hypertension (Landsberg, 2001). In support of this contention, subsequent studies in healthy human volunteers demonstrated that infusions of insulin, with glucose concentrations clamped, increased muscle SNA, an effect which appeared to be independent of the vasodilatory effects of insulin (Anderson et al., 1991; Hausberg et al., 1995). Furthermore, muscle SNA has been reported to be elevated in the obese (Grassi et al., 2007) and in some cases in the absence of elevations in BP. However, it has been known for some time that obesity is a strong predictor of future hypertension (Kannel, 1987). More recently it has been proposed that accumulating adiposity also partly mediates age-related increases in SNA (Seals and Bell, 2004). However, the connection between obesity, insulin resistance and elevated SNA is arguably a ‘chicken and egg’ scenario, and it is possible that elevated SNA can itself lead to insulin resistance, particularly in hypertensive individuals (Julius and Valentini, 1998). Indeed, acute increases in sympathetic activation have been shown to increase plasma insulin concentrations (Jamerson et al., 1993). In addition, it has been proposed that chronic elevations of SNA and subsequent α-adrenergic vasoconstriction may impede postprandial increases in muscle blood flow, thus limiting glucose clearance and causing exaggerated pancreatic insulin release (Julius and Valentini, 1998). In support of this possibility, the administration of vasoactive agents to offset peripheral vasoconstriction in patient populations has been reported to improve insulin sensitivity (Pollare et al., 1989; Pollare et al., 1988). Overall, there is clear evidence to support a linkage between elevated SNA and insulin resistance. This is indeed a rapidly evolving area of research (Grassi et al., 1996; Julius and Valentini, 1998; Tentolouris et al., 2006).
5. Potential mechanisms for sympathetic overactivity
Given the diverse range of pathological conditions associated with sympathetic overactivity and its potential to accelerate the progression of cardiovascular, renal and metabolic pathology, the sympathetic nervous system constitutes an important putative target for arresting the progression of disease (Grassi, 2004a; Grassi, 2004b). To develop effective countermeasures, it is important to identify the signal driving the sympathetic overactivity. However, the mechanisms underlying this sympathetic dysregulation are complex and multifactoral, encompassing alterations in autonomic reflex pathways, central autonomic neuroanatomical sites as well as hormonal factors. Indeed, intense work has focused on the potential role of impairments in sympathetic restraint provided by arterial and cardiopulmonary baroreflexes (Brown, 1980), and of exaggerated sympathetic drive attributable to augmented somatic reflexes (Smith et al., 2006) and central/peripheral chemoreflexes (Schultz et al., 2007). Furthermore, functional alterations in several autonomic nuclei (e.g. RVLM, NTS, and PVN) associated with changes in central concentrations of NO, Ang II, reactive oxygen species (ROS), aldosterone and inflammatory cytokines have also been proposed to contribute to pathological elevations in SNA (Gao et al., 2005b; Guggilam et al., 2008; Patel et al., 2001; Waki et al., 2006; Yu et al., 2008; Zhang et al., 2008; Zimmerman and Davisson, 2004; Zucker, 2006; Zucker et al., 2001). In this regard, accumulating evidence has implicated alterations in central NO in the genesis of central sympathetic overactivity.
Several studies have demonstrated that NO is not only an endothelial-dependent vasodilator but also a key signalling molecule involved in the control of sympathetic outflow from the brainstem (Lepori et al., 1998; Patel et al., 2001; Sander et al., 1997; Sander et al., 1995). All NO synthase (NOS) isoforms are plentiful in brain stem centres involved in the central regulation of SNA and BP (Rodrigo et al., 1994). Indeed, an abundance of animal literature has advanced the hypothesis that centrally-derived NO acts to tonically restrain sympathetic outflow from the brainstem, placing a brake on α-adrenergic vasoconstriction. The administration of NOS inhibitors by intravenous injection (Sakuma et al., 1992), intracisternal injection (el Karib et al., 1993; Togashi et al., 1992), or by direct injection into the RVLM (Zanzinger et al., 1995) or PVN (Zhang and Patel, 1998), has been shown to elicit acute increases in renal SNA and BP, implying a central role of NO on the sympathetic control of BP (Zanzinger, 1999; Zanzinger et al., 1995). A direct influence of NO on central sympathetic neuronal activity has also been derived from studies using rat brainstem slice preparations (Tagawa et al., 1994) and from work demonstrating that gene transfer mediated over expression of NOS at the RVLM decreases urinary noradrenaline in rats (Kishi et al., 2002). Collectively, these studies have demonstrated that alterations in centrally derived NO have functional consequences on central sympathetic outflow.
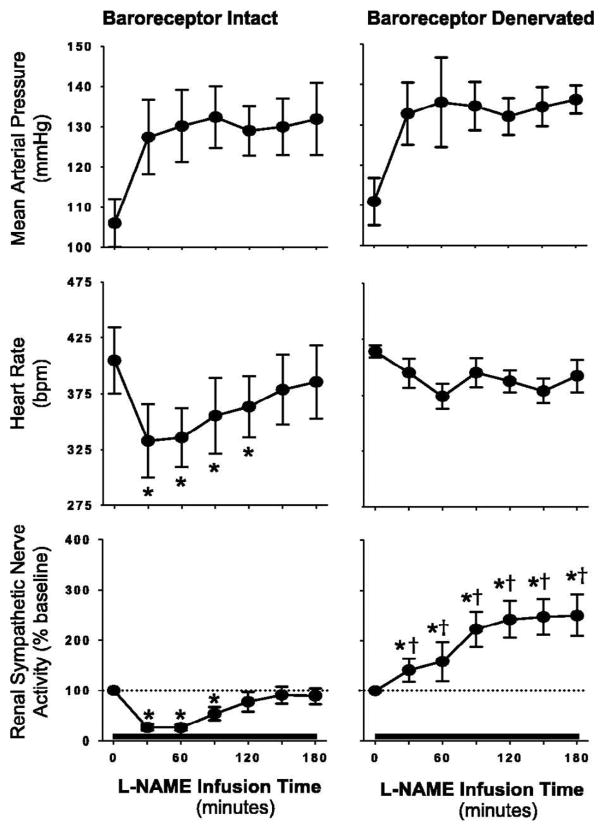
Initial attempts to extrapolate these findings of NO mediated control of central sympathetic outflow to the conscious state provided somewhat equivocal results (Barres et al., 1992; Hansen et al., 1994; Manning et al., 1994; Sander et al., 1995; Zanchi et al., 1995). Part of the explanation for these contradictory findings was likely related to reductions in endothelial-derived NO from the peripheral vasculature when NOS inhibitors were infused systemically. In this regard, experimental systemic NOS inhibition rapidly increases BP due to the removal of endothelial-dependent vasodilation and activates an arterial baroreflex-mediated sympatho-inhibitory response which counteracts any potential increase in SNA from a baroreflex-independent (i.e., central) mechanism (Hansen et al., 1994). Nevertheless, studies of conscious rats with and without sympathectomy during systemic NOS inhibition with NG-nitro-L-arginine (L-NAME) lend clear support for NO control of central SNA in that initial increases in BP were similar in both groups whereas over time BP continued to rise in intact rats but not in the sympathectomized rats (Sander et al., 1997). These results indicate the importance of SNA to the pathogenesis of NO deficient hypertension and have recently been extended to include direct measures of renal SNA (Augustyniak et al., 2006) (Figure 3). During systemic L-NAME, renal SNA exhibited an initial decrease (baroreflex-mediated) but then progressively increased up to 3 hours post infusion, whereas renal SNA remained suppressed throughout when BP was increased to a similar extent with the hypertensive control phenylephrine. Collectively, these data indicate that systemic NOS inhibition and the removal of NO does indeed alter central sympathetic outflow however, arterial baroreflex inhibition and the time for NOS inhibitors to cross the blood brain barrier need to be considered.
Figure 3.
Summary data showing the mean arterial pressure, heart rate, and renal SNA responses to intravenous infusion of NG-nitro-L-arginine methyl ester (L-NAME) in arterial baroreceptor intact rats (left panel) and baroreceptor denervated rats (right panel). In baroreceptor intact rats, systemic L-NAME evoked an initial baroreflex-mediated reduction in renal SNA that returned to baseline at 120 minutes of infusion. In contrast, a marked and sustained increase in renal SNA was found in baroreceptor-denervated rats. *P<0.05 vs. baseline; † P<0.05 vs. baroreceptor intact. Values are means ± SE. From Augustyniak et al., (2006), with permission.
In support of these findings from animal investigations, studies in humans have indicated a dose dependence of the sympathetic response to systemic intravenous administration of a NOS inhibitor, with low dose infusion of NG-monomethyl-L-arginine (L-NMMA) not altering muscle SNA and higher dosages suppressing SNA (Hansen et al., 1994; Lepori et al., 1998; Owlya et al., 1997). The lack of a sympathetic effect at the lower dosage in comparison to the sympatho-inhibition evoked by an equal pressor response elicited by phenylephrine was taken as evidence for a sympatho-excitatory effect of NOS inhibition. Additionally, minimizing the L-NMMA induced increase in BP with the co-infusion of nitroprusside caused SNA to increase. Thus, prevention of the arterial baroreflex-mediated suppression of muscle SNA unmasked a sympatho-excitatory effect of L-NMMA infusion. Further human studies by Sander et al., (Sander et al., 1999) have identified that inhibition of endothelium-dependent vasodilation is the primary mechanism underlying the initiation of the hypertensive response to systemic NOS inhibition but the sympathetic nervous system plays an important role in the full expression and maintenance of the BP raising effect. Recently, our laboratory has provided preliminary evidence that systemic NOS inhibition with L-NAME causes pronounced increases in sympathetic outflow in humans (Young et al., 2008). We made direct recordings of skin SNA during L-NAME infusion and time and hypertensive (phenylephrine) control experiments in healthy subjects. Our rationale for recording skin SNA is that unlike muscle SNA, cutaneous SNA is not under arterial baroreceptor control in normothermic conditions (Wallin et al., 1975) whereas it is responsive to alterations in central sympathetic outflow. We found that skin SNA increased significantly after L-NAME but remained unchanged during time and hypertensive controls. Although it is not possible to determine the precise site at which NO is acting within the central nervous system to alter SNA (e.g. RVLM, PVN, ganglionic synapse), these preliminary findings support a clear role for NO in the tonic restraint of central sympathetic outflow in humans. Given the potential for oestrogen to modulate NOS activity, sex differences may require consideration when examining the regulation of central SNA (Xue et al., 2007).
