Abstract
Circadian rhythm sleep disorders are characterized by complaints of insomnia and excessive sleepiness that are primarily due to alterations in the internal circadian timing system or a misalignment between the timing of sleep and the 24-hour social and physical environment. In addition to physiological and environmental factors, maladaptive behaviors often play an important role in the development of many of the circadian rhythm sleep disorders. This review will focus on the clinical approach to the diagnosis and management of the various circadian rhythm sleep disorders, including delayed sleep phase disorder, advanced sleep phase disorder, non-entrained type, irregular sleep-wake rhythm, shift work sleep disorder and jet lag disorder. Diagnostic tools such as sleep diaries and wrist activity monitoring are often useful in confirming the diagnosis. Because behavioral and environmental factors often are involved in the development of these conditions, a multimodal approach is usually necessary. Interventions include sleep hygiene education, timed exposure to bright light as well as avoidance of bright light at the wrong time of the day and pharmacologic approaches, such as melatonin. However, it should be noted that the use of melatonin is not an FDA-approved indication for the treatment of circadian rhythm sleep disorders.
Keywords: Circadian Rhythm Sleep Disorders, Delayed Sleep Phase Disorder, Advanced Sleep Phase Disorder, Non 24-hr Sleep Wake Syndrome, Irregular Sleep Wake Rhythm, Shift Work Disorder, Jet Lag Syndrome
Introduction
Circadian rhythms are generated by a central pacemaker, the suprachiasmatic nucleus (SCN) in the anterior hypothalamus, and are synchronized to the external environment.1–3 At the molecular level, circadian clock gene proteins oscillate by means of an autoregulatory feedback loop, generating a self-sustained timing system that is highly regulated with a period of about 24 hours.4–6
Current understanding of the sleep-wake cycle indicates that human sleep and wake behaviors are generated by a complex interaction of endogenous circadian and sleep homeostatic processes. The homeostatic process of sleep accumulates as a function of prior wakefulness.7 An essential role of the circadian clock is to promote wakefulness during the day and, thus, facilitate consolidation of sleep during the night.8–10 In humans, the propensity to fall asleep, as measured by multiple sleep latency tests, shows a biphasic circadian rhythm. In most individuals, there is a midday decrease in alertness occurring at around 2–4 p.m., followed by an increase in alertness that peaks during early to mid-evening hours, then it declines to its lowest levels around 4–6 a.m. During the early evening hours, when the homeostatic drive for sleep is high, the circadian alerting signal is also at its highest level.11–14 Thus, the circadian system plays an important role in determining the temporal distribution and duration of sleep and wakefulness.
Optimal sleep quality is achieved when the desired sleep time is aligned with the timing of the endogenous circadian rhythm of sleep and wake propensity. Circadian rhythm sleep disorders (CRSDs) arise from disruption of the circadian timing system or a misalignment between the endogenous circadian timing and the external 24-hour social and physical environment, resulting in complaints of insomnia and/or excessive sleepiness and impairment in important areas of functioning and quality of life. The actual clinical presentation of CRSD is often influenced by a combination of physiological, behavioral, and environmental factors.
The classification and diagnostic criteria for CRSD and its subtypes discussed throughout this manuscript are largely based on published criteria from the International Classification of Sleep Disorders (ICSD-2)15 and Diagnostic and Statistical Manual of Mental Disorders (DSM IV-TR).16 In this section, we will review current clinical approaches for the diagnosis and management of the more commonly encountered CRSDs in clinical practice. However, it is important to note that we currently do not have standardized clinical guidelines for the treatment of these circadian-based sleep disorders.
Circadian Rhythm Sleep Disorder, Delayed Sleep Phase Type (Delayed Sleep Phase Disorder, Delayed Sleep Phase Syndrome)
Clinical Features
Delayed sleep phase disorder (DSPD) is characterized by sleep times that are delayed three to six hours relative to the desired or socially acceptable sleep-wake schedules.17 Patients typically report difficulty falling asleep before 2–6 a.m. and, when free of social obligations, such as on weekends and vacations, would prefer wake times between 10 a.m. and 1 p.m. When following socially enforced sleep-wake times, patients will present with classical symptoms of chronic sleep-onset insomnia and difficulty waking up in the morning for work, school or social obligations.18 Quite often, DSPD patients relate this preference for delayed sleep-wake times remains stable for many years. When using questionnaires to determine diurnal preferences, such as the Horne-Ostberg questionnaire19, DSPD patients rate as “evening types”. However, one must distinguish between an “evening type or owl” without complaints from an individual with DSPD whose delayed sleep and wake times are accompanied by insomnia and excessive sleepiness that result in functional impairments.20
Prevalence
It has been estimated that the prevalence of DSPD in the general population is between 0.13%–0.17%.21, 22 DSPD is more common in adolescents, with a reported prevalence of 7–16%, and may represent approximately 7% of patients presenting to sleep clinics with complaints of chronic insomnia.17, 23
Pathophysiology
Although the exact pathophysiologic mechanisms of DSPD remain poorly understood, it is likely a result of multiple interacting genetic, physiological and behavioral factors. Suggestion of a genetic basis for DSPD is supported by a report of one large family in which the DSPD phenotype was shown to segregate as an autosomal dominant trait24, and recent evidence of polymorphisms in the circadian rhythm genes, such as hPer3, arylalkylamine N-acetyltransferase, HLA and Clock, in individuals with DSPD.25–28
Based on the fundamental properties of circadian and sleep regulation, several mechanisms have been proposed to explain the persistently delayed sleep phase in this disorder. One possible explanation is an alteration in entrainment of the circadian clock to synchronizing agents. For example, individuals with DSPD may have an unusually small advance portion or an unusually large delay portion of the phase response curve (PRC) to light.17 In addition, behavioral factors such as decreased exposure to light during early morning (due to sleeping later in the morning) and prolonged exposure to light later in the evening may contribute to the delayed phase pattern.29 It has also been suggested that the delayed pattern may be due in part to the finding that individuals with DSPD may be more sensitive to evening light.30
Although alterations in the circadian clock are commonly implicated as the underlying cause of DSPD, there is evidence that alterations in homeostatic sleep regulation may also play a role in the pathogenesis of this disorder. It is commonly accepted that sleep architecture is essentially normal in DSPD when patients are allowed to maintain the desired sleep-wake times17, 31–33. However, the finding that following a 24-hour period of sleep deprivation DSPD patients had decreased ability to compensate for sleep loss during the day and the first few hours of the night, 34, 35 suggests that changes in homeostatic regulation of sleep may further contribute to daytime sleepiness and impaired functioning.
Diagnostic Evaluation and Differential Diagnosis
It is necessary to distinguish DSPD patients from “normal” delayed sleep patterns commonly seen in adolescents and young adults who have delayed sleep schedules without experiencing functional impairment or social distress. Although the diagnosis is made primarily by the patient’s history, a sleep diary alone, or in conjunction with actigraphy for at least seven days is recommended to confirm the stable delayed sleep and wake times.15 (Figure 1) Activity monitoring (actigraphy) has become more widely available in recent years and has been shown to be a useful tool in assessing sleep-wake patterns across multiple cycles.36 When the diagnosis is unclear, or when a more accurate determination of circadian phase is indicated, assessment of the timing of physiologic markers, such as dim light melatonin onset (DLMO) or the nadir of the core temperature rhythm can provide additional diagnostic information. A polysomnogram (PSG) is not routinely indicated for the diagnosis of DSPD but may be useful in excluding other sleep disorders. Because of the comorbidity with affective disorders, a careful psychiatric review of systems and a detailed history of psychoactive substance use should be part of the routine evaluation.37
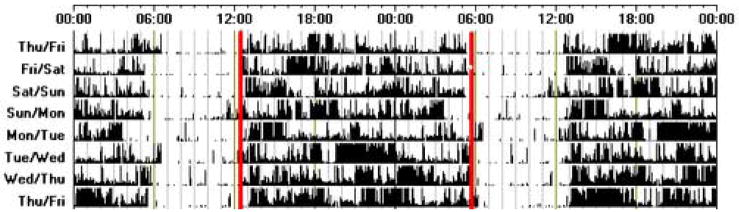
Figure 1.
