Summary
Mitochondrial cardiolipin undergoes extensive remodeling of its acyl groups to generate uniformly substituted species, such as tetralinoleoyl-cardiolipin, but the mechanism of this remodeling has not been elucidated, except for the fact that it requires tafazzin. Here we show that purified recombinant Drosophila tafazzin exchanges acyl groups between cardiolipin and phosphatidylcholine by a combination of forward and reverse transacylations. The acyl exchange is possible in the absence of phospholipase A2 because it requires only trace amounts of lysophospholipids. We show that purified tafazzin reacts with various phospholipid classes and with various acyl groups both in sn-1 and sn-2 position. Expression studies in Sf9 insect cells suggest that the effect of tafazzin on cardiolipin species is dependent on the cellular environment and not on enzymatic substrate specificity. Our data demonstrate that tafazzin catalyzes general acyl exchange between phospholipids, which raises the question whether pattern formation in cardiolipin is the result of the equilibrium distribution of acyl groups between multiple phospholipid species.
Keywords: Cardiolipin, Fatty acids, Mitochondria, Tafazzin
1. Introduction
Phospholipids acquire specific molecular species patterns by remodeling reactions, in which acyl groups are first removed and then replaced by different acyl groups. This process was originally described by Lands [1] for phosphatidylcholine but is thought to be active for many other phospholipids as well [2]. The deacylation reaction of the Lands cycle is catalyzed by phospholipases A2, in particular calcium-independent phospholipase A2 (iPLA2), while the reacylation reaction is catalyzed by a family of acyltransferases that use acyl-coenzyme A as substrate (for reviews see refs. [2] and [3]). Some of these acyltransferases have recently been cloned and characterized [4–8]. The data support, in principle, the idea that acyl specificity may arise from the intrinsic enzymatic properties of the various members of the acyltransferase family.
One phospholipid that undergoes extensive remodeling is mitochondrial cardiolipin (CL, 1′,3′-bis[1,2-diacyl-glycero-3-phosphosphoryl]glycerol; for a review see ref. [9]). Remodeling of CL is unique because it is a dimeric phospholipid with four acyl residues, all of which participate in the process, whereas remodeling of ordinary phospholipids affects primarily the acyl group in sn-2 position. As a result, remodeled CL contains a high proportion of uniformly substituted species, such as (18:2)4-CL (e. g. in human heart; ref. [10]), (16:0)4-CL (e. g. in rat testis; ref. [11]), (22:6)4-CL (e. g. in bivalves; ref. [12]), or (18:1)4-CL (e. g. in yeast; ref. [13]). This phenomenon creates structural uniformity and molecular symmetry among CL species [13]. The significance of CL remodeling has recently come into focus by studies of human Barth syndrome, where mutations in the tafazzin gene cause a remodeling defect affecting primarily CL and perhaps also some other phospholipids [14–16]. These studies have not only established the importance of CL remodeling for human health, they have also identified tafazzin as the key enzyme in this process (for reviews see refs. [17–19]).
We have shown that tafazzin is a transacylase that catalyzes the general reaction PLa + LPLb ↔ LPLa + PLb, where PL and LPL represent phospholipids and lysophospholipids with head groups a and b, respectively [20]. Various other transacylases have already been identified in previous studies, primarily in pathways that form special metabolites, such as bis(monoacylglycero)phosphate [21] or N-acyletanolamine [22]. Transacylase activities also occur as “secondary” functions of A-type phospholipases [23] and lysophospholipases [24], where the presence of a free enzyme-acyl intermediate gives rise to two competing chemical reactions, namely hydrolysis and transesterification. An important characteristic of transacylations is that substrates and products have similar free energies; hence they may proceed in either direction and are highly responsive to the principle of mass action. In this paper, we studied the mechanism of tafazzin in order to understand the remodeling of mitochondrial CL. With the general properties of transacylases in mind, we asked questions like, how can tafazzin cause net transfer of a specific fatty acid into CL; what drives acyl specificity and how do the selected fatty acids distribute equally among all four acyl positions?
2. Materials and methods
2.1. Phospholipids
We synthesized [14C]MLCL ([oleoyl-1-14C]-dilinoleoyl-monolyso-cardiolipin) from DLCL (Avanti Polar Lipids) and [oleoyl-1-14C]-coenzyme A (53 Ci/mol, Amersham Biosciences). A dry residue of DLCL (3 mg) was reconstituted by sonication in 1.8 mL buffer, containing 50 mM Tris (pH 7.4), 0.5 mM EDTA, 10 mM 2-mercaptoethanol, and 10 μM methyl arachidonoyl fluorophosphonate. [Oleoyl-1-14C]-coenzyme A (5 μCi, 100 nmol) and rat liver microsomes (4 mg protein) were added to start the reaction. After 30 minutes of incubation at 37°C, lipids were extracted [25], and separated by two-dimensional thin-layer chromatography on silica gel 60 plates, developed by chloroform-methanol-20% aqueous ammonium hydroxide (65-30-5) in the first direction and chloroform-acetone-methanol-acetic acid-water (50-20-10-10-5) in the second direction. Ninety micrograms of unlabeled MLCL (Avanti Polar Lipids) were added before chromatography for better visualization of the product. Lipid spots were marked after brief exposure to iodine and [14C]MLCL was extracted from silica gel by chloroform-methanol (1:1). 1-Palmitoyl-2-[linoleoyl-1-14C]phosphatidylcholine (53 Ci/mol) and 1-palmitoyl-2-[linoleoyl-1-14C]phosphatidylethanolamine were purchased from Amersham Biosciences. 1-[Palmitoyl-1-14C]-2-lysophosphatidylcholine (55 Ci/mol) was purchased from PerkinElmer Life Sciences. Bovine heart cardiolipin, which is mostly (18:2)4-CL, and synthetic (18:1)4-CL were purchased from Avanti Polar Lipids. The following phospholipid species were also obtained from Avanti Polar Lipids: 16:0-LPC, (16:0)2-PC, (16:1)2-PC, (18:0)2-PC, (18:1)2-PC, 16:0-18:2-PC, 16:0-18:2-PE, (18:2)2-PE, 16:0-18:2-PG, and 16:0-18:2-PA.