Considering that administration of an exogenous NOS inhibitor can increase SNA, it is plausible that physiological accumulation of endogenous NOS inhibitors could also raise SNA. L-NMMA and asymmetric dimethylarginine (ADMA) are naturally occurring NOS inhibitors that circulate in the plasma and are excreted in the urine (Vallance et al., 1992). ADMA is normally found in higher concentrations than L-NMMA and has been reported to be elevated in a number of pathophysiological conditions such as hypercholesterolemia, atherosclerosis, hypertension, chronic renal failure, and chronic heart failure (Boger, 2003). Most striking are the 4 to 12 fold increases in plasma ADMA in patients with end stage renal disease in comparison to healthy subjects with normal renal function (Vallance et al., 1992). Although a strong relationship between plasma concentrations of the sympathetic neurotransmitter noradrenaline and plasma ADMA has been reported among patients with end stage renal disease (Mallamaci et al., 2004), there is currently no direct evidence in humans indicating that excessive ADMA causes a reduction in central NO and increases SNA. However, consistent with this idea are recent findings from Augustyniak et al., demonstrating that systemic intravenous infusion of ADMA increases renal SNA in rats (Augustyniak et al., 2006). Further studies in healthy subjects and patients are needed to examine the potential for elevated ADMA concentrations to influence NO-mediated regulation of central sympathetic outflow.
Aside from changes in NOS activity, peripheral and central NO are also critically dependent upon a balance between ROS and antioxidants. Increased ROS generation will lead to scavenging of NO and thus the removal of central sympathetic inhibition (Lindley et al., 2004; Zimmerman and Davisson, 2004). Coupled with this, increases in ROS have been proposed to directly activate or sensitize sympathetic neurones via alterations in membrane ion channel function (Peterson et al., 2006; Veerasingham and Raizada, 2003; Zimmerman and Davisson, 2004). Conversely, intra-cerebroventricular infusion of the antioxidant tempol (a superoxide dismutase mimetic) has been shown to decrease SNA (Campese et al., 2004). Potential central enzymatic sources of ROS generation include, xanthine oxidase, cyclooxygenase, cytochrome p450, NOS and nicotinamide adenine dinucleotide phosphate (NAD(P)H) oxidase (Zimmerman and Davisson, 2004). The latter being considered as a particularly important source of central ROS (Peterson et al., 2006), and all the NAD(P)H oxidase subunits have been shown to be expressed in sympathetic neuron cultures (Tammariello et al., 2000) and throughout the fore-, mid-, and hindbrain (Serrano et al., 2003). It was first demonstrated that vascular smooth muscle NAD(P)H oxidase is activated by Ang II and subsequently increases vascular ROS levels (Griendling et al., 2000). However, more recent work has shown that a similar phenomenon occurs within the central nervous system where Ang II up-regulates NAD(P)H oxidase via activation of Ang II type 1 (AT1) receptors (Zimmerman and Davisson, 2004; Zucker et al., 2001). Importantly, this process has been shown to occur at neuroanatomical areas implicated in central sympathetic regulation such as the RVLM. Indeed, up-regulation of NAD(P)H oxidase in the RVLM has been shown to increase renal SNA (Gao et al., 2005b). Zucker and colleagues have suggested that this pathway becomes very important in heart failure and contributes to the well known reductions in arterial baroreflex sensitivity and increases in central sympathetic drive present in this disease state (Gao et al., 2005b; Zucker, 2006). In this regard, circulating plasma Ang II is elevated in heart failure and it is thought that the circumventricular organs, which lack a blood brain barrier and are rich in AT1 receptors, are the primary regions where circulating Ang II mediates its central effects by activating NAD(P)H oxidases (Simpson, 1981; Zucker et al., 2001). In addition, an up-regulation of locally produced Ang II and a consequent increase in ROS is also thought to contribute (Zimmerman and Davisson, 2004). Overall, these animal investigations indicate that increases in central sympathetic outflow in heart failure are mediated via Ang II activation of NAD(P)H oxidase and subsequent production of ROS, which may directly activate central SNA pathways along with scavenging NO thereby removing the tonic restraint on sympathetic outflow.
It is important to note that the mechanisms contributing to central sympathetic activation in heart failure, and indeed other disease conditions, are more complex than described above because multiple central neural sites, neurotransmitters and neuromodulators are involved (Weiss et al., 2003). For example, along with the pathophysiological changes in NO signalling reported at the RVLM, NO has been shown to also play a role in other brainstem regions important for the regulation of SNA. Indeed, reductions in neuronal NOS have been reported in the PVN, dorsal pons and hypothalamus in experimental heart failure, which may facilitate an enhanced descending drive to sympathetic pre-motor neurons and enhancement of SNA (Patel et al., 1996; Zhang et al., 1998; Zucker, 2006). A further important consideration is that the actions of NO may be regionally specific. Indeed, work from Paton and colleagues suggests that an Ang II mediated over expression of NO at the NTS decreases arterial baroreflex sensitivity and contributes to the elevated BP in spontaneously hypertensive rats (Paton et al., 2006; Waki et al., 2006). Thus, despite recent progress in this area, future studies in animals and humans focusing on changes in NO as well as potential alterations in Ang II, ROS, aldosterone and inflammatory cytokines are required to better understand the mechanisms contributing to central sympathetic overactivity in disease. Undoubtedly, this research will have a significant impact on future treatments targeting central sympathetic overactivity in disease.
6. Therapeutic strategies for targeting sympathetic over-activity
6.1 Central sympathetic agents
Protecting the heart from sympathetic overactivity by β-adrenergic blockers has proven to be beneficial for survival in hypertensive and heart failure patients and therefore β-blockers have become a mainstay of therapy in these conditions (Clark and Cleland, 2000). Although, angiotensin converting enzyme (ACE) inhibitors are also standard therapy in heart failure and hypertension, their survival benefits may be mediated through non-sympathetic mechanisms as reductions in SNA with ACE inhibition have been shown to be somewhat modest (Benedict et al., 1995; Grassi, 2004b; Swedberg et al., 1990). Likewise, studies in patients with chronic renal failure and essential hypertension have found that following either long term ACE inhibition (Ligtenberg et al., 1999) or AT1 receptor blockade (Klein et al., 2003; Krum et al., 2006) muscle SNA remained significantly elevated. These findings are somewhat surprising given the considerable evidence from animal studies detailing the important role of central Ang II and AT1 receptors in the genesis of elevations in central SNA (Veerasingham and Raizada, 2003; Zucker, 2006) and its amelioration with central administration of ACE inhibitors or AT1 receptor blockers (DiBona et al., 1995; Murakami et al., 1996; Murakami et al., 1997). The reason for these divergent findings is unclear but may reflect differences in central concentrations of the pharmacological agents achieved between animal and human studies (Krum et al., 2006).
An important consideration in the treatment of hypertension is that some pharmacological regimens that effectively reduce BP may actually exacerbate SNA, presumably due to baroreflex unloading (Fu et al., 2005). Thus, to further reduce the deleterious consequences of increases in sympathetic drive, a more effective therapeutic approach may be to inhibit central sympathetic outflow directly by stimulating α2 or imidazoline receptors in the central nervous system. However, even though clonidine (α2 and imidazoline receptor agonist) and moxonidine (selective imidazoline receptor agonist) have both proven to be effective in reducing SNA in chronic heart failure patients, both pharmacological agents have undesirable side effects which limit the potential benefit of these drugs on SNA (Aggarwal et al., 2003; Azevedo et al., 1999; Grassi et al., 2001; Planitz, 1984; Swedberg et al., 2002). In addition, it has been suggested that the excessive blockade of the sympathetic nervous system with moxonidine in heart failure may in fact prove deleterious (i.e. resting SNA may be insufficient to support cardiac output and peripheral resistance) (Floras, 2002). Although further studies titrating the dosage of moxonidine may be beneficial (Aggarwal et al., 2003), other treatment strategies aimed at reducing the sympathetic overactivity are warranted. More recently, the promising beneficial effects of the new imidazoline receptor agonist rilmenidine have come to light (Gerova et al., 2004). Along with acting as a powerful sympatholytic and antihypertensive agent (Head and Burke, 2000), rilmenidine has also been shown to be successful in reducing left ventricular hypertrophy in hypertensives (Koldas et al., 2003), improving arterial distensibility in patients with left ventricular hypertrophy (Trimarco et al., 1995), providing antiarrhythmic protection in the rabbit (Roegel et al., 1998), and improving glycemic control in patients with metabolic syndrome (De Luca et al., 2000). Given the multifaceted beneficial effects of rilmenidine, coupled with the finding that it is generally well tolerated, it is not surprising that it has been purported to be a “first-line antihypertensive option for all groups of hypertensive patient” (Reid, 2000). However, further work is required to explore the extent to which the beneficial effects of rilmenidine extend to other disease states characterised by elevations in central SNA.