An actogram of sleep-wake activity, recorded using wrist activity monitoring, from an individual with DSPD. This individual goes to bed around 5–6 a.m. and wakes between 12–1 p.m. each day. Note that all actigraphy data shown throughout this manuscript is double-plotted; i.e., a period of consecutive 48 hours is represented in each line and then the last 24 hours repeated on the following line.
Clinical Management
Treatment should be tailored to the severity of symptoms, comorbid psychopathology, school schedules, work obligations, ability and willingness of the patient and family to comply with treatment, and social pressures.31, 38, 39 Current treatment options for DSPD include chronotherapy, timed bright light exposure and melatonin. Exogenous melatonin is widely available as a nutritional supplement that is not regulated by the Federal Drug Administration (FDA).
Chronotherapy is a behavioral approach in which the circadian clock is reset by progressively delaying sleep and wake times by approximately three hours every two days, until the desired sleep and wake times are achieved and maintained by adhering to a set sleep-wake schedule and good sleep hygiene practices.17 However, the strict demands of chronotherapy, together with the length of treatment, have limited its applicability and acceptance in clinical practice. For all patients, adherence to a regular sleep wake schedule, avoidance of bright light in the evening and sleep hygiene education are important elements in the approach to the clinical management of DSPD patients.
One of the most commonly used treatments for DSPD is timed bright light exposure. Exposure to bright light can reset the human circadian system, and the effects can be predicted by the human PRC. Bright light exposure in the early morning (advance portion of the light PRC) will advance the phase of circadian rhythms, while exposure to light in the evening (delay portion of the light PRC) will cause phase delays. (Figure 2) Rosenthal and colleagues showed that after two weeks of daily morning (7–9 a.m.) bright light exposure (2,500 lux) combined with restricted evening light, subjects had earlier sleep times and reported subjective improvement in morning alertness.40 Even though light therapy is widely accepted as being effective, there are no established standardized guidelines regarding the duration, intensity or timing of light exposure. The most commonly recommended approach is broad spectrum bright light of 2,000–10,000 lux early morning (6–8 a.m.) for about 1–3 hours.41 In clinical practice, one important limitation of light therapy is poor compliance because of the need to structure social and professional activities around the timing of daily light exposure. Recent reports that both light-induced phase shifts as well as melatonin suppression are most sensitive to short wavelength light of approximately 460 nm (blue) could potentially decrease the intensity and duration of phototherapy and, thus, improve compliance.42, 43 Studies are clearly needed to determine the efficacy and safety of blue-green light for the treatment of DSPD.
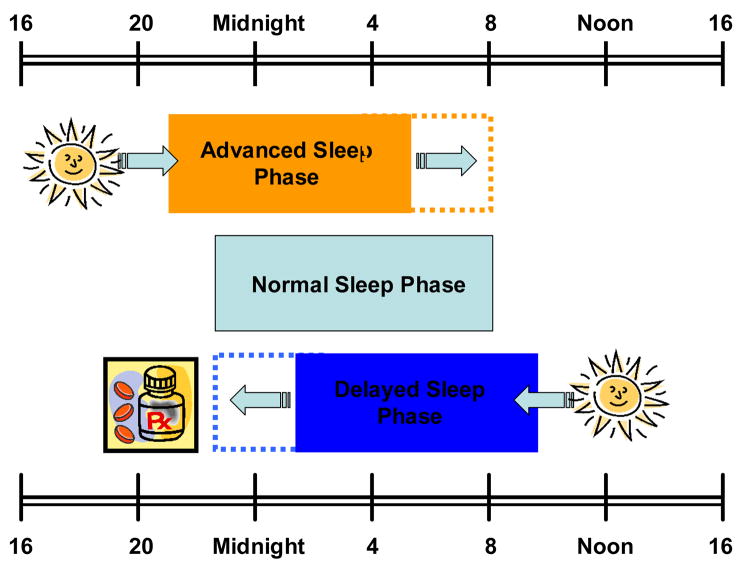
Figure 2.
As predicted by the human PRC (phase response curve) to light, bright light exposure in early mornings will advance the phase of circadian rhythms, while exposure to light in the evening will cause phase delays. As the melatonin PRC is approximately the inverse of PRC to light exposure, melatonin advances the phase rhythm when given in the early evening hours.
In view of the clinical limitations encountered with chronotherapy and poor compliance with phototherapy, melatonin has been increasingly investigated as a treatment venue. When taken in the evening, melatonin advances the timing of circadian rhythms, whereas in the morning it causes a delay in humans.44 It is useful to note that the melatonin PRC is approximately the inverse of the PRC to light. (Figure 2) A recently published study in patients with DSPD showed that the largest phase advance in the timing of circadian rhythms occurred when melatonin (0.3 or 3 mg) was given approximately 6–7 hours before sleep time.45 Despite the potential efficacy of melatonin, a standardized approach for dose, duration and timing of melatonin for the treatment of DSPD is lacking. This is largely due to lack of multicenter randomized placebo-controlled studies and the inconsistent doses and schedules used among the various studies.46–48 Table 1 provides a guideline for the use of melatonin that is based on the literature and on our own clinical experience. Systematic data on the safety of melatonin, particularly with regard to long-term use, is unknown. Side effects may include elevation in blood pressure, headache, dizziness, nausea and drowsiness.49
TABLE 1.
Circadian Rhythm Sleep Disorders – Clinical Features, Diagnostic Evaluation and Treatment Options
| Type | Clinical Features* | Diagnostic Evaluation | Treatment options** |
|---|---|---|---|
| Delayed Sleep Phase Type | Delayed bedtime and wake times Bedtime: 2–6 am Wake time: 10am-1pm More commonly found in adolescents |
Sleep diary or actigraphy monitoring for at least 7 days DLMO and nadir core body temperature may be useful for confirmation of the delayed phase |
Melatonin: 0.3–3mg 5 hours before bedtime Bright light: 1–3 hours of 2,000–10,000 lux between 7–9am Chronothreapy: progressively delay bedtimes |
| Advanced Sleep Phase Type | Advanced bedtime and wake times Bedtime: 6–9pm Wake time: 2–5 am Rare, especially in the young |
Sleep diary or actigraphy monitoring for at least 7 days DLMO and nadir core body temperature may be useful for confirmation of the advanced phase |
Bright light: 1–3 hours of 2,000–10,000 lux between 7–9pm Chronotherapy: progressively advance bedtimes |
| Non-entrained Type | Bedtimes and wake times progressively occur at a later time each day High prevalence in the blind Very rare in sighted individuals |
Sleep diary or actigraphy monitoring for at least 7 days, but it is best to continue monitoring for longer periods to clearly establish a daily drift | Melatonin: 10mg 1 hour before desired bedtime, 0.5mg maintenance dose Structured activity: maintain a regular schedule |
| Irregular sleep-wake Type | No regular bedtimes or wake times Frequent short naps throughout the day and night Most commonly seen in patients with underlying neurologic dysfunction |
Sleep diary or actigraphy monitoring for at least 7 days At least 3 irregular sleep bouts per 24 hours Total sleep time per 24 hours is normal for age |
Bright light: 2 hours of 3,000 to 5,000 lux in the morning Decrease exposure to light and noise during the night Structured activity: increase physical and social activity during the day |
| Shift Work Type | Sleep complains are temporally associated with a work schedule that overlaps usual the time for sleep and are present over the course of at least 1 month | Sleep diary or actigraphymonitoring for at least 7 days demonstrates a disrupted sleep-wake pattern PSG may be useful to exclude co-morbid disorders (i.e, sleep apnea) |
Behavioral strategies: good sleep hygiene, wear sunglasses during commute home, optimize sleep environment Bright light: during night shift 3–6 hours of 1,200–10,000 lux either continuously or intermittently during the first half but no later than 2 hours before end of the shift Melatonin: 3mg before bedtime, if bright light therapy not practical option Stimulants: caffeine or modafinil 200mg at the beginning of the shift Treat co-morbid sleep related disorders |
| Jet Leg Type | Self-limited Severity of symptoms depend on the number of time zones traveled Eastward travel usually more difficult to adjust to |
Objective laboratory testing is usually not indicated | Behavioral strategies: adequate hydration, avoid caffeine and alcohol, good sleep hygiene Strategic exposure to light Melatonin 0.5–5mg for the first 4 days after arrival Short-acting hypnotic use for the first few days after arrival |
When patients allowed to sleep at their desired times.