2.2. Transacylations with isolated mitochondria
Mitochondria were isolated from rat liver by differential centrifugation. Homogenates were prepared in isolation buffer (0.15 M sucrose, 0.05 M KCl, 0.02 M Hepes, 1 mM EDTA, 2 mM 2-mercaptoethanol, pH 7.4) at 4°C, using a tight-fitting Teflon-glass homogenizer. Nuclei and debris were removed by centrifugation at 750 g for 10 minutes. Mitochondria were spun at 17,000 g for 10 minutes, re-suspended in isolation buffer, spun again (17,000 g for 10 min), and finally re-suspended in isolation buffer at a protein concentration of 50 g/L. Mitochondria were stored in aliquots at −80°C until use. Transacylations were measured between [14C]PC and CL in the presence and absence of PLA2 inhibitors. The lipids were mixed, dried and re-suspended by sonication in 0.2 mL aqueous buffer containing 50 mM Tris (pH 7.3), 10 mM 2-mercaptoethanol, 0.5 mM EDTA, and 4 mM ADP. Rat liver mitochondria (1.5 mg protein) were added and the mixtures were incubated for 30 minutes at 37°C. Reactions were stopped by methanol; lipids were extracted [25] and analyzed by two-dimensional thin-layer chromatography on silica gel 60 plates, developed by chloroform-methanol-water (65-25-4) in the first direction and chloroform-acetone-methanol-acetic acid-water (50-20-10-10-5) in the second direction. Lipid spots were marked after brief exposure to iodine and scraped off for liquid scintillation counting with Ecoscint (National Diagnostics). The Transacylation measured by this technique represented genuine mitochondrial activity because the mitochondrial preparation contained only about 15% microsomal contamination and the transacylation activity of liver microsomes is much lower than that of mitochondria [26].
2.3. Transacylations with purified tafazzin
Recombinant tafazzin (MBP-TAZ), consisting of the maltose-binding protein and the full-length isoform of Drosophila melanogaster tafazzin, was expressed in E. coli and purified on amylose resin as described [20]. In the enzyme preparation, phospholipids were present at a concentration of about 10 nmol per mg protein but lysophospholipids were not detectable. To measure transacylation reactions, lipid substrates were dried and then resuspended by sonication in 0.2 mL buffer, containing 50 mM Tris (pH 7.3), 10 mM 2-mercaptoethanol, and 0.5 mM EDTA. Incubations at 37°C were started by addition of 2.5 μg MBP-TAZ and stopped by addition of 2 mL methanol. Lipids were extracted [25] and analyzed on silica gel 60 plates either by one-dimensional thin-layer chromatography with chloroform-methanol-water (65-25-4), or by two-dimensional thin-layer chromatography developed by chloroform-methanol-water (65-25-4) in the first direction and chloroform-acetone-methanol-acetic acid-water (50-20-10-10-5) in the second direction. One-dimensional chromatography was used for PE/LPC, PC/LPC, CL/LPC, MLCL/LPC, and CL/MLCL transacylations, two-dimensional chromatography was used for PC/CL, PC/MLCL, and PC/DLCL transacylations. Lipid spots were marked after brief exposure to iodine and scraped off for liquid scintillation counting with Ecoscint (National Diagnostics). Transacylation activities were proportional to the amount of enzyme from 1 to 3 μg MBP-TAZ.
2.4. Sf9 insect cell cultures
Wild-type Sf9 cells (4×108) were cultured in Baculo-Gold TNM-FH insect medium (BD Biosciences) at 27°C for 48 hours in the presence of different concentrations of bromoenol lactone (BEL). During the incubation, the total number of cells increased to 9×108 (without BEL), 8.5×108 (2.5 μM BEL), or 4.5×108 (10 μM BEL). Drosophila tafazzin (full-length isoform corresponding to transcript RA [27]) and human tafazzin (corresponding to the transcript without exon-5, which is the principal isoforms of human tafazzin) were expressed in Sf9 cells as described previously [20].
2.5. Isolation and identification of MLCL isomers
MLCL isomers were separated by reversed-phase HPLC on an ODS-Hypersil column (250 mm × 4.6 mm, particle size 5 μm). Gradient elution was performed with solvent A containing acetonitrile-methanol-aqueous potassium phosphate (5 mM, pH 7.5) (60-25-15), and solvent B containing acetonitrile-methanol (60–40). The proportion of solvent B increased from zero to hundred percent within hundred minutes. The flow rate was 2 mL/min. UV absorbance was recorded at 205 nm. This method was first applied to commercial MLCL, in which 2-MLCL is the dominant isomer. In order to produce 1-MLCL, bovine heart CL was treated with Rhizopus arrhizus lipase (Fluka) that acts primarily as phospholipase A1. To this end, dry CL (5 μmol) was dispersed in one milliliter of buffer (100 mM sodium borate, 5 mM CaCl2, pH 6.5) by sonication, and one milligram (9.2 U) of Rhizopus arrhizus lipase was added. After 30 minutes at room temperature, the reaction was stopped with methanol. The products were extracted and separated by HPLC. The peaks, corresponding to 1-MLCL and 2-MLCL, were collected and their identity was confirmed by 1H-NMR. For this purpose, the MLCL isomers were dissolved in CDCl3 to an approximate concentration of 2 mM and analyzed with a Bruker AV-400 NMR spectrometer (400 MHz).
2.6. Other methods
The molecular composition of CL was analyzed by fluorescence-HPLC with pre-column derivatization [28]. Fatty acid compositions of total CL and CL molecular species were determined by gas chromatography of fatty acid methyl esters [28]. Protein concentration was determined by the method of Lowry [29].