6.2 Statins
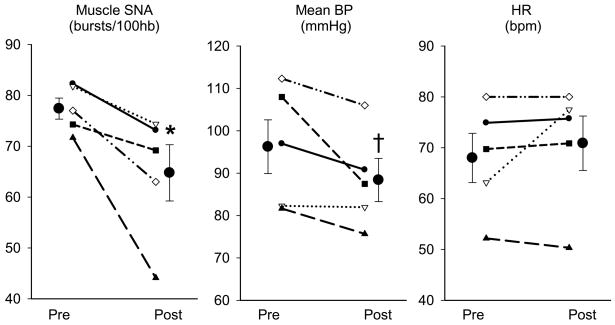
Reductions in LDL cholesterol with 3-hydroxy-3-meythl-glutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) are strongly associated with reductions in coronary heart disease risk (Holme, 1990). However, abundant evidence suggests that statins also have numerous cholesterol independent or “pleiotropic” effects (McFarlane et al., 2002). Indeed, recent findings from clinical trials indicate that statins improve survival in heart failure patients irrespective of cholesterol lowering (Horwich et al., 2004; Horwich and Middlekauff, 2008). Importantly, several studies in pacing-induced heart failure rabbits have demonstrated that statins normalise excessive sympathetic activation in the heart failure state by restoring central sympathetic inhibition and decreasing central sympathetic outflow (Gao et al., 2005a; Pliquett et al., 2003a; Pliquett et al., 2003b). Specifically, simvastatin down-regulates AT1 receptors, NAD(P)H oxidase subunit gene expression and superoxide while up-regulating neuronally derived NOS in the RVLM, in heart failure (Gao et al., 2005a; Gao et al., 2008). Such centrally mediated changes in neural regulation are associated with reductions in SNA and improvements in arterial baroreflex sensitivity and left ventricular function (Gao et al., 2005a). Collectively, this extensive series of studies by Zucker et al., provide clear support that independent of cholesterol lowering stains reduce sympathetic overactivity in heart failure. These are exciting data given the limited success of conventional anti-adrenergic strategies in reducing SNA in heart failure. To examine whether these findings in experimental heart failure could be translated to the clinical setting of human heart failure we recently measured muscle SNA before and after 1 month of simvastatin (40 mg/day) in a small group of heart failure patients (Figure 4). Our preliminary human data suggests that short-term statin therapy is indeed capable of reducing resting central sympathetic outflow in heart failure patients (Fisher et al., 2007). Although very promising, these findings require validation in a larger cohort of patients. In addition, an understanding of the underlying mechanisms and the extension of these SNA-reducing effects of statins to other patient populations with known sympathetic hyperactivity await further investigation.
Figure 4.
Individual and mean data showing changes in muscle sympathetic nerve activity (Muscle SNA; bursts/100 heart beats), mean blood pressure (BP) and heart rate (HR) in 5 heart failure patients before and after one month of Simvastatin (40 mg/day). Muscle SNA was significantly reduced following statin therapy, whereas mean BP was slightly decreased and HR was largely unchanged. * represents P<0.05 vs. Pre-Simvastatin; † represents P=0.08 vs. Pre-Simvastatin. Values are means ± SE.
6.3 Exercise training
Exercise training is a widely prescribed non-pharmacological therapeutic strategy for improving the quality of life in several diseases characterized by high SNA (e.g. heart failure, hypertension, type II diabetes and obesity), and conferring numerous other physiological benefits (Pedersen and Saltin, 2006). In particular, exercise training is well known to produce marked reductions in BP in hypertensive patients (Pescatello et al., 2004). Reductions in BP induced by exercise in healthy individuals have been associated with attenuated noradrenaline spillover and plasma concentrations (Jennings et al., 1986; Mueller, 2007) and in some instances physical activity has also been reported to reduce muscle SNA in healthy individuals (Grassi et al., 1994). However, the majority of evidence from both longitudinal and cross-sectional studies suggests that exercise training has minimal effects on resting muscle SNA in healthy humans (Ray and Hume, 1998). In contrast, exercise training has been shown to produce robust reductions in central sympathetic outflow in recent studies of experimental animals (Gao et al., 2007; Liu et al., 2000; Zucker et al., 2004; Zucker et al., 2001) and patients (Fraga et al., 2007; Roveda et al., 2003) with heart failure. In particular, work by Zucker et al., using an experimental model of heart failure in the rabbit, has made significant contributions to our understanding of the central molecular mechanisms underlying the sympatho-inhibitory effects of exercise in disease (Gao et al., 2007; Liu et al., 2000; Zucker et al., 2004). Recent evidence from this group has demonstrated that exercise training restores the sympatho-inhibitory effects of central NO and enhances arterial baroreflex regulation of renal SNA (Liu et al., 2000). In addition, an exercise induced up-regulation of central antioxidants and down-regulation of NAD(P)H oxidase in the RVLM, with concomitant suppression of central pro-oxidant mechanisms has been reported (Gao et al., 2007). However, it is important to note that other central neural pathways also likely contribute to exercise mediated alterations in central regulation of SNA, and this remains an area of intense research focus (Mueller, 2007). Nevertheless, the emerging evidence from both animal and human studies supports the value of exercise training as a therapeutic strategy for reversal of pathological increases in central sympathetic outflow.
7. Summary
Central sympathetic overactivity has been identified in a plethora of clinical conditions. Aside from a role in the development of hypertension, sympathetic overactivity has been implicated in the initiation and progression of numerous pathophysiological processes independent of increases in BP (e.g. vascular hypertrophy, atherosclerosis, glomerulosclerosis, and insulin resistance). Valuable work to elucidate the mechanisms underlying pathological elevations in central SNA and to develop effective therapeutic strategies is ongoing, which given the increasing prevalence of diseases associated with sympathetic overactivity is clearly a pressing issue.
Table 1.
Potential pathological consequences of elevated central sympathetic nerve activity
| Vascular effects | Cardiac effects |
| VSM cell hypertrophy and proliferation | Cardiac myocyte hypertrophy |
| Medial thickening | Left ventricular hypertrophy |
| Endothelial cell damage | ↑ Incidence of arrhythmia |
| Endothelial dysfunction | Tachycardia |
| Arterial stiffness | |
| ↑ Blood pressure variability | Renal effects |
| ↑ Peripheral vascular resistance | Renal vasoconstriction |
| Hypertension | Sodium and fluid retention |
| Atherosclerosis | Glomerulosclerosis |
| Microalbumineria | |
| Metabolic effects | RAAS activation |
| Insulin resistance | |
| ↑ Plasma insulin concentration | |
| Dyslipidemia |
VSM, vascular smooth muscle; RAAS, Renin-angiotensin-aldosterone system.