Melatonin: To date, there are no established clinical practice parameters for its use, and it is not FDA approved
Overall effectiveness can be improved by using a combination of chronotherapy, morning phototherapy and late afternoon or early evening melatonin.50 It is clear that successful treatment of DSPD requires an individualized approach using behavioral strategies, such as morning bright light exposure, avoidance of evening light, and/or appropriately timed melatonin. In addition, optimizing treatment of comorbid conditions and behavioral interventions, including good sleep hygiene, should be the basic approach for all patients, especially adolescents.
Circadian Rhythm Sleep Disorder, Advanced Sleep Phase Type (Advanced Sleep Phase Disorder, Advanced Sleep Syndrome)
Clinical Features
Advance sleep phase disorder (ASPD) is characterized by habitual and involuntary sleep times (6–9 p.m.) and wake times (2–5 a.m.) that are several hours early relative to conventional and desired times.51, 52 Patients with ASPD typically present with complaints of sleep maintenance insomnia, early morning awakenings and sleepiness in the late afternoon or early evening. In general, individuals with ASPD tend to have less difficulty adjusting to their preferred earlier schedules than those with DSPD. Individuals with ASPD are often described as “larks” and score as morning types on the Horne-Ostberg questionnaire.19
Prevalence
Non-age-related ASPD is believed to be rare, and there are only a few reported cases in the literature.51, 53, 54 However, the prevalence tends to increase with age, and has been estimated to be about 1% in middle age and older adults.55
Pathophysiology
As with DSPD, the exact pathophysiologic mechanisms underlying ASPD are unclear. Proposed mechanisms include an unusually short endogenous circadian period (less than 24 hours)53, or changes in the interaction of circadian timing and sleep homeostatic regulation.56 Furthermore, decreased exposure or weakened responses to entrainment agents such as light and physical activity may also contribute to the advanced sleep phase, particularly in older adults.57–59 Genetic factors are likely to play an important role in ASPD. Familial forms of ASPD have been reported in which the phenotype segregates with an autosomal dominant mode of inheritance.53, 54, 60 To date, mutations in the circadian clock hPer2 and CK1 delta genes have been identified and linked to familial ASPD.61, 62
Diagnostic Evaluation and Differential Diagnosis
In addition to the clinical history, sleep diary and/or actigraphy for at least seven days should be obtained to demonstrate a stable pattern of advanced sleep and wake times. (Figure 3) Other physiologic markers of the circadian phase, such as DLMO and nadir core body temperature show the expected advanced phase. As with most CRSDs, a PSG is not routinely indicated. However, because both ASPD and comorbid sleep disorders, such as obstructive sleep apnea and restless legs syndrome, increase with age, a careful assessment for these conditions is important. Depression should always be considered in the differential diagnosis of ASPD, as it also can present with complaints of early morning awakening.
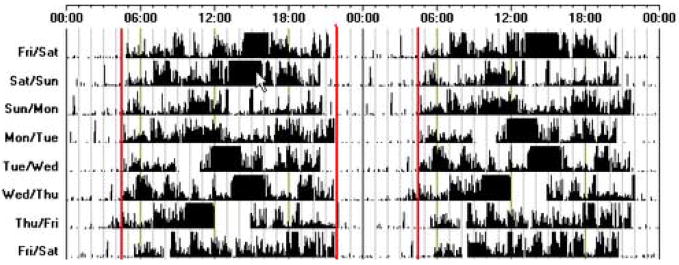
Figure 3.
An actogram of sleep-wake activity, recorded using wrist activity monitoring, from an individual with ASPD. This individual goes to bed between 9–10 p.m. and wakes at 4 a.m. each day. There are two periods when the subject has taken off the wrist activity monitoring (Tuesday for about 2 hours and Thursday for about 3 hours).
Clinical Management
Current treatment options for ASPD include chronotherapy, timed exposure to bright light in the evening and pharmacotherapy with hypnotics or melatonin. Chronotherapy progressively advances sleep time by two hours every day until the desired sleep time is achieved.51 As mentioned previously, chronotherapy is often impractical in clinical settings. The most commonly recommended treatment for ASPD is timed bright light exposure in the evening, usually between 7–9 p.m.41 (Figure 2) It has been shown that just two days of evening light exposure can improve sleep and daytime performance in older adults with early morning insomnia.63 Together, studies indicate that exposure to light in the evening can improve sleep quality, increase total sleep time, and daytime performance in older adults with advanced circadian phase and early morning insomnia.64–66
Little data exists on the clinical usefulness of melatonin for the management of ASPD. In theory, melatonin given in the early morning could delay the timing of circadian rhythms in individuals with ASPD. However, taking melatonin too close to wake-up time could potentially result in next-day residual sedation.
Circadian Rhythm Sleep Disorder, Free-Running Type (Non-entrained type, Non-24-Hour Sleep Wake Syndrome, Hypernychthermal Syndrome)
Clinical Features
Non-24-hour sleep wake syndrome is characterized by a steady daily drift of the major sleep and wake times. Because the endogenous circadian period in humans is usually slightly longer than 24 hours, patients will report a progressive delay in the timing of sleep and wake times. Attempting to maintain a regular sleep-wake schedule can lead to the development of symptoms of insomnia, early morning awakenings and excessive sleepiness that varies in intensity periodically. At times when the endogenous pacemaker is not in phase with the conventional sleep and wake times, patients will report symptoms that cause impairment in social, occupational or other areas of functioning. When the endogenous circadian rhythm is in phase with sleep times, sleep is usually normal.
Epidemiology
It has been estimated that approximately 50% of totally blind people have non-entrained circadian rhythms67 and about 70% have complaints of chronic sleep disturbances.68, 69 In addition to non-entrained rhythms, advanced or delayed circadian rhythms with a period of 24 hours have also been reported in the blind.70 Although rare, non-entrained sleep and wake patterns have also been reported in sighted individuals.71
Pathophysiology
Decreased or lack of photic reception is the most likely cause of non-entrained circadian rhythms in blind people. However, despite absent visual light perception, the circadian clock of some blind individuals can respond to bright light.72 This finding could be partially explained by the recent finding that the major circadian photoreceptors are the melanopsin-containing retinal ganglion cells, rather than the rods and cones.73 Furthermore, because the circadian clock can also be entrained to scheduled social and physical activities, a decrease in or lack of exposure to these nonphotic agents could further contribute to the development of a non-entrained sleep pattern.
In sighted individuals, it has been suggested that non-entrained type could be a severe form of DSPD.18 These individuals may have such an unusually long endogenous circadian period that is beyond entrainment to a 24-hour day.74 This hypothesis is supported by reports of a few DSPD patients who developed free-running rhythms following chronotherapy.75 Other potential causes include a weakened response of the circadian clock to light or decreased exposure to light and/or structured social and physical activities.71
Diagnostic Evaluation
Actigraphy and/or sleep diaries are useful in confirming the non-entrained pattern of sleep and wake, but in order to appreciate the daily drift in the timing of the sleep-wake cycle they should be recorded for at least two weeks.15 (Figure 4) PSG is not required for the diagnosis and results may vary depending on when the study is performed. It should be essentially normal if performed when the endogenous circadian rhythm is in phase with the conventional 24-hour cycle.
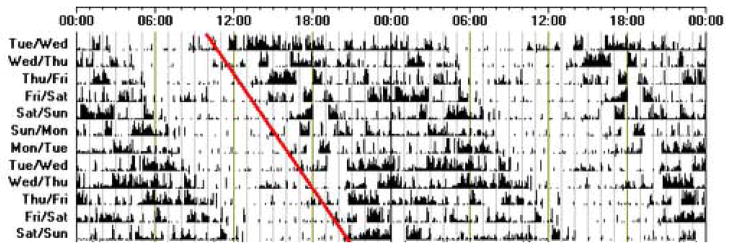
Figure 4.
An actogram of sleep-wake activity, recorded using activity monitoring from an individual with non-24-hour sleep-wake syndrome. Note the consistent delay in bedtime over the recording time.