3. Results
3.1. A novel remodeling mechanism
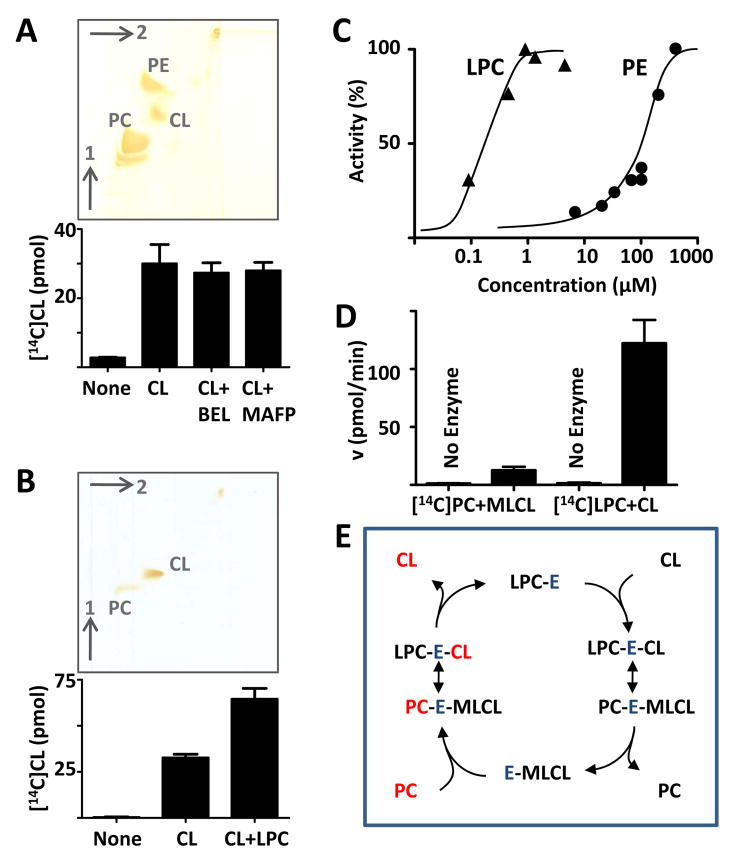
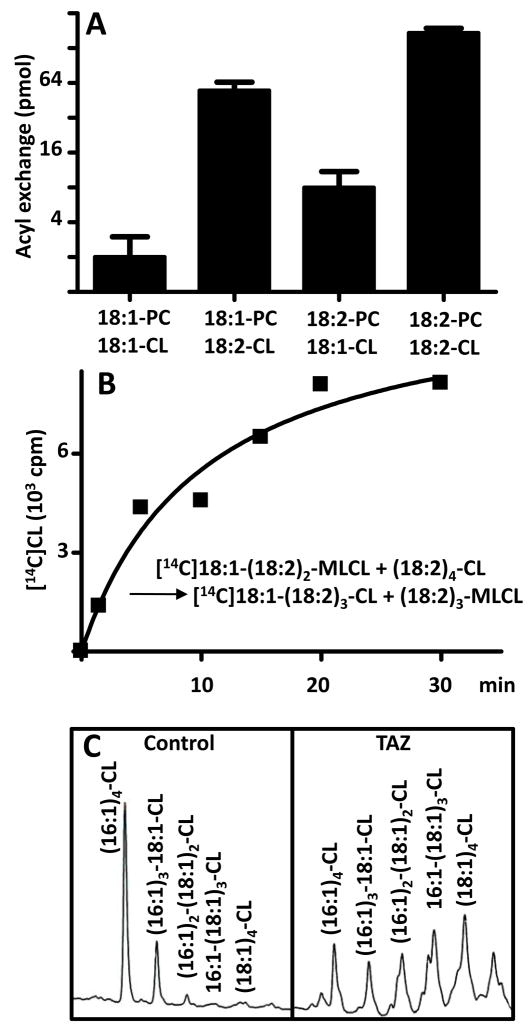
We have previously shown that rat liver mitochondria can remodel exogenous CL with [14C]linoleoyl groups derived from PC [26, 30]. This remodeling has been thought to occur by a deacylation-reacylation cycle, in which the deacylation step is catalyzed by PLA2 and the reacylation step is catalyzed by tafazzin [17]. In order to identify the type of PLA2 that deacylates CL, we added PLA2 inhibitors, such as bromoenol lactone (specific for iPLA2) and methyl arachidonoyl fluorophosphonate (specific for both iPLA2 and cPLA2). These inhibitors suppressed mitochondrial PLA2 activity by about seventy percent (data not shown), but none of them had any significant effect on the remodeling of exogenous CL by rat liver mitochondria (Fig. 1A). Although, this finding did not exclude hydrolysis of CL by other phospholipases, we considered the possibility that tafazzin alone was sufficient to exchange acyl groups between PC and CL. Indeed, we found that purified tafazzin exchanged [14C]linoleoyl groups between [14C]PC and CL (Fig. 1B), although the enzyme preparation in itself did not have any phospholipase activity [20] and no phospholipase was added to stimulate lysophospholipid production.
Fig. 1.
Tafazzin exchanges acyl groups between PC and CL. A, Rat liver mitochondria (1.5 mg protein) were incubated for 30 min in 0.2 mL buffer with 0.1 μCi 16:0-[14C]18:2-PC and, where indicated, CL (10 nmol), bromoenol lactone (BEL, 20 μM), and methyl arachidonoyl fluorophosphonate (MAFP, 10 μM). Phospholipids were separated by two-dimensional thin-layer chromatography and stained with iodine. [14C]CL was isolated to measure radioactivity by liquid scintillation counting. B, Experiment was performed as above, except that purified Drosophila tafazzin (MBP-TAZ, 2.5 μg protein) was added instead of mitochondria. LPC (5 nmol) was added to one set of samples. Tracer PC and CL (10 nmol each) were added before chromatography for better visualization. C, Transacylation rates of purified MBP-TAZ (2.5 μg) were measured by 2 min incubations, either in the presence of 10 μM [14C]16:0-LPC and variable concentrations of 16:0-18:2-PE (circles) or in the presence of 10 μM 16:0-[14C]18:2-PE and variable concentrations of 16:0-LPC (triangles). D, Transacylation rates of purified MBP-TAZ (2.5 μg) were measured by 2 min incubations either with 10 μM [14C]16:0-LPC and 50 μM CL or with 10 μM 16:0-[14C]18:2-PC and 50 μM MLCL. E, Mechanism by which tafazzin (enzyme, E) exchanges acyl groups between CL and PC in the absence of free lysophospholipids. Red color indicates radioactive species in reference to the experiment of panel B. Bar graphs represent means ± SEM (n=3).
These data seemed to contradict our previous conclusion that tafazzin transfers acyl groups from phospholipids to lysophospholipids [20]. However, the fact that lysophospholipids did not have to be added to the medium does not necessarily mean that lysophospholipids were not involved in the tafazzin reaction. In fact, we observed that the PC-CL acyl exchange was stimulated by LPC (Fig. 1B), suggesting that lysophospholipids participated in the reaction, but did not have to be added per se, perhaps because trace amounts of lysophospholipids were sufficient to support the acyl exchange. Thus we hypothesized that the affinity of tafazzin for lysophospholipids is much higher than the affinity for phospholipids. This idea was supported by measurement of the concentration dependence of the transacylation between PE and LPC, which showed that the concentration of PE required for transacylation was about 1000-fold higher than the concentration of LPC (Fig. 1C). The affinity difference between lysophospholipids and phospholipids may also explain why the transacylation rate with 10 μM [14C]LPC and 50 μM CL was much higher than the transacylation rate with 50 μM MLCL and 10 μM [14C]PC (Fig. 1D). The discrepancy in substrate affinities implies that the off-rate of phospholipids at the active site must be higher than the off-rate of lysophospholipids. Thus, after each catalytic cycle, the enzyme may exchange the phospholipid before releasing the lysophospholipid and the lysophospholipid remains available for another catalytic cycle. By this mechanism, acyl groups can be exchanged between PC and CL (Fig. 1E).
