Abstract
Embryonic stem cells represent a potentially unlimited cell source for tissue engineering applications. However, in order to be used for such applications, embryonic stem cells differentiation must be controlled to only the desired lineages. In this study, we examine the effects of nanofibrous architecture and biochemical cues on the osteogenic differentiation of embryonic stem cells compared to the more traditional architecture without the nanofibrous features in two dimensions (thin matrix or flat films) and three dimensions (scaffolds) in vitro. After three weeks of culture the nanofibrous thin matrices were capable of supporting mRNA expression of osteogenic differentiation markers in embryonic stem cells without osteogenic supplements, while solid films required osteogenic supplements and growth factors to achieve mRNA expression of osteogenic differentiation markers. Nanofibrous scaffolds substantially enhanced mRNA expression of osteogenic differentiation markers compared to solid-walled scaffolds, nanofibrous thin matrices or solid films. After 4 weeks of culture, nanofibrous scaffolds were found to contain 3 times more calcium and stronger osteocalcin stain throughout the scaffolds than the solid-walled scaffolds. Overall, the nanofibrous architecture enhanced the osteogenic differentiation and mineralization of embryonic stem cells compared to the solid-walled architecture in both two and three dimensional cultures.
Introduction
The number of surgical procedures to correct bone defects has been increasing over the past 10 years [1]. Currently, these procedures utilize autografts, allografts or metallic and ceramic implants to correct the bone defect. Each of these options has its own drawbacks such as donor site morbidity, pathogen transmission, and mismatching material properties with the native bone respectively [2, 3]. As an alternative to these procedures, tissue engineering has emerged to create de novo tissue by growing cells on three-dimensional (3D) scaffolding [4, 5]. Ideally, this scaffolding should recapitulate the key structural and biochemical signals of the tissue’s natural extracellular matrix (ECM) [6], which is primarily composed of type I collagen in bone. Several fabrication methods are capable of mimicking the size scale of type I collagen [7]. However most are incapable of incorporating a designed 3D macro pore structure. We have developed a synthetic nanofibrous (NF) scaffold capable of well-defined anatomical shapes and pore structures [8].
Once a bone defect reaches a certain size, cells in addition to the scaffolds are required to generate functional tissue. Currently, most cells used for this type of tissue engineering are isolated from an autologous source [9]. This yields a limited number of cells that may lose the ability to generate the desired tissue during cell expansion culture prior to seeding them onto the scaffolding. Embryonic stem cells (ESC) represent a potential advance in cell sourcing for tissue engineering because they proliferate longer than other types of stem cells and possess the ability to differentiate to any tissue type within the body.
Although nano-scale architecture affects cellular proliferation, movement, and orientation [10], few studies have examined the effects of such architecture on ESC [11, 12]. Studies using ESC for tissue engineering have focused mostly on the addition of biochemical cues to control the ESC differentiation [13–16]. However, during embryonic development both the ECM and the biochemical cues play a vital role in tissue development [17]. In this study, we will examine the effects of both the NF architecture and biochemical cues on the osteogenic differentiation of ESC in both two dimensional (2D) and 3D cultures.
Materials and Methods
Poly(L-lactic acid) (PLLA) with an inherent viscosity of 1.6 dl/g was purchased from Alkermes (Medisorb, Cambridge, Massachusetts) and used without further purification. Wax and polysulphonamide for 3D printing were purchased from Solidscape Inc. (Merrimack, New Hampshire). Cyclohexane, dioxane, ethanol, hexane, and methanol were purchased from Fisher Scientific (Pittsburgh, Pennsylvania). Dubecco’s Modified Eagle Media (DMEM), 0.5M EDTA, trypsin, Hank’s buffered salt solution (HBSS) and PCR primers were obtained from Invitrogen (Carlsbad, CA). Fetal Bovine Serum was obtained from Harlan Biological Laboratory (Indianapolis, IN). Human recombinant leukemia inhibitory factor (LIF), and Neuronal Class III β-Tubulin (TUJ1) antibody and goat serum were obtained from Sigma (St. Louis, MO). Human transforming growth factor-beta1 (TGF-β1), insulin-like growth factor I (IGF) and bone morphogenic protein-2 (BMP-2) were obtained from Peprotech (Rocky Hill, New Jersey). Osteocalcin antibody and all secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Both RNeasy Mini Kit and Rnase-Free DNase set were obtained from Qiagen (Valencia, California). TaqMan reverse transcription reagents, real-time PCR primers, and TaqMan Universal PCR Master mix were obtained from Applied Biosystems (Foster City, California). All other chemicals were obtained from Sigma Chemical Company (St. Louis, MO) unless otherwise noted.