Acknowledgments
This review and the original research presented by the authors were, in part, supported by NIH Grant #DK-076636 to PJF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abboud FM. The sympathetic system in hypertension. State-of-the-art review. Hypertension. 1982;4:208–225. [PubMed] [Google Scholar]
- Aggarwal A, Esler MD, Morris MJ, Lambert G, Kaye DM. Regional sympathetic effects of low-dose clonidine in heart failure. Hypertension. 2003;41:553–557. doi: 10.1161/01.HYP.0000055779.93635.A2. [DOI] [PubMed] [Google Scholar]
- Amann K, Rump LC, Simonaviciene A, Oberhauser V, Wessels S, Orth SR, Gross ML, Koch A, Bielenberg GW, Van Kats JP, Ehmke H, Mall G, Ritz E. Effects of low dose sympathetic inhibition on glomerulosclerosis and albuminuria in subtotally nephrectomized rats. J Am Soc Nephrol. 2000;11:1469–1478. doi: 10.1681/ASN.V1181469. [DOI] [PubMed] [Google Scholar]
- Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991;87:2246–2252. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension. 1989;14:177–183. doi: 10.1161/01.hyp.14.2.177. [DOI] [PubMed] [Google Scholar]
- Augustyniak RA, Victor RG, Morgan DA, Zhang W. L-NAME- and ADMA-induced sympathetic neural activation in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R726–732. doi: 10.1152/ajpregu.00768.2004. [DOI] [PubMed] [Google Scholar]
- Azevedo ER, Newton GE, Parker JD. Cardiac and systemic sympathetic activity in response to clonidine in human heart failure. J Am Coll Cardiol. 1999;33:186–191. doi: 10.1016/s0735-1097(98)00524-5. [DOI] [PubMed] [Google Scholar]
- Barres C, Julien C, Sassard J. Arterial pressure control in the sympathectomized rat. Kidney Int Suppl. 1992;37:S19–23. [PubMed] [Google Scholar]
- Barretto AC, Santos AC, Munhoz R, Rondon MU, Franco FG, Trombetta IC, Roveda F, de Matos LN, Braga AM, Middlekauff HR, Negrao CE. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol. 2008 doi: 10.1016/j.ijcard.2008.03.056. [DOI] [PubMed] [Google Scholar]
- Barton DA, Dawood T, Lambert EA, Esler MD, Haikerwal D, Brenchley C, Socratous F, Kaye DM, Schlaich MP, Hickie I, Lambert GW. Sympathetic activity in major depressive disorder: identifying those at increased cardiac risk? J Hypertens. 2007;25:2117–2124. doi: 10.1097/HJH.0b013e32829baae7. [DOI] [PubMed] [Google Scholar]
- Benedict CR, Francis GS, Shelton B, Johnstone DE, Kubo SH, Kirlin P, Nicklas J, Liang CS, Konstam MA, Greenberg B, et al. Effect of long-term enalapril therapy on neurohormones in patients with left ventricular dysfunction. SOLVD Investigators. Am J Cardiol. 1995;75:1151–1157. doi: 10.1016/s0002-9149(99)80748-6. [DOI] [PubMed] [Google Scholar]
- Bevan RD. Effect of sympathetic denervation on smooth muscle cell proliferation in the growing rabbit ear artery. Circ Res. 1975;37:14–19. doi: 10.1161/01.res.37.1.14. [DOI] [PubMed] [Google Scholar]
- Bevan RD. Trophic effects of peripheral adrenergic nerves on vascular structure. Hypertension. 1984;6:III19–26. doi: 10.1161/01.hyp.6.6_pt_2.iii19. [DOI] [PubMed] [Google Scholar]
- Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999a;33:1111–1117. doi: 10.1161/01.hyp.33.5.1111. [DOI] [PubMed] [Google Scholar]
- Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999b;99:2434–2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- Boger RH. The emerging role of asymmetric dimethylarginine as a novel cardiovascular risk factor. Cardiovasc Res. 2003;59:824–833. doi: 10.1016/s0008-6363(03)00500-5. [DOI] [PubMed] [Google Scholar]
- Boutouyrie P, Lacolley P, Girerd X, Beck L, Safar M, Laurent S. Sympathetic activation decreases medium-sized arterial compliance in humans. Am J Physiol. 1994;267:H1368–1376. doi: 10.1152/ajpheart.1994.267.4.H1368. [DOI] [PubMed] [Google Scholar]
- Brown AM. Receptors under pressure. An update on baroreceptors. Circ Res. 1980;46:1–10. doi: 10.1161/01.res.46.1.1. [DOI] [PubMed] [Google Scholar]
- Burns J, Sivananthan MU, Ball SG, Mackintosh AF, Mary DA, Greenwood JP. Relationship between central sympathetic drive and magnetic resonance imaging-determined left ventricular mass in essential hypertension. Circulation. 2007;115:1999–2005. doi: 10.1161/CIRCULATIONAHA.106.668863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campese VM, Ye S, Zhong H, Yanamadala V, Ye Z, Chiu J. Reactive oxygen species stimulate central and peripheral sympathetic nervous system activity. Am J Physiol Heart Circ Physiol. 2004;287:H695–703. doi: 10.1152/ajpheart.00619.2003. [DOI] [PubMed] [Google Scholar]
- Clark AL, Cleland JG. The control of adrenergic function in heart failure: therapeutic intervention. Heart Fail Rev. 2000;5:101–114. doi: 10.1023/A:1009854325711. [DOI] [PubMed] [Google Scholar]
- Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- Converse RL, Jr, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, Victor RG. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327:1912–1918. doi: 10.1056/NEJM199212313272704. [DOI] [PubMed] [Google Scholar]
- Cowley AW, Jr, Liard JF, Guyton AC. Role of baroreceptor reflex in daily control of arterial blood pressure and other variables in dogs. Circ Res. 1973;32:564–576. doi: 10.1161/01.res.32.5.564. [DOI] [PubMed] [Google Scholar]
- Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–2090. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- Dao HH, Lemay J, de Champlain J, deBlois D, Moreau P. Norepinephrine-induced aortic hyperplasia and extracellular matrix deposition are endothelin-dependent. J Hypertens. 2001;19:1965–1973. doi: 10.1097/00004872-200111000-00006. [DOI] [PubMed] [Google Scholar]
- De Luca N, Izzo R, Fontana D, Iovino G, Argenziano L, Vecchione C, Trimarco B. Haemodynamic and metabolic effects of rilmenidine in hypertensive patients with metabolic syndrome X. A double-blind parallel study versus amlodipine. J Hypertens. 2000;18:1515–1522. doi: 10.1097/00004872-200018100-00021. [DOI] [PubMed] [Google Scholar]
- DiBona GF. Sympathetic nervous system and the kidney in hypertension. Curr Opin Nephrol Hypertens. 2002;11:197–200. doi: 10.1097/00041552-200203000-00011. [DOI] [PubMed] [Google Scholar]
- DiBona GF, Jones SY, Brooks VL. ANG II receptor blockade and arterial baroreflex regulation of renal nerve activity in cardiac failure. Am J Physiol. 1995;269:R1189–1196. doi: 10.1152/ajpregu.1995.269.5.R1189. [DOI] [PubMed] [Google Scholar]
- Dyson KS, Shoemaker JK, Hughson RL. Effect of acute sympathetic nervous system activation on flow-mediated dilation of brachial artery. Am J Physiol Heart Circ Physiol. 2006;290:H1446–1453. doi: 10.1152/ajpheart.00771.2005. [DOI] [PubMed] [Google Scholar]
- el Karib AO, Sheng J, Betz AL, Malvin RL. The central effects of a nitric oxide synthase inhibitor (N omega-nitro-L-arginine) on blood pressure and plasma renin. Clin Exp Hypertens. 1993;15:819–832. doi: 10.3109/10641969309041644. [DOI] [PubMed] [Google Scholar]
- Esler M, Julius S, Zweifler A, Randall O, Harburg E, Gardiner H, DeQuattro V. Mild high-renin essential hypertension. Neurogenic human hypertension? N Engl J Med. 1977;296:405–411. doi: 10.1056/NEJM197702242960801. [DOI] [PubMed] [Google Scholar]
- Esler M, Lambert G, Brunner-La Rocca HP, Vaddadi G, Kaye D. Sympathetic nerve activity and neurotransmitter release in humans: translation from pathophysiology into clinical practice. Acta Physiol Scand. 2003;177:275–284. doi: 10.1046/j.1365-201X.2003.01089.x. [DOI] [PubMed] [Google Scholar]
- Esler M, Lambert G, Jennings G. Increased regional sympathetic nervous activity in human hypertension: causes and consequences. J Hypertens Suppl. 1990;8:S53–57. [PubMed] [Google Scholar]
- Failla M, Grappiolo A, Emanuelli G, Vitale G, Fraschini N, Bigoni M, Grieco N, Denti M, Giannattasio C, Mancia G. Sympathetic tone restrains arterial distensibility of healthy and atherosclerotic subjects. J Hypertens. 1999;17:1117–1123. doi: 10.1097/00004872-199917080-00011. [DOI] [PubMed] [Google Scholar]
- Feihl F, Liaudet L, Levy BI, Waeber B. Hypertension and microvascular remodelling. Cardiovasc Res. 2008;78:274–285. doi: 10.1093/cvr/cvn022. [DOI] [PubMed] [Google Scholar]
- Fischer D, Rossa S, Landmesser U, Spiekermann S, Engberding N, Hornig B, Drexler H. Endothelial dysfunction in patients with chronic heart failure is independently associated with increased incidence of hospitalization, cardiac transplantation, or death. Eur Heart J. 2005;26:65–69. doi: 10.1093/eurheartj/ehi001. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Junor C, Ahmed A, Gallagher KM, Fadel PJ. The influence of statin therapy on resting sympathetic nerve activity in patients with heart failure. FASEB J. 2007;21:910–919. [Google Scholar]
- Floras JS. The “unsympathetic” nervous system of heart failure. Circulation. 2002;105:1753–1755. doi: 10.1161/01.cir.0000013788.71817.16. [DOI] [PubMed] [Google Scholar]