Since PC and CL can exchange acyl groups in the absence of PLA2, the question arises whether PLA2 is required at all for CL remodeling. Mitochondria express iPLA2’s that may hydrolyze CL [31–33]. Evidence for CL hydrolysis has been obtained by the detection of MLCL in apoptotic cells [34, 35] and in mutants with tafazzin deficiency [16, 36]. However, genetic ablation of iPLA2γ from mouse led only to a small decrease of the remodeling product (18:2)4-CL [37] and inhibition of iPLA2’s in Sf9 cells led only to a small decrease of the remodeling product (16:1)4-CL (Table 1). Although these experiments demonstrated some small effects of iPLA2’s on the CL composition (e.g. increase of non-remodeled CL from 5 to 15% in Sf9 cells), they strongly suggested that iPLA2 is not essential for the formation of uniformly substituted CL species. Furthermore, we deleted the only iPLA2 homolog (CG6718) in the Drosophila genome but did not find any change in the CL composition although CG6718 deletion decreased the MLCL/CL ratio [38]. In summary, these observations suggest that iPLA2 is required for the formation of MLCL, but not for the remodeling of CL.
Table 1.
Effect of bromoenol lactone (BEL) on the CL composition of Sf9 insect cells
| BEL (μM) | CL molecular species (%) | |||
|---|---|---|---|---|
| (16:1)4-CL | (16:1)3-18:1-CL | (16:1)2-(18:1)2-CL | Non-remodeled CL | |
| 0 | 65.8±1.1 | 24.6±2.7 | 4.8±2.0 | 4.8±3.5 |
| 2.5 | 60.2±0.4 | 27.9±1.0 | 7.5±1.0 | 4.4±1.5 |
| 10 | 49.0±2.6 | 26.5±3.0 | 8.2±1.3 | 15.2±7.9 |
3.2. Acyl specificity
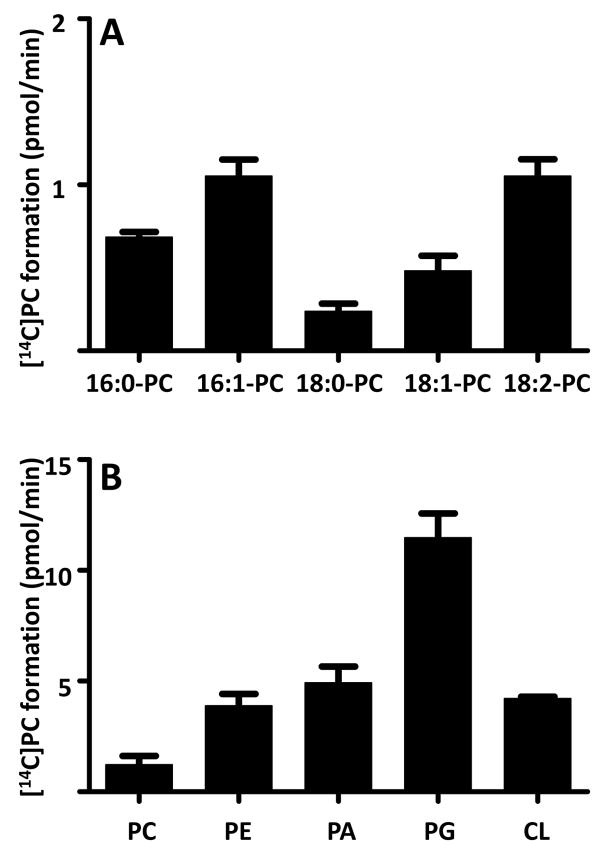
In order to determine the intrinsic acyl specificity of Drosophila tafazzin, we purified the enzyme and measured its activity in the presence of [14C]LPC and various molecular species of PC (Fig. 2A). We found variable transacylation rates for individual acyl groups (18:2>18:1>18:0; 16:1>16:0), but the differences were modest and the specificity profile of tafazzin did not match the acyl pattern of Drosophila-CL. For instance, 16:1 was only slightly preferred over 16:0 in vitro, but 16:1 was about ten times as abundant as 16:0 in Drosophila CL and Sf9-CL [20, 27]. Likewise, Drosophila tafazzin has only an about twofold preference for 18:2 over 18:1, whereas Drosophila CL shows a high preference for 18:2 over 18:1 even though 18:2 is a minor and 18:1 is a major fatty acid in Drosophila. Furthermore, tafazzin reacts with a number of different phospholipid classes, and its activity is affected by the type of head group (Fig. 2B). Collectively, the data suggest that tafazzin can transfer a variety of fatty acids between different phospholipids, but the fatty acid composition of CL cannot be explained by the acyl specificity of tafazzin.
Fig. 2.
Substrate specificity of isolated Drosophila tafazzin. A, Apparent acyl specificity of purified Drosophila tafazzin (MBP-TAZ, 2.5 μg) was determined by 5-min incubations with [14C]LPC (5 μM, 0.05 μCi) and various molecular species of PC (50 μM). B, Apparent head group specificity of purified Drosophila tafazzin (MBP-TAZ, 2.5 μg) was determined by 5-min incubations with [14C]LPC (5 μM, 0.05 μCi) and linoleoyl species of various phospholipids (50 μM). Recovery of radioactivity was measured in PC. Bar graphs represent means ± SEM (n=3).
3.3. Regiospecificity
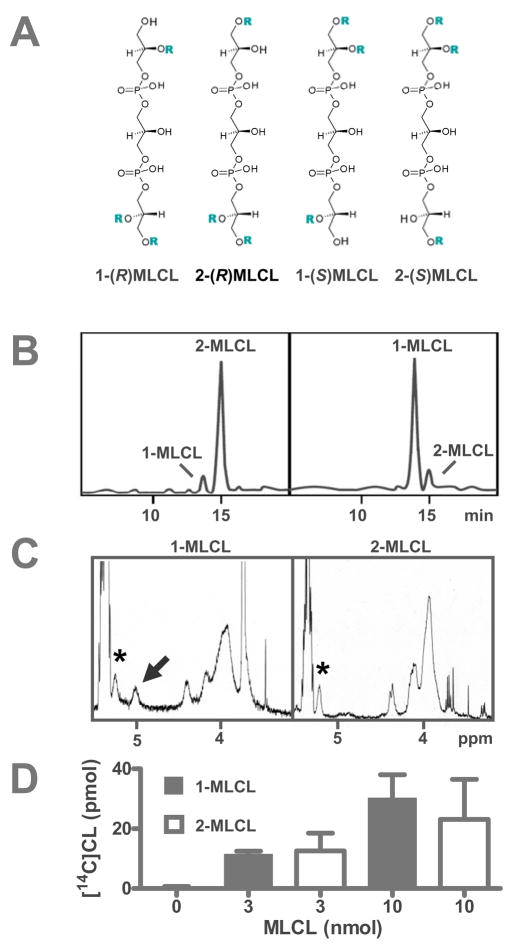
CL is unique among phospholipids in the sense that all four of its acyl groups participate in the remodeling process, which requires a mechanism that does not discriminate between sn-1 and sn-2 ester bonds. To determine whether tafazzin can in fact transfer acyl groups into sn-1 and sn-2 positions, we measured its activity in the presence of different MLCL isomers. MLCL has four isomeric structures with respect to the position of the lysofunction and the chirality of the central carbon atom (Fig. 3A), but none of these isomers have been isolated yet. We used reversed-phase HPLC to resolve the isomers present in commercial MLCL and obtained two peaks that were tentatively assigned to 1-MLCL and 2-MLCL, respectively (Fig. 3B). Both peaks behaved like authentic MLCL in thin-layer chromatography. The 1-MLCL/2-MLCL ratio of commercial MLCL was 1:9, which was in agreement to the 1-LPC/2-LPC ratio observed in commercial LPC preparations [39, 40]. To specifically create 1-MLCL, we treated CL with Rhizopus arrhizus lipase, which removes acyl groups preferentially from the sn-1 position. Upon this treatment, the first peak that was thought to be 1-MLCL, increased relative to the second peak, corroborating the isomer assignment (Fig. 3B). We further confirmed the identity of the MLCL isomers by 1H-NMR spectroscopy and found, in agreement with published data on LPC [39], that the 1-lyso product, but not the 2-lyso product, yielded a specific resonance at 5.0 ppm, which is characteristic of the >CH-O-CO proton in the 1-lysophosphatidyl moiety (Fig. 3C). To determine the regiospecificity of tafazzin, we isolated the MLCL isomers and incubated them with purified enzyme and 2-[14C]linoleoyl-PC. The experiment showed that 1-MLCL and 2-MLCL were equally effective acyl acceptors, suggesting that tafazzin is able to transfer acyl groups both into sn-1 and sn-2 positions (Fig. 3D).