Clinical Management
Treatment options for non-entrained type circadian rhythm disorders in blind people include attempting to synchronize the circadian pacemaker by using behavioral approaches such as maintaining a regular schedule of sleep-wake times and social/work activities and pharmacologic approaches, such as melatonin. Education regarding sleep hygiene, particularly maintaining a regular schedule of activities is essential. Melatonin 10 mg given one hour before bedtime has been shown to synchronize sleep-wake behaviors to a 24-hour period in blind people with non-entrained circadian patterns.76 Entrainment can be maintained with lower doses of 0.5 mg of melatonin given nightly.77
Much less is known regarding treatment approaches for sighted patients with non-24-hour sleep-wake disorder. Attempts should be made to increase the regularity of work and sleep-wake schedules, as well as the intensity and duration of bright light exposure during the day. Very little is known regarding the use of melatonin in sighted persons. In a recent case report, administration of melatonin in the evening to a sighted adolescent with non-24-hour sleep-wake schedule disorder restored a normal sleep-wake cycle.78
Circadian Rhythm Sleep Disorder, Irregular Sleep-Wake Type (Irregular Sleep-Wake Rhythm)
Irregular sleep-wake rhythm is characterized by the lack of a clearly identifiable circadian pattern of sleep and wake times. Although total sleep time over 24 hours may be normal for age, sleep and wake periods occur in short bouts throughout the day and night.
This disorder is most commonly seen in association with dementia, mental retardation and brain injury.79–81 It has been suggested that both dysfunction of the central processes responsible for the generation of circadian rhythms, as well as decreased exposure to external synchronizing agents, such as light and social activities, play a role in the development and maintenance of the irregular sleep-wake rhythm. Therefore, irregular sleep-wake rhythm should be differentiated from poor sleep hygiene and voluntary maintenance of irregular schedules.
The goal of treatment is to consolidate nocturnal sleep and improve daytime alertness. Strategies involve increasing the intensity and duration of light exposure during the day, limiting evening light exposure and structured social and physical activities.59, 82–85 Results of light treatment studies in dementia indicate that increasing morning or evening light exposure can improve circadian amplitude and consolidate nocturnal sleep as measured by actigraphy.86, 87 Based on hourly recordings by nursing staff, morning bright light exposure (2 hours at 3,000–5,000 lux) for four weeks increased nocturnal sleep time and decreased daytime sleep time in patients with dementia.88 Therefore, behavioral interventions such as increasing light exposure and physical and social activities during the day and decreasing exposure to light and noise during the night are first line approaches for all patients with irregular sleep-wake rhythm.
Melatonin has also been investigated for the treatment of irregular sleep-wake rhythm. In children with psychomotor retardation, evening administration of melatonin can improve consolidation and regularity of sleep-wake patterns.89 In general, the effects of melatonin on sleep of older adults with dementia have yielded inconsistent results. The largest multicenter placebo-controlled study of melatonin failed to show significant improvements in sleep measures (a trend towards improvement was seen with the higher dose of melatonin of 10 mg).90
Circadian Rhythm Sleep Disorder, Shift Work Type (Shift Work Disorder, Shift Work Sleep Disorder)
Clinical Features
Sleep disturbances are common complaints among shift workers, but not all shift workers have sleep difficulties severe enough to interfere with work performance and/or social functioning. The ability to cope with shift work varies from individual to individual and is influenced by multiple factors, such as age, domestic responsibilities, commute times, type of work schedule, diurnal preference and family responsibilities.91
Shift work disorder (SWD) typically presents with complaints of unrefreshing sleep, excessive sleepiness and insomnia that vary depending on the work schedule. The relationship between sleep problems and work schedule should be evident by the history. SWD is most commonly seen in association with night and early morning (before 6 a.m.) shifts. Patients usually report decreased total sleep time, poor sleep quality and excessive sleepiness at work.92, 93 Chronic sleep deprivation may lead to impaired performance at work and safety concerns at work and/or during the commute home.92, 94, 95
Prevalence
Approximately 20% of the workforce in industrialized countries is estimated to work non-standard hours.96 A recent study suggests that the prevalence of shift work sleep disorder is approximately 10% in night and rotating shift workers.97
Pathophysiology
SWD results when individuals are required to work and sleep at times that are in opposition to the circadian propensity for sleep and alertness, leading to symptoms of insomnia and excessive sleepiness. Patients may complain of problems initiating and maintaining sleep as they are attempting to sleep at a time of low circadian sleep propensity. Symptoms of insomnia and excessive sleepiness may persist for several days after the last night shift or on days off, even after sleep has been restored to conventional times. This continued difficulty is likely due to a partial adjustment of the circadian system. Successful adaptation to shift work will be influenced by the number of consecutive night shifts and the speed and direction of the shift rotation.
Diagnostic Evaluation and Differential Diagnosis
Diagnosis of SWD is based on a temporal relationship between the clinical presentation and a history of shift work. The sleep disturbance persists for the duration of the shift work and may continue after the individual resumes a more conventional work schedule. A sleep diary and/or actigraphy are useful to demonstrate the disrupted sleep and napping behavior. PSG is indicated when a patient’s history suggests comorbid disorders, such as sleep apnea. Increased association with depression, alcohol or drug misuse or abuse, gastrointestinal symptoms (e.g., dyspepsia, constipation, diarrhea), sleep apnea, obesity and miscarriage have been reported in shift workers and patients should be screened for such comorbidities.98, 99
Clinical Management
Treatment for SWD is based on two main approaches: (1) attempting to re-align the circadian propensity for sleep and wakefulness with the work schedule (light and melatonin) and (2) improving sleep and alertness (behavioral and pharmacologic approaches). In night shift workers, bright light or melatonin can accelerate the adaptation of circadian rhythms.100–103 Treatment approaches have predominantly been focused on the night shift worker.
Various degrees of success have been demonstrated with bright light exposure during the night shift using intensities from 1,200 to 10,000 lux for a duration of 3–6 hours.100–103 A practical approach could include intermittent bright light exposure (about 20 min/hr blocks) throughout the night shift but terminating the exposure approximately two hours before the end of the shift.104, 105 Furthermore, circadian adjustment can be accelerated by the avoidance of bright light in the early morning by using dark sunglasses on the way home after a night shift.104
The inability to sleep during the day is a common problem in patients with SWD. To improve the duration and quality of sleep, daytime sleep should be initiated as soon as possible after the night shift in a dark and quiet environment. Sleep hygiene education as well as social and family support to protect and maximize the shift worker’s sleep are essential for all patients. Patients may also have comorbid insomnia that requires cognitive behavioral therapy (e.g., cognitive therapy, relaxation techniques, stimulus control and bedtime restriction) alone or in combination with short-acting hypnotic medications.
Melatonin has also been studied in SWD. In night shift workers, melatonin administered in the morning can accelerate re-entrainment by delaying the timing of circadian rhythms, and also improve sleep with its hypnotic effects. Although melatonin taken during the day can improve daytime sleep, it has not been shown to significantly increase nighttime alertness.100
Increasing sleep duration and improving sleep quality is an important anchor for managing sleepiness during work. Short scheduled naps during the shift has also been shown to improve alertness and performance.106 For individuals in whom behavioral strategies are insufficient, particularly when safety is a concern, pharmacological agents may also be used to alleviate excessive sleepiness. Caffeine (250–400 mg) during the first two hours of the night shift has been shown to improve nighttime alertness without subjective impairment of subsequent daytime sleep.107 Modafinil, a non-sympathomimetic stimulant has also been studied for the treatment of excessive sleepiness associated with shift work sleep disorder. Studies show that a 200 mg dose of modafinil taken 30 to 60 minutes before the start of the night shift increased alertness, reduced neurobehavioral deficits and did not interfere with subsequent sleep.108, 109 Modafinil is approved by the FDA for the treatment of excessive sleepiness associated with SWD. However, it is important to note that although wake-promoting agents improve sleepiness, alertness is usually not normalized.
Treatment should always be individualized to the needs of each patient, and all aspects of the individual’s life need to be taken into consideration when preparing a treatment plan to obtain maximal success. A multicomponent approach composed of sleep hygiene education, measures to optimize the sleep environment, treatment of insomnia and excessive sleepiness using behavioral and/or pharmacologic therapies, and enlisting the support of family, friends and employer is necessary for the successful management of SWD.