- Folkow B. Physiological aspects of primary hypertension. Physiol Rev. 1982;62:347–504. doi: 10.1152/physrev.1982.62.2.347. [DOI] [PubMed] [Google Scholar]
- Fraga R, Franco FG, Roveda F, de Matos LN, Braga AM, Rondon MU, Rotta DR, Brum PC, Barretto AC, Middlekauff HR, Negrao CE. Exercise training reduces sympathetic nerve activity in heart failure patients treated with carvedilol. Eur J Heart Fail. 2007;9:630–636. doi: 10.1016/j.ejheart.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Frenneaux M, Williams L. Ventricular-arterial and ventricular-ventricular interactions and their relevance to diastolic filling. Prog Cardiovasc Dis. 2007;49:252–262. doi: 10.1016/j.pcad.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Fu Q, Zhang R, Witkowski S, Arbab-Zadeh A, Prasad A, Okazaki K, Levine BD. Persistent sympathetic activation during chronic antihypertensive therapy: a potential mechanism for long term morbidity? Hypertension. 2005;45:513–521. doi: 10.1161/01.HYP.0000158312.63381.c1. [DOI] [PubMed] [Google Scholar]
- Furlan R, Ardizzone S, Palazzolo L, Rimoldi A, Perego F, Barbic F, Bevilacqua M, Vago L, Bianchi Porro G, Malliani A. Sympathetic overactivity in active ulcerative colitis: effects of clonidine. Am J Physiol Regul Integr Comp Physiol. 2006;290:R224–232. doi: 10.1152/ajpregu.00442.2005. [DOI] [PubMed] [Google Scholar]
- Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Simvastatin therapy normalizes sympathetic neural control in experimental heart failure: roles of angiotensin II type 1 receptors and NAD(P)H oxidase. Circulation. 2005a;112:1763–1770. doi: 10.1161/CIRCULATIONAHA.105.552174. [DOI] [PubMed] [Google Scholar]
- Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Sympathoexcitation by central ANG II: roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am J Physiol Heart Circ Physiol. 2005b;288:H2271–2279. doi: 10.1152/ajpheart.00949.2004. [DOI] [PubMed] [Google Scholar]
- Gao L, Wang W, Liu D, Zucker IH. Exercise training normalizes sympathetic outflow by central antioxidant mechanisms in rabbits with pacing-induced chronic heart failure. Circulation. 2007;115:3095–3102. doi: 10.1161/CIRCULATIONAHA.106.677989. [DOI] [PubMed] [Google Scholar]
- Gao L, Wang W, Zucker IH. Simvastatin inhibits central sympathetic outflow in heart failure by a nitric-oxide synthase mechanism. J Pharmacol Exp Ther. 2008;326:278–285. doi: 10.1124/jpet.107.136028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerova M, Torok J, Pechaoova O, Matuskova J. Rilmenidine prevents blood pressure increase in rats with compromised nitric oxide production. Acta Pharmacol Sin. 2004;25:1640–1646. [PubMed] [Google Scholar]
- Goldstein DS. Plasma catecholamines and essential hypertension. An analytical review. Hypertension. 1983;5:86–99. doi: 10.1161/01.hyp.5.1.86. [DOI] [PubMed] [Google Scholar]
- Graham LN, Smith PA, Huggett RJ, Stoker JB, Mackintosh AF, Mary DA. Sympathetic drive in anterior and inferior uncomplicated acute myocardial infarction. Circulation. 2004;109:2285–2289. doi: 10.1161/01.CIR.0000129252.96341.8B. [DOI] [PubMed] [Google Scholar]
- Grassi G. Role of the sympathetic nervous system in human hypertension. J Hypertens. 1998;16:1979–1987. doi: 10.1097/00004872-199816121-00019. [DOI] [PubMed] [Google Scholar]
- Grassi G. Counteracting the sympathetic nervous system in essential hypertension. Curr Opin Nephrol Hypertens. 2004a;13:513–519. doi: 10.1097/00041552-200409000-00006. [DOI] [PubMed] [Google Scholar]
- Grassi G. Sympathetic and baroreflex function in hypertension: implications for current and new drugs. Curr Pharm Des. 2004b;10:3579–3589. doi: 10.2174/1381612043382756. [DOI] [PubMed] [Google Scholar]
- Grassi G, Cattaneo BM, Seravalle G, Colombo M, Cavagnini F, Mancia G. Obesity and the sympathetic nervous system. Blood Press Suppl. 1996;1:43–46. [PubMed] [Google Scholar]
- Grassi G, Cattaneo BM, Seravalle G, Lanfranchi A, Mancia G. Baroreflex control of sympathetic nerve activity in essential and secondary hypertension. Hypertension. 1998;31:68–72. doi: 10.1161/01.hyp.31.1.68. [DOI] [PubMed] [Google Scholar]
- Grassi G, Dell’Oro R, Quarti-Trevano F, Scopelliti F, Seravalle G, Paleari F, Gamba PL, Mancia G. Neuroadrenergic and reflex abnormalities in patients with metabolic syndrome. Diabetologia. 2005;48:1359–1365. doi: 10.1007/s00125-005-1798-z. [DOI] [PubMed] [Google Scholar]
- Grassi G, Esler M. How to assess sympathetic activity in humans. J Hypertens. 1999;17:719–734. doi: 10.1097/00004872-199917060-00001. [DOI] [PubMed] [Google Scholar]
- Grassi G, Giannattasio C, Failla M, Pesenti A, Peretti G, Marinoni E, Fraschini N, Vailati S, Mancia G. Sympathetic modulation of radial artery compliance in congestive heart failure. Hypertension. 1995;26:348–354. doi: 10.1161/01.hyp.26.2.348. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Calhoun DA, Mancia G. Physical training and baroreceptor control of sympathetic nerve activity in humans. Hypertension. 1994;23:294–301. doi: 10.1161/01.hyp.23.3.294. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Quarti-Trevano F, Scopelliti F, Dell’Oro R, Bolla G, Mancia G. Excessive sympathetic activation in heart failure with obesity and metabolic syndrome: characteristics and mechanisms. Hypertension. 2007;49:535–541. doi: 10.1161/01.HYP.0000255983.32896.b9. [DOI] [PubMed] [Google Scholar]
- Grassi G, Turri C, Seravalle G, Bertinieri G, Pierini A, Mancia G. Effects of chronic clonidine administration on sympathetic nerve traffic and baroreflex function in heart failure. Hypertension. 2001;38:286–291. doi: 10.1161/01.hyp.38.2.286. [DOI] [PubMed] [Google Scholar]
- Greenwood JP, Scott EM, Walker JJ, Stoker JB, Mary DA. The magnitude of sympathetic hyperactivity in pregnancy-induced hypertension and preeclampsia. Am J Hypertens. 2003;16:194–199. doi: 10.1016/s0895-7061(02)03256-9. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- Guggilam A, Patel KP, Haque M, Ebenezer PJ, Kapusta DR, Francis J. Cytokine blockade attenuates sympathoexcitation in heart failure: cross-talk between nNOS, AT-1R and cytokines in the hypothalamic paraventricular nucleus. Eur J Heart Fail. 2008;10:625–634. doi: 10.1016/j.ejheart.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Jacobsen TN, Victor RG. Is nitric oxide involved in the tonic inhibition of central sympathetic outflow in humans? Hypertension. 1994;24:439–444. doi: 10.1161/01.hyp.24.4.439. [DOI] [PubMed] [Google Scholar]
- Hausberg M, Mark AL, Hoffman RP, Sinkey CA, Anderson EA. Dissociation of sympathoexcitatory and vasodilator actions of modestly elevated plasma insulin levels. J Hypertens. 1995;13:1015–1021. doi: 10.1097/00004872-199509000-00012. [DOI] [PubMed] [Google Scholar]
- Head GA, Burke SL. I1 imidazoline receptors in cardiovascular regulation: the place of rilmenidine. Am J Hypertens. 2000;13:89S–98S. doi: 10.1016/s0895-7061(00)00224-7. [DOI] [PubMed] [Google Scholar]
- Henle J. Ueber die Contractilitat der Gefasse. Wschr ges Heilk. 1840;21:1. [Google Scholar]
- Heymans CJF, Folkow B. Vasomotor control and the regulation of blood pressure. In: Fishman AP, Richards DW, editors. Circulation of the blood: Men and ideas. American Physiological Society; Bethesda, Maryland: 1982. pp. 407–486. [Google Scholar]
- Hijmering ML, Stroes ES, Olijhoek J, Hutten BA, Blankestijn PJ, Rabelink TJ. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J Am Coll Cardiol. 2002;39:683–688. doi: 10.1016/s0735-1097(01)01786-7. [DOI] [PubMed] [Google Scholar]
- Holme I. An analysis of randomized trials evaluating the effect of cholesterol reduction on total mortality and coronary heart disease incidence. Circulation. 1990;82:1916–1924. doi: 10.1161/01.cir.82.6.1916. [DOI] [PubMed] [Google Scholar]
- Horwich TB, MacLellan WR, Fonarow GC. Statin therapy is associated with improved survival in ischemic and non-ischemic heart failure. J Am Coll Cardiol. 2004;43:642–648. doi: 10.1016/j.jacc.2003.07.049. [DOI] [PubMed] [Google Scholar]
- Horwich TB, Middlekauff HR. Potential autonomic nervous system effects of statins in heart failure. Heart Fail Clin. 2008;4:163–170. doi: 10.1016/j.hfc.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggett RJ, Scott EM, Gilbey SG, Stoker JB, Mackintosh AF, Mary DA. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation. 2003;108:3097–3101. doi: 10.1161/01.CIR.0000103123.66264.FE. [DOI] [PubMed] [Google Scholar]
- Jamerson KA, Julius S, Gudbrandsson T, Andersson O, Brant DO. Reflex sympathetic activation induces acute insulin resistance in the human forearm. Hypertension. 1993;21:618–623. doi: 10.1161/01.hyp.21.5.618. [DOI] [PubMed] [Google Scholar]