Fig. 3.
Both 1-MLCL and 2-MLCL are substrates of tafazzin. A, MLCL has four isomers with different positions of the acyl groups (R), which affects the chirality of the central carbon atom. B, MLCL isomers were separated by reversed-phase HPLC. Absorbance at 205 nm was plotted against retention time. The left chromatogram shows a commercial preparation of MLCL. The right chromatogram shows MLCL generated by treatment of bovine heart CL with Rhizopus arrhizus. C, 1H-NMR spectra showed that 1-MLCL contained two >CH-O-CO groups, one in the phosphatidyl moiety (asterisk) and one in the 1-lysophophatidyl moiety (arrow). 2-MLCL contained only one >CH-O-CO group in the phosphatidyl moiety (asterisk). Line assignments were made according to 1H-NMR data of the analogous LPC isomers [39] and NMR simulation. D, Both 1-MLCL and 2-MLCL reacted with purified tafazzin (MBP-TAZ). MBP-TAZ (2.5 μg) was incubated with the isomers and 2 nmol 16:0-[14C]18:2-PC (0.1 μCi) in 0.2 mL buffer for 30 minutes. Results are expressed as mean ± range of duplicate determinations.
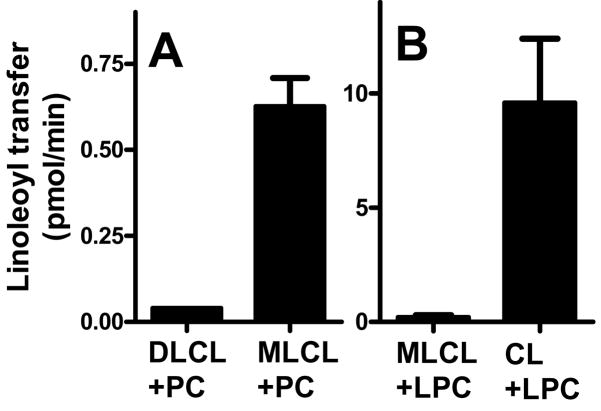
Since the enzymatic mechanism of tafazzin allows remodeling of all acyl groups of CL, one may ask whether the two sides of the CL molecule (i.e. 1′-phosphoglycerol and 3′-phosphoglycerol moieties) transacylate independently or whether transacylation of one side affects transacylation of the other side. If the two sides reacted independently, the phosphatidyl moiety of MLCL should function as acyl donor and the lysophosphatidyl moiety as acyl acceptor. However, we found that purified tafazzin catalyzed the reaction [14C]PC + MLCL ↔ LPC + [14C]CL, but not the reaction [14C]PC + DLCL ↔ LPC + [14C]MLCL, suggesting that MLCL did, but DLCL did not function as acyl acceptor (Fig. 4A). Due to the reversibility of transacylations, this conclusion implies that only CL, but not MLCL can be an acyl donor, a postulate that was directly confirmed by the experiment shown in Fig. 4B. Thus, despite bearing structural elements of a phospholipid and a lysophospholipid, the role of MLCL is limited to that of an acyl acceptor, implying that deacylation of one glycerol moiety of CL inhibits deacylation of the other. In summary, the data show that tafazzin catalyzes inter-conversions between CL and MLCL involving all four acyl groups, which produces (i) acyl exchange and (ii) positional isomerisation.
Fig. 4.
MLCL is an acyl acceptor but not an acyl donor of tafazzin. A, Purified tafazzin (MBP-TAZ, 2.5 μg) was incubated for 5 min with 10 μM 1-palmitoyl-2[14C]linoleoyl-PC (0.1 μCi) and 50 μM of DLCL or MLCL, followed by quantification of the transacylation products ([14C]MLCL or [14C]CL). B, MBP-TAZ (2.5 μg) was incubated for 5 min with 5 μM[14C]LPC (0.05 μCi) and 50 μM of MLCL or CL, followed by quantification of [14C]PC. Results are expressed as mean ± SEM of triplicate determinations.
3.4. Forward and reverse transacylations
The question arises as to whether it is conceivable that forward and reverse transacylations carry different fatty acids and therefore change the molecular composition of CL. To demonstrate that this is possible in principle, we measured the transacylation between CL and PC either with 18:1-CL/18:2-PC or with 18:2-CL/18:1-PC. We found that in both incubations, 18:1 and 18:2 were exchanged by isolated tafazzin, suggesting that tafazzin alone can alter the molecular composition of an artificial CL/PC membrane by transferring 18:1 and 18:2 in opposite directions (Fig. 5A). However, more acyl groups were exchanged between CL and PC when tafazzin interacted with a liposome that contained primarily 18:2 (18:2-CL/[14C]18:1-PC) as opposed to a liposome that contained primarily 18:1 (18:1-CL/[14C]18:2-PC). In agreement with that, more acyl groups were exchanged between 18:2-CL and [14C]18:2-PC than between 18:1-CL and [14C]18:1-PC, suggesting that the dominant acyl group of the liposome affected the activity of the exchange reaction. Furthermore, tafazzin may transform the molecular composition of CL even in the absence of PC, since it may couple the acylation of MLCL to the deacylation of CL (Fig. 5B). We concluded that tafazzin can alter the molecular composition of CL in vitro by a combination of forward and reverse transacylations.