Two-Dimensional Thin Matrix and Film Preparation for Cell Culture
PLLA was dissolved in tetrahydrofuran at 60°C to make a 10% (wt/v) PLLA solution. The NF PLLA matrix (thickness~40μm) was fabricated by first casting 0.4 mL of the PLLA solution on a glass support plate which had been pre-heated at 45°C for 10 min and then sealing the polymer solution on the glass support plate by covering it with another pre-heated glass plate. The polymer solution was phase separated at −20°C for 2 hrs and then immersed into an ice-water mixture to exchange tetrahydrofuran for 24 hrs. The matrix was washed with distilled water at room temperature for 24 hrs with water changed every 8 hrs. The matrix was then freeze-dried.
The matrices were cut to fit into a 35 mm Petri dish and secured in place with a disk of silicone elastomer from Dow Corning (Midland, MI) containing a 1.5 mm by 1.5 mm opening. The matrices were sterilized with ethylene oxide, wet with HBSS 2 times for 0.5 hrs each and rinsed with differentiation media (DMEM supplemented with 20% FBS 10−4M β-mercaptoethanol, and 1.33μg/ml HEPES) for 1 hr.
PLLA thin flat (solid) films were fabricated in a similar manner excluding the phase separation step. Instead, the solvent was evaporated at room temperature in a fume hood. The thin solid films were then treated similar to the NF matrices.
Three-Dimensional Scaffold Preparation for Cell Culture
Scaffolds were fabricated as previously described [8]. Briefly, negative molds were designed and converted into stereolithography data using Rhinoceros software (Robert McNeel & Associates, Seattle, Washington), and then imported into Modelworks software (Solidscape) to convert the files for 3D printing. Molds were fabricated in a layer-by-layer fashion with the molten wax and polysulphonamide printed separately using a Modelmaker II (Solidscape). The polysulphonamide was later dissolved in ethanol.
External dimensions of the scaffolds were (L×W×H) 6.6×6.6×2.45 mm. Internally, the scaffold contains partially overlapping orthogonally stacked layers of parallel rectangular channels with open channels of (W×H) 400×300 μm and closed struts of (W×H) 350×300 μm.
For NF scaffolds, a 9% (wt/v) solution of PLLA in 4:1 (v/v) dioxane:methanol was stirred at 60 °C until homogeneous. Dioxane was dripped into the mold to wet the wax surface, the polymer solution was cast into the mold, and the polymer/mold composite was phase separated overnight at −20 °C. The solvent was extracted with cold ethanol (−20 °C) for 1 d and ice-cold water for 1 d. Excess polymer was trimmed with a razor blade, and the polymer/mold composite was washed in 37 °C cyclohexane to dissolve the wax mold, followed by washings in 37 °C ethanol and water, and subsequent freeze-drying.
For SW scaffolds, a 9% (wt/v) PLLA/dioxane solution was similarly cast and phase separated. The polymer/mold composites were lyophilized at −5 to −10 °C to remove dioxane crystals. Excess polymer was trimmed with a razor blade and wax molds were dissolved away similarly to those in NF samples.
D3 Culture and Seeding
D3 mouse ESC [18] were cultured on 0.1% gelatin-coated tissue culture flasks in ESC media (DMEM supplemented with 10% FBS, 10−4M β-mercaptoethanol, 0.224 μg/ml L-glutamine, 1.33 μg/ml HEPES, and 1,000 units/ml human recombinant LIF). Media formulations used are provided in Table 1.
Table 1.