- Jennings G, Nelson L, Nestel P, Esler M, Korner P, Burton D, Bazelmans J. The effects of changes in physical activity on major cardiovascular risk factors, hemodynamics, sympathetic function, and glucose utilization in man: a controlled study of four levels of activity. Circulation. 1986;73:30–40. doi: 10.1161/01.cir.73.1.30. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Charkoudian N, Wallin BG. A sympathetic view of the sympathetic nervous system and human blood pressure regulation. Exp Physiol. 2008;93:715–724. doi: 10.1113/expphysiol.2007.039545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judy WV, Watanabe AM, Murphy WR, Aprison BS, Yu PL. Sympathetic nerve activity and blood pressure in normotensive backcross rats genetically related to the spontaneously hypertensive rat. Hypertension. 1979;1:598–604. doi: 10.1161/01.hyp.1.6.598. [DOI] [PubMed] [Google Scholar]
- Julius S, Valentini M. Consequences of the increased autonomic nervous drive in hypertension, heart failure and diabetes. Blood Press Suppl. 1998;3:5–13. doi: 10.1080/080370598438410-1. [DOI] [PubMed] [Google Scholar]
- Kannel WB. Some lessons in cardiovascular epidemiology from Framingham. Am J Cardiol. 1976;37:269–282. doi: 10.1016/0002-9149(76)90323-4. [DOI] [PubMed] [Google Scholar]
- Kannel WB. Metabolic risk factors for coronary heart disease in women: perspective from the Framingham Study. Am Heart J. 1987;114:413–419. doi: 10.1016/0002-8703(87)90511-4. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB. Using ethological principles to study psychosocial influences on coronary atherosclerosis in monkeys. Acta Physiol Scand Suppl. 1997;640:96–99. [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Adams MR, Weingand KW, Clarkson TB. Inhibition of coronary atherosclerosis by propranolol in behaviorally predisposed monkeys fed an atherogenic diet. Circulation. 1987;76:1364–1372. doi: 10.1161/01.cir.76.6.1364. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Clarkson TB, Lusso FM, Taub DM, Miller EW. Social stress and atherosclerosis in normocholesterolemic monkeys. Science. 1983;220:733–735. doi: 10.1126/science.6836311. [DOI] [PubMed] [Google Scholar]
- Kinlay S, Creager MA, Fukumoto M, Hikita H, Fang JC, Selwyn AP, Ganz P. Endothelium-derived nitric oxide regulates arterial elasticity in human arteries in vivo. Hypertension. 2001;38:1049–1053. doi: 10.1161/hy1101.095329. [DOI] [PubMed] [Google Scholar]
- Kishi T, Hirooka Y, Ito K, Sakai K, Shimokawa H, Takeshita A. Cardiovascular effects of overexpression of endothelial nitric oxide synthase in the rostral ventrolateral medulla in stroke-prone spontaneously hypertensive rats. Hypertension. 2002;39:264–268. doi: 10.1161/hy0202.102701. [DOI] [PubMed] [Google Scholar]
- Klein IH, Ligtenberg G, Oey PL, Koomans HA, Blankestijn PJ. Enalapril and losartan reduce sympathetic hyperactivity in patients with chronic renal failure. J Am Soc Nephrol. 2003;14:425–430. doi: 10.1097/01.asn.0000045049.72965.b7. [DOI] [PubMed] [Google Scholar]
- Koldas L, Ayan F, Ikitimur B. Short-term effects of rilmenidine on left ventricular hypertrophy and systolic and diastolic function in patients with essential hypertension: comparison with an angiotensin converting enzyme inhibitor and a calcium antagonist. Jpn Heart J. 2003;44:693–704. doi: 10.1536/jhj.44.693. [DOI] [PubMed] [Google Scholar]
- Kotanko P. Cause and consequences of sympathetic hyperactivity in chronic kidney disease. Blood Purif. 2006;24:95–99. doi: 10.1159/000089444. [DOI] [PubMed] [Google Scholar]
- Krum H, Lambert E, Windebank E, Campbell DJ, Esler M. Effect of angiotensin II receptor blockade on autonomic nervous system function in patients with essential hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H1706–1712. doi: 10.1152/ajpheart.00885.2005. [DOI] [PubMed] [Google Scholar]
- Landsberg L. Diet, obesity and hypertension: an hypothesis involving insulin, the sympathetic nervous system, and adaptive thermogenesis. Q J Med. 1986;61:1081–1090. [PubMed] [Google Scholar]
- Landsberg L. Insulin-mediated sympathetic stimulation: role in the pathogenesis of obesity-related hypertension (or, how insulin affects blood pressure, and why) J Hypertens. 2001;19:523–528. doi: 10.1097/00004872-200103001-00001. [DOI] [PubMed] [Google Scholar]
- Leimbach WN, Jr, Wallin BG, Victor RG, Aylward PE, Sundlof G, Mark AL. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation. 1986;73:913–919. doi: 10.1161/01.cir.73.5.913. [DOI] [PubMed] [Google Scholar]
- Lepori M, Sartori C, Trueb L, Owlya R, Nicod P, Scherrer U. Haemodynamic and sympathetic effects of inhibition of nitric oxide synthase by systemic infusion of N(G)-monomethyl-L-arginine into humans are dose dependent. J Hypertens. 1998;16:519–523. doi: 10.1097/00004872-199816040-00013. [DOI] [PubMed] [Google Scholar]
- Levy BI, Benessiano J, Poitevin P, Safar ME. Endothelium-dependent mechanical properties of the carotid artery in Wistar-Kyoto and spontaneously hypertensive rats. J Hypertens Suppl. 1989;7:S118–119. doi: 10.1097/00004872-198900076-00055. [DOI] [PubMed] [Google Scholar]
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- Ligtenberg G, Blankestijn PJ, Oey PL, Klein IH, Dijkhorst-Oei LT, Boomsma F, Wieneke GH, van Huffelen AC, Koomans HA. Reduction of sympathetic hyperactivity by enalapril in patients with chronic renal failure. N Engl J Med. 1999;340:1321–1328. doi: 10.1056/NEJM199904293401704. [DOI] [PubMed] [Google Scholar]
- Lindley TE, Doobay MF, Sharma RV, Davisson RL. Superoxide is involved in the central nervous system activation and sympathoexcitation of myocardial infarction-induced heart failure. Circ Res. 2004;94:402–409. doi: 10.1161/01.RES.0000112964.40701.93. [DOI] [PubMed] [Google Scholar]
- Liu JL, Irvine S, Reid IA, Patel KP, Zucker IH. Chronic exercise reduces sympathetic nerve activity in rabbits with pacing-induced heart failure: A role for angiotensin II. Circulation. 2000;102:1854–1862. doi: 10.1161/01.cir.102.15.1854. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart Disease and Stroke Statistics--2009 Update. A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008 doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- Loewy AD. Central autonomic pathways. In: Loewy AD, Spyer KM, editors. Central Regulation of Autonomic Functions. Oxford University Press; New York: 1990. pp. 88–103. [Google Scholar]
- London GM, Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME. Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38:434–438. doi: 10.1161/01.hyp.38.3.434. [DOI] [PubMed] [Google Scholar]
- Lown B, Verrier RL. Neural activity and ventricular fibrillation. N Engl J Med. 1976;294:1165–1170. doi: 10.1056/NEJM197605202942107. [DOI] [PubMed] [Google Scholar]
- Mallamaci F, Tripepi G, Maas R, Malatino L, Boger R, Zoccali C. Analysis of the relationship between norepinephrine and asymmetric dimethyl arginine levels among patients with end-stage renal disease. J Am Soc Nephrol. 2004;15:435–441. doi: 10.1097/01.asn.0000106717.58091.f6. [DOI] [PubMed] [Google Scholar]
- Mancia G, Giannattasio C, Grassi G. Arterial distensibility in cardiovascular diseases. J Nephrol. 1998;11:284–288. [PubMed] [Google Scholar]
- Mancia G, Parati G. The role of blood pressure variability in end-organ damage. J Hypertens Suppl. 2003;21:S17–23. doi: 10.1097/00004872-200307006-00004. [DOI] [PubMed] [Google Scholar]
- Mangoni AA, Mircoli L, Giannattasio C, Mancia G, Ferrari AU. Effect of sympathectomy on mechanical properties of common carotid and femoral arteries. Hypertension. 1997;30:1085–1088. doi: 10.1161/01.hyp.30.5.1085. [DOI] [PubMed] [Google Scholar]
- Manning RD, Jr, Hu L, Williamson TD. Mechanisms involved in the cardiovascular-renal actions of nitric oxide inhibition. Hypertension. 1994;23:951–956. doi: 10.1161/01.hyp.23.6.951. [DOI] [PubMed] [Google Scholar]
- McFarlane SI, Muniyappa R, Francisco R, Sowers JR. Clinical review 145: Pleiotropic effects of statins: lipid reduction and beyond. J Clin Endocrinol Metab. 2002;87:1451–1458. doi: 10.1210/jcem.87.4.8412. [DOI] [PubMed] [Google Scholar]
- Mueller PJ. Exercise training and sympathetic nervous system activity: evidence for physical activity dependent neural plasticity. Clin Exp Pharmacol Physiol. 2007;34:377–384. doi: 10.1111/j.1440-1681.2007.04590.x. [DOI] [PubMed] [Google Scholar]
- Murakami H, Liu JL, Zucker IH. Blockade of AT1 receptors enhances baroreflex control of heart rate in conscious rabbits with heart failure. Am J Physiol. 1996;271:R303–309. doi: 10.1152/ajpregu.1996.271.1.R303. [DOI] [PubMed] [Google Scholar]
- Murakami H, Liu JL, Zucker IH. Angiotensin II blockade [corrected] enhances baroreflex control of sympathetic outflow in heart failure. Hypertension. 1997;29:564–569. doi: 10.1161/01.hyp.29.2.564. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, Somers VK. The sympathetic nervous system and obstructive sleep apnea: implications for hypertension. J Hypertens. 1997;15:1613–1619. doi: 10.1097/00004872-199715120-00062. [DOI] [PubMed] [Google Scholar]