Fig. 5.
Effect of tafazzin on the molecular composition of CL. A, Acyl exchange between PC and CL was determined in the presence of MBP-TAZ (2.5 μg), 10 μM [14C]acyl labeled PC, and 60 μM CL. The amount of [14C]CL was measured after 30 min. Bar graph represents means ± SEM (n=3). B, CL-MLCL transacylation was measured in a mixture containing MBP-TAZ (2.5 μg), 0.01 μCi [14C]MLCL and 20 nmol CL in a volume of 0.2 mL. C, Molecular composition of CL was determined by fluorescence-HPLC in control Sf9 cells and in Sf9 cells expressing Drosophila tafazzin (TAZ). In each chromatogram, the fluorescence yield is plotted against the retention time from 30 to 50 min.
Next, we investigated the effect of tafazzin in vivo, by overexpressing both human tafazzin and Drosophila tafazzin in Sf9 insect cells. In wild-type Sf9 cells, (16:1)4-CL was the dominant CL species, while 18:1 was the dominant fatty acid of other phospholipids [20]. Overexpression of foreign tafazzin caused a reversal of CL remodeling as it decreased the proportion of (16:1)4-CL in favor of multiple 18:1-containing molecular species (Fig. 5C). Specifically, the 16:1/18:1 ratio of CL decreased from 15±2 (n=3) in wild-type cells to 1.3±0.1 (n=3) in cells expressing human tafazzin and to 0.5±0.2 (n=3) in cells expressing Drosophila tafazzin. Thus, overexpression of human and Drosophila tafazzin induced an increase of 18:1 in Sf9 CL, despite the fact that human tafazzin enriches mostly 18:2 in human CL [10] and Drosophila tafazzin enriches mostly 16:1 and 18:2 in Drosophila CL [13]. The data confirm that CL remodeling is reversible and that the apparent acyl specificity of CL remodeling depends on the environment of tafazzin rather than its enzymatic specificity. To explain the reversal of CL remodeling we are postulating that the high abundance of tafazzin in the Sf9 overexpression system, causes equilibration of fatty acids between cellular phospholipid pools, making the fatty acid composition of CL more equal to the fatty acid composition of PE and PC (see also data in ref. [20]).
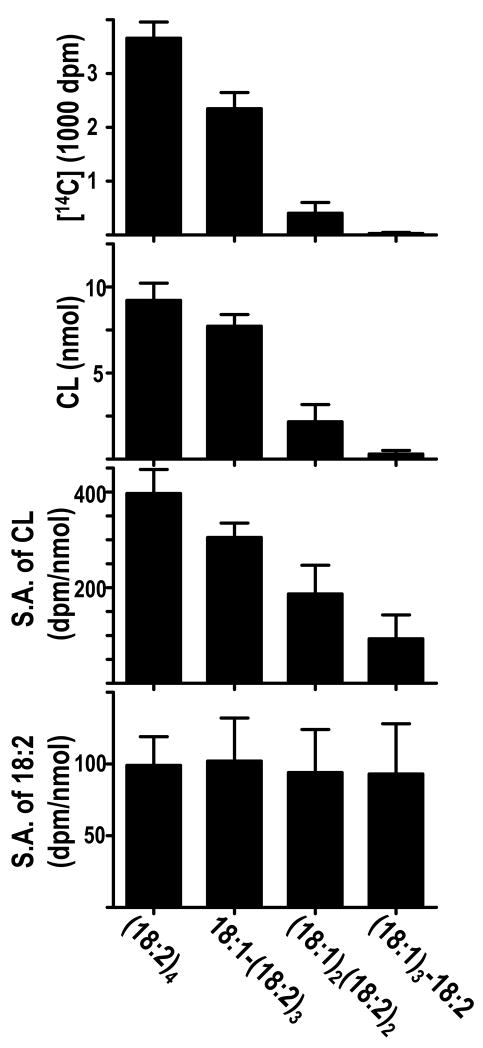
Further evidence for the equilibrium nature of CL remodeling was obtained in isolated rat liver mitochondria. We incubated mitochondria with (18:1)4-CL and [14C]18:2-PC, and measured radioactivity in the remodeling product ([14C]18:2)4-CL and the remodeling intermediates (18:1)3-[14C]18:2-CL, (18:1)2-([14C]18:2)2-CL, and 18:1-([14C]18:2)3-CL. We found that the specific radioactivity of 18:2 residues was similar in all four species, suggesting that these species were in equilibrium (Fig. 6). This result opposes the idea of a sequential pathway, in which one would expect a gradient of specific radioactivities from the first to the final remodeling product. However, the result is consistent with the idea that the molecular composition of remodeled CL reflects the chemical equilibrium state of multiple transacylation reactions.
Fig. 6.
Recovery of [14C]18:2 in CL species formed by remodeling of (18:1)4-CL by rat liver mitochondria. Mitochondria (6 mg protein) were incubated at 37 C for 40 minutes in 1.2 mL buffer containing 60 nmol (18:1)4-CL, 0.6 μCi 16:0-[14C]18:2-PC, 50 mM Tris (pH 7.3), 10 mM 2-mercaptoethanol, 0.5 mM EDTA, and 4 mM ADP. Lipids were extracted and [14C]CL was isolated by two-dimensional thin-layer chromatography. [14C]CL species were resolved by reversed-phase HPLC and detected by absorbance at 205 nm [30]. Individual fractions were collected and their radioacticity was measured by liquid scintillation counting. The amount of molecular species was estimated from the UV chromatograms with external calibration. Results are expressed as mean ± range of duplicate determinations. S.A., specific radioactivity.
4. Discussion
The creation of characteristic fatty acid profiles in phospholipids has generally been attributed to the substrate specificities of acyltransferases involved in phospholipid remodeling. Here we propose a new hypothesis for CL remodeling, according to which acyl specificity arises from the chemical equilibrium of transacylations. The new idea has evolved as a result of three critical observations. First, we found that isolated tafazzin catalyzes the exchange of acyl groups between CL and PC by a novel acyl shuttling mechanism, in which lysophospholipids occur only as catalytic intermediates. Thus, tafazzin acts in effect as a phospholipid-phospholipid acyl exchanger. Since this process does not require continuous supply of lysophospholipids, it may explain, why genetic and pharmacologic manipulation of PLA2 has relatively little effect on CL remodeling. Second, we found that the acyl-specificity of tafazzin does not provide a plausible explanation for the acyl specificity of CL, and that expression of tafazzin in a foreign host does not replicate the CL composition of the tafazzin source, suggesting that it is not the enzymatic specificity of tafazzin that is driving the remodeling process. Third, we found lack of regiospecificity suggesting that all four acyl groups of CL engage in the transacylation. This is an important prerequisite for uniformity and symmetry in CL acyl distribution.