Circadian Rhythm Sleep Disorder, Jet Lag Type (Jet Lag Syndrome)
Jet lag is the result of the external environment being temporarily altered in relation to the timing of the endogenous circadian rhythm by rapid traveling across time zones. It is characterized by symptoms such as daytime fatigue and sleepiness, nighttime insomnia, mood changes, difficulty concentrating, general malaise and gastrointestinal problems.110 During eastward travel, difficulty falling asleep is more prominent, and during westward travel complaint of sleep maintenance is most common. Symptoms are transient and should resolve as the traveler’s circadian clock re-establishes a normal phase relationship with the local time.
Although there is substantial inter-individual variability in the severity of jet lag symptoms, the direction of travel and the number of time zones crossed are important factors to consider.111 For example, westward travel generally causes less disruption than eastward travel, which can be explained by the fact that the free-running period (tau) of humans is slightly longer than 24 hours, making it easier to delay rather than advance the circadian rhythms.112
A combination of approaches to accelerate circadian alignment, including timed light exposure and melatonin113, 114 and/or behavioral strategies, has been used to improve sleep and daytime function. Travelers should be instructed to wear loose fitting clothes, drink plenty of fluid, and avoid alcohol and excessive caffeinated beverages or foods during the flight to minimize travel fatigue. Upon arrival, all efforts should be made to eat meals at local times, exercise and maintain good sleep habits to decrease the severity of the symptoms.115
Independent of the direction of travel, travelers should remain awake while it is daytime at the destination. Eastward travelers should avoid bright light in the early morning, and increase exposure to bright light in the evening while westward travelers should get as much exposure to bright light as possible in the early morning and try to sleep when it gets dark, avoiding bright light in the evening.112 Advancing one’s circadian rhythm using intermittent morning bright light prior to eastward flight has been proposed by Eastman et al. as a possible way to prevent or reduce jet lag symptoms.116
Pharmacologic approaches have also been studied. Melatonin has been shown to alleviate jet lag symptoms with doses of 0.5–5 mg taken close to local bedtime for up to four days117. However, subjective sleep quality and sleep latency significantly improved with only the 5 mg dose.118 The short-term use of short-acting non-benzodiazepine hypnotic medications can be useful in alleviating symptoms of insomnia, and caffeine is often consumed to improve alertness. In one study, zolpidem 10 mg taken for three consecutive nights after arrival has been shown to improve sleep after travel.119 However, the combination of zolpidem and melatonin did not significantly improve symptoms of jet lag, and was associated with increased adverse effects when compared to melatonin alone.120
Summary
CRSDs should be considered in the differential diagnosis of every patient presenting with symptoms of excessive sleepiness and/or insomnia. A misalignment between the endogenous circadian timing system and the external environment constitutes the basis for most of these disorders. Management focuses on realignment of the circadian timing system with the desired or socially acceptable sleep and wake times. However, social and behavioral factors play an important role in perpetuating or exacerbating these disorders and, thus, successful management relies on a multimodal approach. The treatment of CRSDs remains a challenge in part because large multicenter placebo-controlled clinical studies using phototherapy or pharmacologic agents are scarce. Although melatonin receptor agonists, such as ramelteon, have recently been approved for the treatment of insomnia, it has not been studied in patients with CRSDs.
Recent rapid advances in our understanding of the neurobiology and genetics of circadian rhythm and sleep regulation should lead to improved diagnostic tools and treatments that will establish evidence-based as well as practical practice guidelines for the evaluation and treatment of CRSDs.
Footnotes
Disclosure Statement
Ana Barion has nothing to disclose.
Phyllis C. Zee has served as a consultant for Boeringher Ingelheim, Glaxo Smith Kline, Neurocrine Biosciences, Pfizer, Sanofi-Aventis, Sepracor and Takeda Pharmaceutical. Dr. Zee has also served as an investigator on research grants funded by Boeringher Ingelheim, Glaxo Smith Kline, Neurocrine Biosciences, Sanofi-Aventis and Takeda Pharmaceuticals.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42(1):201–6. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 2.Moore RY. Circadian rhythms: basic neurobiology and clinical applications. Annu Rev Med. 1997;48:253–66. doi: 10.1146/annurev.med.48.1.253. [DOI] [PubMed] [Google Scholar]
- 3.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972;69(6):1583–6. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96(2):271–90. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 5.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–41. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 6.Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet. 2004;5:407–41. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dijk DJ, Brunner DP, Beersma DG, Borbely AA. Electroencephalogram power density and slow wave sleep as a function of prior waking and circadian phase. Sleep. 1990;13(5):430–40. doi: 10.1093/sleep/13.5.430. [DOI] [PubMed] [Google Scholar]
- 8.Czeisler CA, Weitzman E, Moore-Ede MC, Zimmerman JC, Knauer RS. Human sleep: its duration and organization depend on its circadian phase. Science. 1980;210(4475):1264–7. doi: 10.1126/science.7434029. [DOI] [PubMed] [Google Scholar]
- 9.Zulley J, Wever R, Aschoff J. The dependence of onset and duration of sleep on th circadian rhythm of rectal temperature. Pflugers Arch. 1981;391(4):314–8. doi: 10.1007/BF00581514. [DOI] [PubMed] [Google Scholar]
- 10.Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166(1):63–8. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- 11.Carskadon MA, Dement WC. Sleep studies on a 90-minute day. Electroencephalography Clinical Neurophysiology. 1975;39(2):145–55. doi: 10.1016/0013-4694(75)90004-8. [DOI] [PubMed] [Google Scholar]
- 12.Strogatz SH, Kronauer RE, Czeisler CA. Circadian pacemaker interferes with sleep onset at specific times each day: role in insomnia. Am J Physiol. 1987;253(1 Pt 2):R172–8. doi: 10.1152/ajpregu.1987.253.1.R172. [DOI] [PubMed] [Google Scholar]
- 13.Dantz B, Edgar DM, Dement WC. Circadian rhythms in narcolepsy: studies on a 90 minute day. Electroencephalogr Clin Neurophysiol. 1994;90(1):24–35. doi: 10.1016/0013-4694(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 14.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15(5 Pt 1):3526–38. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The International Classification of Sleep Disorders: Diagnostic & Coding Manual, ICSD-2. 2. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 16.American Psychiatric Association. American Psychiatric Association Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 17.Weitzman ED, Czeisler CA, Coleman RM, et al. Delayed sleep phase syndrome. A chronobiological disorder with sleep- onset insomnia. Arch Gen Psychiatry. 1981;38(7):737–46. doi: 10.1001/archpsyc.1981.01780320017001. [DOI] [PubMed] [Google Scholar]