- Neunteufl T, Heher S, Katzenschlager R, Wolfl G, Kostner K, Maurer G, Weidinger F. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol. 2000;86:207–210. doi: 10.1016/s0002-9149(00)00857-2. [DOI] [PubMed] [Google Scholar]
- Neunteufl T, Katzenschlager R, Hassan A, Klaar U, Schwarzacher S, Glogar D, Bauer P, Weidinger F. Systemic endothelial dysfunction is related to the extent and severity of coronary artery disease. Atherosclerosis. 1997;129:111–118. doi: 10.1016/s0021-9150(96)06018-2. [DOI] [PubMed] [Google Scholar]
- Owlya R, Vollenweider L, Trueb L, Sartori C, Lepori M, Nicod P, Scherrer U. Cardiovascular and sympathetic effects of nitric oxide inhibition at rest and during static exercise in humans. Circulation. 1997;96:3897–3903. doi: 10.1161/01.cir.96.11.3897. [DOI] [PubMed] [Google Scholar]
- Packer M. Sudden unexpected death in patients with congestive heart failure: a second frontier. Circulation. 1985;72:681–685. doi: 10.1161/01.cir.72.4.681. [DOI] [PubMed] [Google Scholar]
- Pagani M, Montano N, Porta A, Malliani A, Abboud FM, Birkett C, Somers VK. Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation. 1997;95:1441–1448. doi: 10.1161/01.cir.95.6.1441. [DOI] [PubMed] [Google Scholar]
- Parati G, Mancia G, Di Rienzo M, Castiglioni P. Point: cardiovascular variability is/is not an index of autonomic control of circulation. J Appl Physiol. 2006;101:676–678. doi: 10.1152/japplphysiol.00446.2006. discussion 681–672. [DOI] [PubMed] [Google Scholar]
- Patel KP, Li YF, Hirooka Y. Role of nitric oxide in central sympathetic outflow. Exp Biol Med (Maywood) 2001;226:814–824. doi: 10.1177/153537020122600902. [DOI] [PubMed] [Google Scholar]
- Patel KP, Zhang K, Zucker IH, Krukoff TL. Decreased gene expression of neuronal nitric oxide synthase in hypothalamus and brainstem of rats in heart failure. Brain Res. 1996;734:109–115. [PubMed] [Google Scholar]
- Paton JF, Lonergan T, Deuchars J, James PE, Kasparov S. Detection of angiotensin II mediated nitric oxide release within the nucleus of the solitary tract using electron-paramagnetic resonance (EPR) spectroscopy. Auton Neurosci. 2006;126–127:193–201. doi: 10.1016/j.autneu.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports. 2006;16(Suppl 1):3–63. doi: 10.1111/j.1600-0838.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, Ferraro A, Chello M, Mastroroberto P, Verdecchia P, Schillaci G. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191–196. doi: 10.1161/01.cir.104.2.191. [DOI] [PubMed] [Google Scholar]
- Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;36:533–553. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- Peterson JR, Sharma RV, Davisson RL. Reactive oxygen species in the neuropathogenesis of hypertension. Curr Hypertens Rep. 2006;8:232–241. doi: 10.1007/s11906-006-0056-1. [DOI] [PubMed] [Google Scholar]
- Pettersson K, Bejne B, Bjork H, Strawn WB, Bondjers G. Experimental sympathetic activation causes endothelial injury in the rabbit thoracic aorta via beta 1-adrenoceptor activation. Circ Res. 1990;67:1027–1034. doi: 10.1161/01.res.67.4.1027. [DOI] [PubMed] [Google Scholar]
- Planitz V. Crossover comparison of moxonidine and clonidine in mild to moderate hypertension. Eur J Clin Pharmacol. 1984;27:147–152. doi: 10.1007/BF00544037. [DOI] [PubMed] [Google Scholar]
- Pliquett RU, Cornish KG, Peuler JD, Zucker IH. Simvastatin normalizes autonomic neural control in experimental heart failure. Circulation. 2003a;107:2493–2498. doi: 10.1161/01.CIR.0000065606.63163.B9. [DOI] [PubMed] [Google Scholar]
- Pliquett RU, Cornish KG, Zucker IH. Statin therapy restores sympathovagal balance in experimental heart failure. J Appl Physiol. 2003b;95:700–704. doi: 10.1152/japplphysiol.00265.2003. [DOI] [PubMed] [Google Scholar]
- Pollare T, Lithell H, Berne C. A comparison of the effects of hydrochlorothiazide and captopril on glucose and lipid metabolism in patients with hypertension. N Engl J Med. 1989;321:868–873. doi: 10.1056/NEJM198909283211305. [DOI] [PubMed] [Google Scholar]
- Pollare T, Lithell H, Selinus I, Berne C. Application of prazosin is associated with an increase of insulin sensitivity in obese patients with hypertension. Diabetologia. 1988;31:415–420. doi: 10.1007/BF00271585. [DOI] [PubMed] [Google Scholar]
- Ramirez AJ, Bertinieri G, Belli L, Cavallazzi A, Di Rienzo M, Pedotti A, Mancia G. Reflex control of blood pressure and heart rate by arterial baroreceptors and by cardiopulmonary receptors in the unanaesthetized cat. J Hypertens. 1985;3:327–335. doi: 10.1097/00004872-198508000-00004. [DOI] [PubMed] [Google Scholar]
- Ray CA, Hume KM. Sympathetic neural adaptations to exercise training in humans: insights from microneurography. Med Sci Sports Exerc. 1998;30:387–391. doi: 10.1097/00005768-199803000-00008. [DOI] [PubMed] [Google Scholar]
- Reid JL. Rilmenidine: a clinical overview. Am J Hypertens. 2000;13:106S–111S. doi: 10.1016/s0895-7061(00)00226-0. [DOI] [PubMed] [Google Scholar]
- Reuben DB, Talvi SL, Rowe JW, Seeman TE. High urinary catecholamine excretion predicts mortality and functional decline in high-functioning, community-dwelling older persons: MacArthur Studies of Successful Aging. J Gerontol A Biol Sci Med Sci. 2000;55:M618–624. doi: 10.1093/gerona/55.10.m618. [DOI] [PubMed] [Google Scholar]
- Rodrigo J, Springall DR, Uttenthal O, Bentura ML, Abadia-Molina F, Riveros-Moreno V, Martinez-Murillo R, Polak JM, Moncada S. Localization of nitric oxide synthase in the adult rat brain. Philos Trans R Soc Lond B Biol Sci. 1994;345:175–221. doi: 10.1098/rstb.1994.0096. [DOI] [PubMed] [Google Scholar]
- Roegel JC, Yannoulis N, De Jong W, Feldman J, Bousquet P. Preventive effect of rilmenidine on the occurrence of neurogenic ventricular arrhythmias in rabbits. J Hypertens Suppl. 1998;16:S39–43. [PubMed] [Google Scholar]
- Roveda F, Middlekauff HR, Rondon MU, Reis SF, Souza M, Nastari L, Barretto AC, Krieger EM, Negrao CE. The effects of exercise training on sympathetic neural activation in advanced heart failure: a randomized controlled trial. J Am Coll Cardiol. 2003;42:854–860. doi: 10.1016/s0735-1097(03)00831-3. [DOI] [PubMed] [Google Scholar]
- Sakuma I, Togashi H, Yoshioka M, Saito H, Yanagida M, Tamura M, Kobayashi T, Yasuda H, Gross SS, Levi R. NG-methyl-L-arginine, an inhibitor of L-arginine-derived nitric oxide synthesis, stimulates renal sympathetic nerve activity in vivo. A role for nitric oxide in the central regulation of sympathetic tone? Circ Res. 1992;70:607–611. doi: 10.1161/01.res.70.3.607. [DOI] [PubMed] [Google Scholar]
- Salzer DA, Medeiros PJ, Craen R, Shoemaker JK. Neurogenic-nitric oxide interactions affecting brachial artery mechanics in humans: roles of vessel distensibility vs. diameter. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1181–1187. doi: 10.1152/ajpregu.90333.2008. [DOI] [PubMed] [Google Scholar]
- Sander M, Chavoshan B, Victor RG. A large blood pressure-raising effect of nitric oxide synthase inhibition in humans. Hypertension. 1999;33:937–942. doi: 10.1161/01.hyp.33.4.937. [DOI] [PubMed] [Google Scholar]
- Sander M, Hansen J, Victor RG. The sympathetic nervous system is involved in the maintenance but not initiation of the hypertension induced by N(omega)-nitro-L-arginine methyl ester. Hypertension. 1997;30:64–70. doi: 10.1161/01.hyp.30.1.64. [DOI] [PubMed] [Google Scholar]
- Sander M, Hansen PG, Victor RG. Sympathetically mediated hypertension caused by chronic inhibition of nitric oxide. Hypertension. 1995;26:691–695. doi: 10.1161/01.hyp.26.4.691. [DOI] [PubMed] [Google Scholar]
- Santos AC, Alves MJ, Rondon MU, Barretto AC, Middlekauff HR, Negrao CE. Sympathetic activation restrains endothelium-mediated muscle vasodilatation in heart failure patients. Am J Physiol Heart Circ Physiol. 2005;289:H593–599. doi: 10.1152/ajpheart.01240.2004. [DOI] [PubMed] [Google Scholar]
- Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- Schultz HD, Li YL, Ding Y. Arterial chemoreceptors and sympathetic nerve activity: implications for hypertension and heart failure. Hypertension. 2007;50:6–13. doi: 10.1161/HYPERTENSIONAHA.106.076083. [DOI] [PubMed] [Google Scholar]
- Seals DR, Bell C. Chronic sympathetic activation: consequence and cause of age-associated obesity? Diabetes. 2004;53:276–284. doi: 10.2337/diabetes.53.2.276. [DOI] [PubMed] [Google Scholar]
- Sen S, Young D. Effect of sodium deprivation on cardiac hypertrophy in spontaneously hypertensive rats: influence of aging. J Mol Cell Cardiol. 1991;23:695–704. doi: 10.1016/0022-2828(91)90979-v. [DOI] [PubMed] [Google Scholar]