One important question is why MLCL accumulates in human Barth syndrome [36] and other models of tafazzin deficiency [16]. Since tafazzin connects cardiolipin and other phospholipids via transacylation equilibrium, it may “spread” the effects of deacylation and acyl-CoA dependent reacylation among all participants of the transacylation equilibrium. Thus, one possible reason, why MLCL increases in the absence of tafazzin is that acyl-CoA reacylation of LPC (and other lysophospholipids) can no longer drive the reacylation of MLCL. Another possible reason is that abnormal CL may be more susceptible to degradation. Regardless of the mechanism, our studies in Drosophila suggest that CL hydrolysis to MLCL is not part of the remodeling process because MLCL formation was affected by iPLA2 deficiency whereas CL remodeling was not (unpublished data).
The idea that acyl remodeling, i.e. the formation of specific acylation patterns, may arise from a transacylation equilibrium, is a novel hitherto unrecognized concept that will be dealt with mathematically in the accompanying paper [41].
Acknowledgments
This work was supported in part by grants from the National Heart Lung and Blood Institute (R01 HL078788-01) and from the Barth Syndrome Foundation.
Abbreviations
- Acyl species are abbreviated X, Y
where X specifies the number of carbon atoms and Y specifies the number of double bonds
- BEL
bromoenol lactone
- CL
cardiolipin
- DLCL
dilyso-cardiolipin
- HPLC
high-performance liquid chromatography
- LPC
lyso-phosphatidylcholine
- MBP-TAZ
fusion protein consisting of maltose-binding protein and full-length Drosophila tafazzin
- MLCL
monolyso-cardiolipin
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PLA2
phospholipase A2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lands WE. Metabolism of glycerolipids. II. The enzymatic acylation of lysolecithin. J Biol Chem. 1960;235:2233–2237. [PubMed] [Google Scholar]
- 2.Yamashita A, Sugiura T, Waku K. Acyltransferases and transacylases involved in fatty acid remodeling of phospholipids and metabolism of bioactive lipids in mammalian cells. J Biochem. 1997;122:1–16. doi: 10.1093/oxfordjournals.jbchem.a021715. [DOI] [PubMed] [Google Scholar]
- 3.Winstead MV, Balsinde J, Dennis EA. Calcium-independent phospholipase A2: structure and function. Biochim Biophys Acta. 2000;1488:28–39. doi: 10.1016/s1388-1981(00)00107-4. [DOI] [PubMed] [Google Scholar]
- 4.Cao J, Liu Y, Lockwood J, Burn P, Shi Y. A novel cardiolipin-remodeling pathway revealed by a gene encoding an endoplasmic reticulum-associated acyl-CoA:lysocardiolipin acyltransferase (ALCAT1) in mouse. J Biol Chem. 2004;279:31727–31734. doi: 10.1074/jbc.M402930200. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y, Cao J, Shi Y. Identification and characterization of a gene encoding human LPGAT1, an endoplasmic reticulum-associated lysophosphatidylglycerol acyltransferase. J Biol Chem. 2004;279:55866–55874. doi: 10.1074/jbc.M406710200. [DOI] [PubMed] [Google Scholar]
- 6.Nakanishi H, Shindou H, Hishikawa D, Harayama T, Ogasawara R, Suwabe A, Taguchi R, Shimizu T. Cloning and characterization of mouse lung-type acyl-CoA:lysophosphatidylcholine acyltransferase 1 (LPCAT1). Expression in alveolar type II cells and possible involvement in surfactant production. J Biol Chem. 2006;281:20140–20147. doi: 10.1074/jbc.M600225200. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Hyatt BA, Mucenski ML, Mason RJ, Shannon JM. Identification and characterization of a lysophosphatidylcholine acyltransferase in alveolar type II cells. Proc Natl Acad Sci USA. 2006;103:11724–11729. doi: 10.1073/pnas.0604946103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riekhof WR, Wu J, Jones JL, Voelker DR. Identification and characterization of the major lysophosphatidylethanolamine acyltransferase in Saccharomyces cerevisiae. J Biol Chem. 2007;282:28344–28352. doi: 10.1074/jbc.M705256200. [DOI] [PubMed] [Google Scholar]
- 9.Schlame M. Cardiolipin synthesis for the assembly of bacterial and mitochondrial membranes. J Lipid Res. 2008;49:1607–1620. doi: 10.1194/jlr.R700018-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlame M, Towbin JA, Heerdt PM, Jehle R, DiMauro S, Blanck TJJ. Deficiency of tetralinoleoyl-cardiolipin in Barth syndrome. Ann Neurol. 2002;51:634–637. doi: 10.1002/ana.10176. [DOI] [PubMed] [Google Scholar]
- 11.Wang HJ, Jackson SN, Woods AS. Direct MALDI-MS analysis of cardiolipin from rat organs sections. J Am Soc Mass Spectrom. 2007;18:567–577. doi: 10.1016/j.jasms.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kraffe E, Soudant P, Marty Y, Kervarec N, Jehan P. Evidence of a tetradocosahexaenoic cardiolipin in some marine bivalves. Lipids. 2002;37:507–514. doi: 10.1007/s11745-002-0925-z. [DOI] [PubMed] [Google Scholar]
- 13.Schlame M, Ren M, Xu Y, Greenberg ML, Haller I. Molecular symmetry in mitochondrial cardiolipins. Chem Phys Lipids. 2005;138:38–49. doi: 10.1016/j.chemphyslip.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Vreken P, Valianpour F, Nijtmans LG, Grivell LA, Plecko B, Wanders RJA, Barth PG. Defective remodeling of cardiolipin and phosphatidylglycerol in Barth syndrome. Biochem Biophys Res Comm. 2000;279:378–382. doi: 10.1006/bbrc.2000.3952. [DOI] [PubMed] [Google Scholar]
- 15.Schlame M, Kelley RI, Feigenbaum A, Towbin Ja, Heerdt PM, Schieble T, Wanders RJA, DiMauro S, Blanck TJJ. Phospholipid abnormalities in children with Barth syndrome. J Am Coll Cardiol. 2003;42:1994–1999. doi: 10.1016/j.jacc.2003.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Gu Z, Valianpour F, Chen S, Vaz FM, Hakkaart GA, Wanders RJA, Greenberg ML. Aberrant cardiolipin metabolism in the yeast taz1 mutant: a model for Barth syndrome. Mol Microbiol. 2004;51:149–158. doi: 10.1046/j.1365-2958.2003.03802.x. [DOI] [PubMed] [Google Scholar]