- 18.Regestein QR, Monk TH. Delayed sleep phase syndrome: a review of its clinical aspects. Am J Psychiatry. 1995;152(4):602–8. doi: 10.1176/ajp.152.4.602. [DOI] [PubMed] [Google Scholar]
- 19.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 20.Dagan Y. Circadian rhythm sleep disorders (CRSD) Sleep Med Rev. 2002;6(1):45–54. doi: 10.1053/smrv.2001.0190. [DOI] [PubMed] [Google Scholar]
- 21.Yazaki M, Shirakawa S, Okawa M, Takahashi K. Demography of sleep disturbances associated with circadian rhythm disorders in Japan. Psychiatry Clin Neurosci. 1999;53(2):267–8. doi: 10.1046/j.1440-1819.1999.00533.x. [DOI] [PubMed] [Google Scholar]
- 22.Schrader H, Bovim G, Sand T. The prevalence of delayed and advanced sleep phase syndromes. J Sleep Res. 1993;2(1):51–5. doi: 10.1111/j.1365-2869.1993.tb00061.x. [DOI] [PubMed] [Google Scholar]
- 23.Pelayo R, Thorpy MJ, Govinski P. Prevalence of delayed sleep phase syndrome among adolescents. Sleep Res. 1988;17:392. [Google Scholar]
- 24.Ancoli-Israel S, Schnierow B, Kelsoe J, Fink R. A pedigree of one family with delayed sleep phase syndrome. Chronobiol Int. 2001;18(5):831–40. doi: 10.1081/cbi-100107518. [DOI] [PubMed] [Google Scholar]
- 25.Ebisawa T, Uchiyama M, Kajimura N, et al. Association of structural polymorphisms in the human period3 gene with delayed sleep phase syndrome. EMBO Rep. 2001;2(4):342–6. doi: 10.1093/embo-reports/kve070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwase T, Kajimura N, Uchiyama M, et al. Mutation screening of the human Clock gene in circadian rhythm sleep disorders. Psychiatry Res. 2002;109(2):121–8. doi: 10.1016/s0165-1781(02)00006-9. [DOI] [PubMed] [Google Scholar]
- 27.Archer SN, Robilliard DL, Skene DJ, et al. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003;26(4):413–5. doi: 10.1093/sleep/26.4.413. [DOI] [PubMed] [Google Scholar]
- 28.Hohjoh H, Takasu M, Shishikura K, Takahashi Y, Honda Y, Tokunaga K. Significant association of the arylalkylamine N-acetyltransferase (AA-NAT) gene with delayed sleep phase syndrome. Neurogenetics. 2003;4(3):151–3. doi: 10.1007/s10048-002-0141-9. [DOI] [PubMed] [Google Scholar]
- 29.Ozaki S, Uchiyama M, Shirakawa S, Okawa M. Prolonged interval from body temperature nadir to sleep offset in patients with delayed sleep phase syndrome. Sleep. 1996;19(1):36–40. [PubMed] [Google Scholar]
- 30.Aoki H, Ozeki Y, Yamada N. Hypersensitivity of melatonin suppression in response to light in patients with delayed sleep phase syndrome. Chronobiol Int. 2001;18(2):263–71. doi: 10.1081/cbi-100103190. [DOI] [PubMed] [Google Scholar]
- 31.Thorpy MJ, Korman E, Spielman AJ, Glovinsky PB. Delayed sleep phase syndrome in adolescents. J Adolesc Health Care. 1988;9(1):22–7. doi: 10.1016/0197-0070(88)90014-9. [DOI] [PubMed] [Google Scholar]
- 32.Uchiyama M, Okawa M, Shirakawa S, Sugishita M, Takahashi K. A polysomnographic study on patients with delayed sleep phase syndrome (DSPS) Jpn J Psychiatry Neurol. 1992;46(1):219–21. doi: 10.1111/j.1440-1819.1992.tb00841.x. [DOI] [PubMed] [Google Scholar]
- 33.Alvarez B, Dahlitz M, Vignau J. The delayed sleep phase syndrome: clinical and investigative findings in 14 subjects. J Neurol Neurosurg Psychiatry. 1992;55:665–70. doi: 10.1136/jnnp.55.8.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uchiyama M, Okawa M, Shibui K, et al. poor recovery sleep after sleep deprivation in delayed sleep phase syndrome. Psychiatry Clin Neurosci. 1999;53(2):195–7. doi: 10.1046/j.1440-1819.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- 35.Uchiyama M, Okawa M, Shibui K, et al. poor compensatory function for sleep loss as a pathogenic factor in patients with delayed sleep phase syndrome. Sleep. 2000;23(4):553–8. [PubMed] [Google Scholar]
- 36.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 37.Dagan Y, Borodkin K. Behavioral and psychiatric consequences of sleep-wake schedule disorders. Dialogues Clin Neurosci. 2005;7(4):357–65. doi: 10.31887/DCNS.2005.7.4/ydagan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohta T, Iwata T, Kayukawa Y, Okada T. Daily activity and persistent sleep-wake schedule disorders. Prog Neuropsychopharmacol Biol Psychiatry. 1992;16(4):529–37. doi: 10.1016/0278-5846(92)90058-m. [DOI] [PubMed] [Google Scholar]
- 39.Regestein QR, Pavlova M. Treatment of delayed sleep phase syndrome. Gen Hosp Psychiatry. 1995;17(5):335–45. doi: 10.1016/0163-8343(95)00062-v. [DOI] [PubMed] [Google Scholar]
- 40.Rosenthal NE, Joseph-Vanderpool JR, Levendosky AA, et al. Phase-shifting effects of bright morning light as treatment for delayed sleep phase syndrome. Sleep. 1990;13(4):354–61. [PubMed] [Google Scholar]
- 41.Chesson AL, Jr, Littner M, Davila D, et al. Practice parameters for the use of light therapy in the treatment of sleep disorders. Standards of Practice Committee. American Academy of Sleep Medicine Sleep. 1999;22(5):641–60. doi: 10.1093/sleep/22.5.641. [DOI] [PubMed] [Google Scholar]
- 42.Warman VL, Dijk DJ, Warman GR, Arendt J, Skene DJ. Phase advancing human circadian rhythms with short wavelength light. Neurosci Lett. 2003;342(1–2):37–40. doi: 10.1016/s0304-3940(03)00223-4. [DOI] [PubMed] [Google Scholar]
- 43.Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003;88(9):4502–5. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- 44.Lewy AJ, Ahmed S, Jackson JM, Sack RL. Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiol Int. 1992;9(5):380–92. doi: 10.3109/07420529209064550. [DOI] [PubMed] [Google Scholar]
- 45.Mundey K, Benloucif S, Harsanyi K, Dubocovich ML, Zee PC. Phase-dependent treatment of delayed sleep phase syndrome with melatonin. Sleep. 2005;28(10):1271–8. doi: 10.1093/sleep/28.10.1271. [DOI] [PubMed] [Google Scholar]
- 46.Dahlitz M, Alvarez B, Vignau J, English J, Arendt J, Parkes JD. Delayed sleep phase syndrome response to melatonin. Lancet. 1991;337(8750):1121–4. doi: 10.1016/0140-6736(91)92787-3. [DOI] [PubMed] [Google Scholar]
- 47.Oldani A, Ferini-Strambi L, Zucconi M, Stankov B, Fraschini F, Smirne S. Melatonin and delayed sleep phase syndrome: ambulatory polygraphic evaluation. Neuroreport. 1994;6(1):132–4. doi: 10.1097/00001756-199412300-00034. [DOI] [PubMed] [Google Scholar]
- 48.Nagtegaal JE, Laurant MW, Kerkhof GA, Smits MG, van der Meer YG, Coenen AM. Effects of melatonin on the quality of life in patients with delayed sleep phase syndrome. J Psychosom Res. 2000;48(1):45–50. doi: 10.1016/s0022-3999(99)00075-6. [DOI] [PubMed] [Google Scholar]
- 49.Buscemi N, Vandermeer B, Hooton N, et al. Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta-analysis. Bmj. 2006;332(7538):385–93. doi: 10.1136/bmj.38731.532766.F6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Revell VL, Burgess HJ, Gazda CJ, Smith MR, Fogg LF, Eastman CI. Advancing human circadian rhythms with afternoon melatonin and morning intermittent bright light. J Clin Endocrinol Metab. 2006;91(1):54–9. doi: 10.1210/jc.2005-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moldofsky H, Musisi S, Phillipson EA. Treatment of a case of advanced sleep phase syndrome by phase advance chronotherapy. Sleep. 1986;9(1):61–5. doi: 10.1093/sleep/9.1.61. [DOI] [PubMed] [Google Scholar]