- Serrano J, Encinas JM, Fernandez AP, Castro-Blanco S, Alonso D, Fernandez-Vizarra P, Richart A, Bentura ML, Santacana M, Cuttitta F, Martinez A, Rodrigo J. Distribution of immunoreactivity for the adrenomedullin binding protein, complement factor H, in the rat brain. Neuroscience. 2003;116:947–962. doi: 10.1016/s0306-4522(02)00773-x. [DOI] [PubMed] [Google Scholar]
- Simpson JB. The circumventricular organs and the central actions of angiotensin. Neuroendocrinology. 1981;32:248–256. doi: 10.1159/000123167. [DOI] [PubMed] [Google Scholar]
- Simpson P. Norepinephrine-stimulated hypertrophy of cultured rat myocardial cells is an alpha 1 adrenergic response. J Clin Invest. 1983;72:732–738. doi: 10.1172/JCI111023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PA, Graham LN, Mackintosh AF, Stoker JB, Mary DA. Relationship between central sympathetic activity and stages of human hypertension. Am J Hypertens. 2004;17:217–222. doi: 10.1016/j.amjhyper.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp Physiol. 2006;91:89–102. doi: 10.1113/expphysiol.2005.032367. [DOI] [PubMed] [Google Scholar]
- Stelling C. Experimentelle Untersuchungen uber den Einflus des Nervus depressor auf die Herzthatigkeit und den Blurdruck. Dorpat: Laakmann; 1867. [Google Scholar]
- Strack AM, Sawyer WB, Hughes JH, Platt KB, Loewy AD. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res. 1989;491:156–162. doi: 10.1016/0006-8993(89)90098-x. [DOI] [PubMed] [Google Scholar]
- Strojek K, Grzeszczak W, Gorska J, Leschinger MI, Ritz E. Lowering of microalbuminuria in diabetic patients by a sympathicoplegic agent: novel approach to prevent progression of diabetic nephropathy? J Am Soc Nephrol. 2001;12:602–605. doi: 10.1681/ASN.V123602. [DOI] [PubMed] [Google Scholar]
- Swedberg K, Bristow MR, Cohn JN, Dargie H, Straub M, Wiltse C, Wright TJ. Effects of sustained-release moxonidine, an imidazoline agonist, on plasma norepinephrine in patients with chronic heart failure. Circulation. 2002;105:1797–1803. doi: 10.1161/01.cir.0000014212.04920.62. [DOI] [PubMed] [Google Scholar]
- Swedberg K, Eneroth P, Kjekshus J, Wilhelmsen L. Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. CONSENSUS Trial Study Group. Circulation. 1990;82:1730–1736. doi: 10.1161/01.cir.82.5.1730. [DOI] [PubMed] [Google Scholar]
- Tagawa T, Imaizumi T, Harada S, Endo T, Shiramoto M, Hirooka Y, Takeshita A. Nitric oxide influences neuronal activity in the nucleus tractus solitarius of rat brainstem slices. Circ Res. 1994;75:70–76. doi: 10.1161/01.res.75.1.70. [DOI] [PubMed] [Google Scholar]
- Tammariello SP, Quinn MT, Estus S. NADPH oxidase contributes directly to oxidative stress and apoptosis in nerve growth factor-deprived sympathetic neurons. J Neurosci. 2000;20:RC53. doi: 10.1523/JNEUROSCI.20-01-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tentolouris N, Liatis S, Katsilambros N. Sympathetic system activity in obesity and metabolic syndrome. Ann N Y Acad Sci. 2006;1083:129–152. doi: 10.1196/annals.1367.010. [DOI] [PubMed] [Google Scholar]
- Thijssen DH, de Groot P, Kooijman M, Smits P, Hopman MT. Sympathetic nervous system contributes to the age-related impairment of flow-mediated dilation of the superficial femoral artery. Am J Physiol Heart Circ Physiol. 2006;291:H3122–3129. doi: 10.1152/ajpheart.00240.2006. [DOI] [PubMed] [Google Scholar]
- Thrasher TN. Arterial baroreceptor input contributes to long-term control of blood pressure. Curr Hypertens Rep. 2006;8:249–254. doi: 10.1007/s11906-006-0058-z. [DOI] [PubMed] [Google Scholar]
- Togashi H, Sakuma I, Yoshioka M, Kobayashi T, Yasuda H, Kitabatake A, Saito H, Gross SS, Levi R. A central nervous system action of nitric oxide in blood pressure regulation. J Pharmacol Exp Ther. 1992;262:343–347. [PubMed] [Google Scholar]
- Trimarco B, Morisco C, Sarno D, Iovino G, Argenziano L, Russo R, De Luca N, Volpe M. Rilmenidine in patients with left ventricular hypertrophy: beyond the reduction of left ventricular mass. J Cardiovasc Pharmacol. 1995;26(Suppl 2):S29–33. [PubMed] [Google Scholar]
- Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–575. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Veerasingham SJ, Raizada MK. Brain renin-angiotensin system dysfunction in hypertension: recent advances and perspectives. Br J Pharmacol. 2003;139:191–202. doi: 10.1038/sj.bjp.0705262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waki H, Murphy D, Yao ST, Kasparov S, Paton JF. Endothelial NO synthase activity in nucleus tractus solitarii contributes to hypertension in spontaneously hypertensive rats. Hypertension. 2006;48:644–650. doi: 10.1161/01.HYP.0000238200.46085.c6. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Esler M, Dorward P, Eisenhofer G, Ferrier C, Westerman R, Jennings G. Simultaneous measurements of cardiac noradrenaline spillover and sympathetic outflow to skeletal muscle in humans. J Physiol. 1992;453:45–58. doi: 10.1113/jphysiol.1992.sp019217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin BG, Sundlof G, Delius W. The effect of carotid sinus nerve stimulation on muscle and skin nerve sympathetic activity in man. Pflugers Arch. 1975;358:101–110. doi: 10.1007/BF00583921. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Thompson JM, Jennings GL, Esler MD. Renal noradrenaline spillover correlates with muscle sympathetic activity in humans. J Physiol. 1996;491 (Pt 3):881–887. doi: 10.1113/jphysiol.1996.sp021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss ML, Kenney MJ, Musch TI, Patel KP. Modifications to central neural circuitry during heart failure. Acta Physiol Scand. 2003;177:57–67. doi: 10.1046/j.1365-201X.2003.01047.x. [DOI] [PubMed] [Google Scholar]
- Xue B, Johnson AK, Hay M. Sex differences in angiotensin II- induced hypertension. Braz J Med Biol Res. 2007;40:727–734. doi: 10.1590/s0100-879x2007000500018. [DOI] [PubMed] [Google Scholar]
- Young CN, Fisher JP, Gallagher KM, Fadel PJ. Pharmacological inhibition of nitric oxide synthase increases sympathetic nerve activity in healthy humans. FASEB J. 2008;22:740–713. [Google Scholar]
- Yu Y, Wei SG, Zhang ZH, Gomez-Sanchez E, Weiss RM, Felder RB. Does aldosterone upregulate the brain renin-angiotensin system in rats with heart failure? Hypertension. 2008;51:727–733. doi: 10.1161/HYPERTENSIONAHA.107.099796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanchi A, Schaad NC, Osterheld MC, Grouzmann E, Nussberger J, Brunner HR, Waeber B. Effects of chronic NO synthase inhibition in rats on renin-angiotensin system and sympathetic nervous system. Am J Physiol. 1995;268:H2267–2273. doi: 10.1152/ajpheart.1995.268.6.H2267. [DOI] [PubMed] [Google Scholar]
- Zanzinger J. Role of nitric oxide in the neural control of cardiovascular function. Cardiovasc Res. 1999;43:639–649. doi: 10.1016/s0008-6363(99)00085-1. [DOI] [PubMed] [Google Scholar]
- Zanzinger J, Czachurski J, Seller H. Inhibition of basal and reflex-mediated sympathetic activity in the RVLM by nitric oxide. Am J Physiol. 1995;268:R958–962. doi: 10.1152/ajpregu.1995.268.4.R958. [DOI] [PubMed] [Google Scholar]
- Zhang K, Patel KP. Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: role of GABA. Am J Physiol. 1998;275:R728–734. doi: 10.1152/ajpregu.1998.275.3.R728. [DOI] [PubMed] [Google Scholar]
- Zhang K, Zucker IH, Patel KP. Altered number of diaphorase (NOS) positive neurons in the hypothalamus of rats with heart failure. Brain Res. 1998;786:219–225. doi: 10.1016/s0006-8993(97)01449-2. [DOI] [PubMed] [Google Scholar]
- Zhang ZH, Yu Y, Kang YM, Wei SG, Felder RB. Aldosterone acts centrally to increase brain renin-angiotensin system activity and oxidative stress in normal rats. Am J Physiol Heart Circ Physiol. 2008;294:H1067–1074. doi: 10.1152/ajpheart.01131.2007. [DOI] [PubMed] [Google Scholar]
- Zimmerman MC, Davisson RL. Redox signaling in central neural regulation of cardiovascular function. Prog Biophys Mol Biol. 2004;84:125–149. doi: 10.1016/j.pbiomolbio.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Zoccali C, Mallamaci F, Parlongo S, Cutrupi S, Benedetto FA, Tripepi G, Bonanno G, Rapisarda F, Fatuzzo P, Seminara G, Cataliotti A, Stancanelli B, Malatino LS. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation. 2002;105:1354–1359. doi: 10.1161/hc1102.105261. [DOI] [PubMed] [Google Scholar]
- Zucker IH. Novel mechanisms of sympathetic regulation in chronic heart failure. Hypertension. 2006;48:1005–1011. doi: 10.1161/01.HYP.0000246614.47231.25. [DOI] [PubMed] [Google Scholar]
- Zucker IH, Patel KP, Schultz HD, Li YF, Wang W, Pliquett RU. Exercise training and sympathetic regulation in experimental heart failure. Exerc Sport Sci Rev. 2004;32:107–111. doi: 10.1097/00003677-200407000-00006. [DOI] [PubMed] [Google Scholar]
- Zucker IH, Wang W, Pliquett RU, Liu JL, Patel KP. The regulation of sympathetic outflow in heart failure. The roles of angiotensin II, nitric oxide, and exercise training. Ann N Y Acad Sci. 2001;940:431–443. [PubMed] [Google Scholar]