- 17.Schlame M, Ren M. Barth syndrome, a human disorder of cardiolipin metabolism. FEBS Lett. 2006;580:5450–5455. doi: 10.1016/j.febslet.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 18.Hauff KD, Hatch GM. Cardiolipin metabolism and Barth syndrome. Progr Lipid Res. 2006;45:91–101. doi: 10.1016/j.plipres.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Li G, Chen S, Thompson MN, Greenberg ML. New insights into the regulation of cardiolipin biosynthesis in yeast: Implications for Barth syndrome. Biochim Biophys Acta. 2007;1771:432–441. doi: 10.1016/j.bbalip.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Xu Y, Malhotra A, Ren M, Schlame M. The enzymatic function of tafazzin. J Biol Chem. 2006;281:39217–39224. doi: 10.1074/jbc.M606100200. [DOI] [PubMed] [Google Scholar]
- 21.Amidon B, Brown A, Waite M. Transacylase and phospholipases in the synthesis of bis(monoacylglycero)phosphate. Biochemistry. 1996;35:13995–14002. doi: 10.1021/bi961164o. [DOI] [PubMed] [Google Scholar]
- 22.Schmid HH. Pathways and mechanisms of N-acylethanolamine biosynthesis: can anandamide be generated selectively? Chem Phys Lipids. 2000;108:71–87. doi: 10.1016/s0009-3084(00)00188-2. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh M, Tucker DE, Burchett SA, Leslie CC. Properties of the group IV phospholipase A2 family. Progr Lipid Res. 2006;45:487–510. doi: 10.1016/j.plipres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Wang A, Dennis EA. Mammalian lysophospholipases. Biochim Biophys Acta. 1999;1439:1–16. doi: 10.1016/s1388-1981(99)00063-3. [DOI] [PubMed] [Google Scholar]
- 25.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 26.Schlame M, Rustow B. Lysocardiolipin formation and reacylation in isolated rat liver mitochondria. Biochem J. 1990;272:589–595. doi: 10.1042/bj2720589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Y, Condell M, Plesken H, Edelman-Novemsky I, Ma J, Ren M, Schlame M. A Drosophila model of Barth syndrome. Proc Natl Acad Sci USA. 2006;103:11584–11588. doi: 10.1073/pnas.0603242103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlame M, Shanske S, Doty S, Konig T, Sculco T, DiMauro S, Blanck TJJ. Microanalysis of cardiolipin in small biopsies including skeletal muscle from patients with mitochondrial disease. J Lipid Res. 1999;40:1585–1592. [PubMed] [Google Scholar]
- 29.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 30.Xu Y, Kelley RI, Blanck TJJ, Schlame M. Remodeling of cardiolipin by phospholipid transacylation. J Biol Chem. 2003;278:51380–51385. doi: 10.1074/jbc.M307382200. [DOI] [PubMed] [Google Scholar]
- 31.Broekemeier KM, Iben JR, LeVan EG, Crouser ED, Pfeiffer DR. Pore formation and uncoupling initiate a Ca2+-independent degradation of mitochondrial phospholipids. Biochemistry. 2002;41:7771–7780. doi: 10.1021/bi020157z. [DOI] [PubMed] [Google Scholar]
- 32.Williams SD, Gottlieb RA. Inhibition of mitochondrial calcium-independent phospholipase A2 (iPLA2) attenuates mitochondrial phospholipid loss and is cardioprotective. Biochem J. 2002;362:23–32. doi: 10.1042/0264-6021:3620023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mancuso DJ, Han X, Jenkins CM, Lehman JJ, Sambandam N, Sims HF, Yang J, Yan W, Yang K, Green K, Abendschein DR, Saffitz JE, Gross RW. Dramatic accumulation of triglycerides and precipitation of cardiac hemodynamic dysfunction during brief caloric restriction in transgenic myocardium expressing human calcium-independent phospholipase A2γ. J Biol Chem. 2007;282:9216–9227. doi: 10.1074/jbc.M607307200. [DOI] [PubMed] [Google Scholar]
- 34.Esposti MD, Cristea IM, Gaskell SJ, Nakao Y, Dive C. Proapoptotic Bid binds to monolysocardiolipin, a new molecular connection between mitochondrial membranes and cell death. Cell Death Diff. 2003;10:1300–1309. doi: 10.1038/sj.cdd.4401306. [DOI] [PubMed] [Google Scholar]
- 35.Tyurin VA, Tyurina YY, Osipov AN, Belikova NA, Basova LV, Kapralov AA, Bayir H, Kagan VE. Interactions of cardiolipin and lyso-cardiolipins with cytochrome c and tBid: conflict or assistance in apoptosis. Cell Death Diff. 2007;14:872–875. doi: 10.1038/sj.cdd.4402068. [DOI] [PubMed] [Google Scholar]
- 36.Valianpour F, Mitsakos V, Schlemmer D, Towbin JA, Taylor JM, Ekert PG, Thorburn DR, Munnich A, Wanders RJA, Barth PG, Vaz FM. Monolysocardiolipins accumulate in Barth syndrome but do not lead to enhanced apoptosis. J Lipid Res. 2005;46:1182–1195. doi: 10.1194/jlr.M500056-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Mancuso DJ, Sims HF, Han X, Jenkins CM, Guan SP, Yang K, Moon SH, Pietka T, Abumrad NA, Schlesinger PH, Gross RW. Genetic ablation of calcium-independent phospholipase A2γ leads to alterations in mitochondrial lipid metabolism and function resulting in a deficient mitochondrial bioenergetic phenotype. J Biol Chem. 2007;282:34611–34622. doi: 10.1074/jbc.M707795200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malhotra A, Edelman-Novemsky I, Xu Y, Plesken H, Ma J, Schlame M, Ren M. Role of calcium-independent phospholipase A2 in the pathogenesis of Barth syndrome. Proc Natl Acad Sci USA. 2009 doi: 10.1073/pnas.0811224106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pluckthun A, Dennis EA. Acyl and phosphoryl migration in lysophospholipids: Importance in phospholipid synthesis and phospholipase specificity. Biochemistry. 1982;21:1743–1750. doi: 10.1021/bi00537a007. [DOI] [PubMed] [Google Scholar]
- 40.Creer MH, Gross RW. Separation of isomeric lysophospholipids by reverse phase HPLC. Lipids. 1985;20:922–928. doi: 10.1007/BF02534778. [DOI] [PubMed] [Google Scholar]
- 41.Schlame M. Formation of molecular species of mitochondrial cardiolipin. 2. A statistical model of pattern formation by phospholipid transacylation. Biochim Biophys Acta. doi: 10.1016/j.bbalip.2009.01.018. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]