- 52.Kamei Y, Urata J, Uchiyaya M, et al. Clinical characteristics of circadian rhythm sleep disorders. Psychiatry Clin Neurosci. 1998;52(2):234–5. doi: 10.1111/j.1440-1819.1998.tb01049.x. [DOI] [PubMed] [Google Scholar]
- 53.Jones CR, Campbell SS, Zone SE, et al. Familial advanced sleep-phase syndrome: A short-period circadian rhythm variant in humans. Nat Med. 1999;5(9):1062–5. doi: 10.1038/12502. [DOI] [PubMed] [Google Scholar]
- 54.Reid KJ, Chang AM, Dubocovich ML, Turek FW, Takahashi JS, Zee PC. Familial advanced sleep phase syndrome. Arch Neurol. 2001;58(7):1089–94. doi: 10.1001/archneur.58.7.1089. [DOI] [PubMed] [Google Scholar]
- 55.Ando K, Kripke DF, Ancoli-Israel S. Estimated prevalence of delayed and advanced sleep phase syndromes. Sleep Res. 1995;24:509. [Google Scholar]
- 56.Duffy JF, Czeisler CA. Age-related change in the relationship between circadian period, circadian phase, and diurnal preference in humans. Neurosci Lett. 2002;318:117–20. doi: 10.1016/s0304-3940(01)02427-2. [DOI] [PubMed] [Google Scholar]
- 57.Ancoli-Israel S, Kripke DF. Prevalent sleep problems in the aged. Biofeedback Self Regul. 1991;16(4):349–59. doi: 10.1007/BF00999989. [DOI] [PubMed] [Google Scholar]
- 58.Moore RY. A clock for the ages. Science. 1999;284(5423):2102–3. doi: 10.1126/science.284.5423.2102. [DOI] [PubMed] [Google Scholar]
- 59.Naylor E, Penev PD, Orbeta L, et al. Daily social and physical activity increases slow-wave sleep and daytime neuropsychological performance in the elderly. Sleep. 2000;23(1):87–95. [PubMed] [Google Scholar]
- 60.Satoh K, Mishima K, Inoue Y, Ebisawa T, Shimizu T. Two pedigrees of familial advanced sleep phase syndrome in Japan. Sleep. 2003;26(4):416–7. doi: 10.1093/sleep/26.4.416. [DOI] [PubMed] [Google Scholar]
- 61.Toh KL, Jones CR, He Y, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291(5506):1040–3. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- 62.Xu Y, Padiath QS, Shapiro RE, et al. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature. 2005;434(7033):640–4. doi: 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
- 63.Lack L, Wright H, Kemp K, Gibbon S. The treatment of early-morning awakening insomnia with 2 evenings of bright light. Sleep. 2005;28(5):616–23. doi: 10.1093/sleep/28.5.616. [DOI] [PubMed] [Google Scholar]
- 64.Campbell SS, Dawson D, Anderson MW. Alleviation of sleep maintenance insomnia with timed exposure to bright light. J Am Geriatr Soc. 1993;41(8):829–36. doi: 10.1111/j.1532-5415.1993.tb06179.x. [DOI] [PubMed] [Google Scholar]
- 65.Murphy PJ, Campbell SS. Enhanced performance in elderly subjects following bright light treatment of sleep maintenance insomnia. J Sleep Res. 1996;5(3):165–72. doi: 10.1046/j.1365-2869.1996.t01-1-00001.x. [DOI] [PubMed] [Google Scholar]
- 66.Lack L, Wright H. The effect of evening bright light in delaying the circadian rhythms and lengthening the sleep of early morning awakening insomniacs. Sleep. 1993;16(5):436–43. doi: 10.1093/sleep/16.5.436. [DOI] [PubMed] [Google Scholar]
- 67.Sack RL, Lewy AJ, Blood ML, Keith LD, Nakagawa H. Circadian rhythm abnormalities in totally blind people: incidence and clinical significance. J Clin Endocrinol Metab. 1992;75(1):127–34. doi: 10.1210/jcem.75.1.1619000. [DOI] [PubMed] [Google Scholar]
- 68.Miles LE, Raynal DM, Wilson MA. Blind man living in normal society has circadian rhythms of 24.9 hours. Science. 1977;198(4315):421–3. doi: 10.1126/science.910139. [DOI] [PubMed] [Google Scholar]
- 69.Martens H, Endlich H, Hildebrandt G. Sleep/wake distribution in blind subjects with and without sleep complaints. Sleep Research. 1990;9:398. [Google Scholar]
- 70.Lockley SW, Skene DJ, Butler LJ, Arendt J. Sleep and activity rhythms are related to circadian phase in the blind. Sleep. 1999;22(5):616–23. doi: 10.1093/sleep/22.5.616. [DOI] [PubMed] [Google Scholar]
- 71.McArthur AJ, Lewy AJ, Sack RL. Non-24-hour sleep-wake syndrome in a sighted man: circadian rhythm studies and efficacy of melatonin treatment. Sleep. 1996;19(7):544–53. doi: 10.1093/sleep/19.7.544. [DOI] [PubMed] [Google Scholar]
- 72.Czeisler CA, Shanahan TL, Klerman EB, et al. Suppression of melatonin secretion in some blind patients by exposure to bright light. N Engl J Med. 1995;332(1):6–11. doi: 10.1056/NEJM199501053320102. [DOI] [PubMed] [Google Scholar]
- 73.Freedman MS, Lucas RJ, Soni B, et al. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284(5413):502–4. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- 74.Uchiyama M, Shibui K, Hayakawa T, et al. Larger phase angle between sleep propensity and melatonin rhythms in sighted humans with non-24-hour sleep-wake syndrome. Sleep. 2002;25(1):83–8. doi: 10.1093/sleep/25.1.83. [DOI] [PubMed] [Google Scholar]
- 75.Oren DA, Wehr TA. Hypernyctohemeral syndrome after chronotherapy for delayed sleep phase syndrome. N Engl J Med. 1992;327(24):1762. doi: 10.1056/NEJM199212103272417. [DOI] [PubMed] [Google Scholar]
- 76.Sack RL, Brandes RW, Kendall AR, Lewy AJ. Entrainment of free-running circadian rhythms by melatonin in blind people. New England Journal Medicine. 2000;343(15):1070–7. doi: 10.1056/NEJM200010123431503. [DOI] [PubMed] [Google Scholar]
- 77.Lewy AJ, Bauer VK, Hasler BP, Kendall AR, Pires ML, Sack RL. Capturing the circadian rhythms of free-running blind people with 0.5 mg melatonin. Brain Res. 2001;918(1–2):96–100. doi: 10.1016/s0006-8993(01)02964-x. [DOI] [PubMed] [Google Scholar]
- 78.Dagan Y, Ayalon L. Case study: psychiatric misdiagnosis of non-24-hours sleep-wake schedule disorder resolved by melatonin. J Am Acad Child Adolesc Psychiatry. 2005;44(12):1271–5. doi: 10.1097/01.chi.0000181040.83465.48. [DOI] [PubMed] [Google Scholar]
- 79.Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol Psychiatry. 1990;27(6):563–72. doi: 10.1016/0006-3223(90)90523-5. [DOI] [PubMed] [Google Scholar]
- 80.Hoogendijk WJ, van Someren EJ, Mirmiran M, et al. Circadian rhythm-related behavioral disturbances and structural hypothalamic changes in Alzheimer’s disease. Int Psychogeriatr. 1996;8(Suppl 3):245–52. doi: 10.1017/s1041610297003426. discussion 69–72. [DOI] [PubMed] [Google Scholar]
- 81.Wagner DR. Disorders of the circadian sleep-wake cycle. Neurol Clin. 1996;14(3):651–70. doi: 10.1016/s0733-8619(05)70278-4. [DOI] [PubMed] [Google Scholar]
- 82.Okawa M, Mishima K, Hishikawa Y, Hozumi S, Hori H, Takahashi K. Circadian rhythm disorders in sleep-waking and body temperature in elderly patients with dementia and their treatment. Sleep. 1991;14(6):478–85. doi: 10.1093/sleep/14.6.478. [DOI] [PubMed] [Google Scholar]
- 83.Schnelle JF, Cruise PA, Alessi CA, Ludlow K, al-Samarrai NR, Ouslander JG. Sleep hygiene in physically dependent nursing home residents: behavioral and environmental intervention implications. Sleep. 1998;21(5):515–23. [PubMed] [Google Scholar]
- 84.Van Someren EJ, Swaab DF, Colenda CC, Cohen W, McCall WV, Rosenquist PB. Bright light therapy: improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol Int. 1999;16(4):505–18. doi: 10.3109/07420529908998724. [DOI] [PubMed] [Google Scholar]
- 85.Ancoli-Israel S, Martin JL, Kripke DF, Marler M, Klauber MR. Effect of light treatment on sleep and circadian rhythms in demented nursing home patients. J Am Geriatr Soc. 2002;50(2):282–9. doi: 10.1046/j.1532-5415.2002.50060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Van Someren EJ, Kessler A, Mirmiran M, Swaab DF. Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biological Psychiatry. 1997;41:955–63. doi: 10.1016/S0006-3223(97)89928-3. [DOI] [PubMed] [Google Scholar]
- 87.Gehrman PR, Connor D, Marler M, et al. Bright light improves sleep but melatonin does not in severe alzheimer’s disease. Sleep. 2002;25:A438. [Google Scholar]
- 88.Mishima K, Okawa M, Hishikawa Y, Hozumi S, Hori H, Takahashi K. Morning bright light therapy for sleep and behavior disorders in elderly patients with dementia. Acta Psychiatr Scand. 1994;89(1):1–7. doi: 10.1111/j.1600-0447.1994.tb01477.x. [DOI] [PubMed] [Google Scholar]
- 89.Pillar G, Shahar E, Peled N, Ravid S, Lavie P, Etzioni A. Melatonin improves sleep-wake patterns in psychomotor retarded children. Pediatr Neurol. 2000;23(3):225–8. doi: 10.1016/s0887-8994(00)00161-2. [DOI] [PubMed] [Google Scholar]
- 90.Singer C, Tractenberg RE, Kaye J, et al. A multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer’s disease. Sleep. 2003;26(7):893–901. doi: 10.1093/sleep/26.7.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harma MI, Hakola T, Akerstedt T, Laitinen JT. Age and adjustment to night work. Occup Environ Med. 1994;51(8):568–73. doi: 10.1136/oem.51.8.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Akerstedt T. Shift work and disturbed sleep/wakefulness. Occup Med (Lond) 2003;53(2):89–94. doi: 10.1093/occmed/kqg046. [DOI] [PubMed] [Google Scholar]
- 93.Knauth P, Landau K, Droge C, Schwitteck M, Widynski M, Rutenfranz J. Duration of sleep depending on the type of shift work. Int Arch Occup Environ Health. 1980;46(2):167–77. doi: 10.1007/BF00378195. [DOI] [PubMed] [Google Scholar]
- 94.Akerstedt T. Increased risk of accidents during night shift. An underestimated problem are fatigue-induced accidents. Lakartidningen. 1995;92(20):2103–4. [PubMed] [Google Scholar]
- 95.Scott AJ. Shift work and health. Prim Care. 2000;27(4):1057–79. doi: 10.1016/s0095-4543(05)70189-5. [DOI] [PubMed] [Google Scholar]
- 96.Presser HB. Towards a 24 Hour Economy. Science. 1999;284:1778–9. [Google Scholar]
- 97.Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27(8):1453–62. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 98.Moore-Ede MC, Richardson GS. Medical implications of shift-work. Annu Rev Med. 1985;36:607–17. doi: 10.1146/annurev.me.36.020185.003135. [DOI] [PubMed] [Google Scholar]
- 99.Reinberg A, Vieux N, Andlauer P. Tolerance to shiftwork: a chronobiological approach. Adv Biol Psychiatry. 1983;11:35. [Google Scholar]
- 100.Burgess HJ, Sharkey KM, Eastman CI. Bright light, dark and melatonin can promote circadian adaptation in night shift workers. Sleep Med Rev. 2002;6(5):407–20. [PubMed] [Google Scholar]
- 101.Dawson D, Campbell SS. Timed exposure to bright light improves sleep and alertness during simulated night shifts. Sleep. 1991;14(6):511–6. doi: 10.1093/sleep/14.6.511. [DOI] [PubMed] [Google Scholar]
- 102.Dawson D, Encel N, Lushington K. Improving adaptation to simulated night shift: timed exposure to bright light versus daytime melatonin administration. Sleep. 1995;18(1):11–21. doi: 10.1093/sleep/18.1.11. [DOI] [PubMed] [Google Scholar]
- 103.Sharkey KM, Fogg LF, Eastman CI. Effects of melatonin administration on daytime sleep after simulated night shift work. J Sleep Res. 2001;10(3):181–92. doi: 10.1046/j.1365-2869.2001.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Crowley SJ, Lee C, Tseng CY, Fogg LF, Eastman CI. Combinations of bright light, scheduled dark, sunglasses, and melatonin to facilitate circadian entrainment to night shift work. J Biol Rhythms. 2003;18(6):513–23. doi: 10.1177/0748730403258422. [DOI] [PubMed] [Google Scholar]
- 105.Boivin DB, James FO. Intermittent exposure to bright light in field conditions. Aviat Space Environ Med. 2004;75(3 Suppl):A158–60. [PubMed] [Google Scholar]
- 106.Purnell MT, Feyer AM, Herbison GP. The impact of a nap opportunity during the night shift on the performance and alertness of 12-h shift workers. J Sleep Res. 2002;11(3):219–27. doi: 10.1046/j.1365-2869.2002.00309.x. [DOI] [PubMed] [Google Scholar]
- 107.Walsh JK, Muehlbach MJ, Schweitzer PK. Hypnotics and caffeine as countermeasures for shiftwork-related sleepiness and sleep disturbance. J Sleep Res. 1995;4(S2):80–3. doi: 10.1111/j.1365-2869.1995.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 108.Czeisler CA, Walsh JK, Roth T, et al. Modafinil for excessive sleepiness associated with shift-work sleep disorder. N Engl J Med. 2005;353(5):476–86. doi: 10.1056/NEJMoa041292. [DOI] [PubMed] [Google Scholar]
- 109.Walsh JK, Randazzo AC, Stone KL, Schweitzer PK. Modafinil improves alertness, vigilance, and executive function during simulated night shifts. Sleep. 2004;27(3):434–9. doi: 10.1093/sleep/27.3.434. [DOI] [PubMed] [Google Scholar]
- 110.Winget CM, DeRoshia CW, Markley CL, Holley DC. A review of human physiological and performance changes associated with desynchronosis of biological rhythms. Aviat Space Environ Med. 1984;55(12):1085–96. [PubMed] [Google Scholar]
- 111.Boulos Z, Campbell SS, Lewy AJ, Terman M, Dijk DJ, Eastman CI. Light treatment for sleep disorders: consensus report. VII. Jet lag. J Biol Rhythms. 1995;10(2):167–76. doi: 10.1177/074873049501000209. [DOI] [PubMed] [Google Scholar]
- 112.Herxheimer A, Waterhouse J. The prevention and treatment of jet lag. Bmj. 2003;326(7384):296–7. doi: 10.1136/bmj.326.7384.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Burgess HJ, Crowley SJ, Gazda CJ, Fogg LF, Eastman CI. Preflight adjustment to eastward travel: 3 days of advancing sleep with and without morning bright light. J Biol Rhythms. 2003;18(4):318–28. doi: 10.1177/0748730403253585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Beaumont M, Batejat D, Pierard C, et al. Caffeine or melatonin effects on sleep and sleepiness after rapid eastward transmeridian travel. J Appl Physiol. 2004;96(1):50–8. doi: 10.1152/japplphysiol.00940.2002. [DOI] [PubMed] [Google Scholar]
- 115.Waterhouse J, Reilly T, Atkinson G. Jet-lag. Lancet. 1997;350(9091):1611–6. doi: 10.1016/S0140-6736(97)07569-7. [DOI] [PubMed] [Google Scholar]
- 116.Eastman CI, Gazda CJ, Burgess HJ, Crowley SJ, Fogg LF. Advancing circadian rhythms before eastward flight: a strategy to prevent or reduce jet lag. Sleep. 2005;28(1):33–44. doi: 10.1093/sleep/28.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Herxheimer A, Petrie KJ. Melatonin for the prevention and treatment of jet lag. Cochrane Database Syst Rev. 2006;(2):CD00484. doi: 10.1002/14651858.CD001520. [DOI] [PubMed] [Google Scholar]
- 118.Suhner A, Schlagenhauf P, Johnson R, Tschopp A, Steffen R. Comparative study to determine the optimal melatonin dosage form for the alleviation of jet lag. Chronobiol Int. 1998;15(6):655–66. doi: 10.3109/07420529808993201. [DOI] [PubMed] [Google Scholar]
- 119.Jamieson AO, Zammit GK, Rosenberg RS, Davis JR, Walsh JK. Zolpidem reduces the sleep disturbance of jet lag. Sleep Med. 2001;2(5):423–30. doi: 10.1016/s1389-9457(00)00073-3. [DOI] [PubMed] [Google Scholar]
- 120.Suhner A, Schlagenhauf P, Hofer I, Johnson R, Tschopp A, Steffen R. Effectiveness and tolerability of melatonin and zolpidem for the alleviation of jet lag. Aviat Space Environ Med. 2001;72(7):638–46. [PubMed] [Google Scholar]