Media formulations used for ESC culture and differentiation
| Media Title | Media Formulations |
|---|---|
| ESC Media | DMEM supplemented with 10% FBS, 10−4M β-mercaptoethanol, 0.224 μg/ml L-glutamine, 1.33 μg/ml HEPES and 1,000 units/ml human recombinant LIF. |
| EB Media | DMEM supplemented with 10% FBS, 10−4M β-mercaptoethanol, 0.224 μg/ml L-glutamine, and 1.33 μg/ml HEPES. For osteogenic cultures 1 μM dexamethasone, 50 mg/mL ascorbic acid and 10 mM β-glycerol phosphate were added. |
| Differentiation Media | DMEM supplemented with 20% FBS, 10−4M β-mercaptoethanol, 0.224 μg/ml L-glutamine, and 1.33 μg/ml HEPES. |
| Osteogenic Media | DMEM supplemented with 20% FBS, 10−4M β-mercaptoethanol, 0.224 μg/ml L-glutamine, 1.33 μg/ml HEPES 1 μM dexamethasone, 50 mg/mL ascorbic acid and 10 mM β-glycerol phosphate. |
| BMP Media | DMEM supplemented with 20% FBS, 10−4M β-mercaptoethanol, 0.224 μg/ml L-glutamine, 1.33 μg/ml HEPES 1 μM dexamethasone, 50 mg/mL ascorbic acid, 10 mM β-glycerol phosphate and 25ng/mL of BMP-2 for days 6–9 of differentiation. |
| TBI Media | DMEM supplemented with 20% FBS, 10−4M β-mercaptoethanol, 0.224 μg/ml L-glutamine, 1.33 μg/ml HEPES1 μM dexamethasone, 50 mg/mL ascorbic acid, 10 mM β-glycerol phosphate 2.5 ng/mL of TGF-β1 for 2–5 days of differentiation, 25 ng/mL of BMP-2 for days 6–9 of differentiation and 100ng/mL of IGF-1 for days 10–13 of differentiation. |
Embryoid bodies (EBs) were formed by seeding 3×106 cells into a 60 mm polystyrene dish containing EB media (ESC media without LIF supplement with the addition of 1 μM dexamethasone, 50 mg/mL ascorbic acid and 10 mM β-glycerol phosphate in osteogenic cultures). The media was changed every 2 to 3 days. After 5 days, EBs were dissociated with 0.25% trypsin/1mM EDTA. 1.5×105 EB-derived cells were seeded on each of the prepared 2D matrices, films or 0.1% gelatin-coated tissue culture dish controls, while 2×106 EB-derived cells were seeded onto each 3D scaffold. Upon seeding, cells were cultured in differentiation media, osteogenic media, BMP media or TBI media (Table 1 contains media formulations). TBI media was used unless otherwise stated. On the thin matrices and controls the media was changed 12 hrs after seeding and then every other day for the remainder of the culture period. For scaffolds, the media was changed every 12 hrs for 72 hrs. The scaffolds were then transferred from the Telfon seeding trays to 6-well plates and the media was changed every other day.
PCR and Real time PCR
Total RNA was isolated using an RNeasy Mini Kit with Rnase-Free DNase set according to the manufacturer’s protocol after thin matrices and scaffolds were mechanically homogenized with a Tissue-Tearor (BioSpec Products, Bartlesville, OK) while cells cultured on gelatin-coated tissue culture plate controls were harvested with a cell scraper. The cDNA was made using a Geneamp PCR (Applied Biosystems) with TaqMan reverse transcription reagents and 10 min incubation at 25 °C, 30 min reverse transcription at 48 °C, and 5 min inactivation at 95 °C. 5μL of each reaction was subject to PCR using AmpliTaq Gold DNA polymerase (Applied Biosystems) for each of the following: collagen type I ( 5′-gaagtcagctgcatacac-3′ and 5′-aggaagtccaggctgtcc-3′); runx2 (5′-ccgcacgacaaccgcaccat-3′ and 5′-cgctccggcccacaaatctc-3′); bone sailoprotein (5′-gtcaacggcaccagcaccaa-3′ and 5′-gtagctgtattcgtcctcat-3′); osteocalcin (5′-cggccctgagtctgacaaa-3′ and 5′-accttattgccctcctgcctt-3′) and β actin ( 5′-caggattccatacccaagaag-3′ and 5′-aaccctaaggccaaccgtg-3′). The cycling conditions used were 94°C for 5mins followed by 94°C for 30s, 55°C for 60s, 72°C for 60s 35 times for β actin, and 94°C for 30s, 55°C for 60s, 72°C for 60s 30 times for runx2, bone sialoprotein, and osteocalcin. These amplifications were followed by a 10min extension at 72°C.
Real-time PCR was set up using TaqMan Universal PCR Master mix and specific primer sequence for collagen type I, runx2, bone sialoprotien, osteocalcin and β-actin with 2 min incubation at 50°C, a 10 min Taq Activation at 95°C, and 50 cycles of denaturation for 15 s at 95°C followed by an extension for 1 min at 72°C on an ABI Prism 7500 Real-Time PCR System (Applied Biosystems). Target genes were normalized against β-actin.
Immunofluorescence and Histological Staining
Cells growing on NF matrices, flat (solid) films and gelatin coated tissue culture plastic controls were fixed with 2% paraformaldehyde/PBS, washed, and stored at 4°C in PBS. For histological analysis, cells grown on scaffolds were fixed in 10% neutral buffered formalin solution (Sigma, St. Louis, Missouri), dehydrated through an ethanol gradient, and embedded in paraffin. Samples were cut as 5 μm sections. The paraffin was dissolved with xylene and the sections were rehydrated through an ethanol gradient. The sections were then incubated in 0.5% pepsin for 10min at 30°C for antigen retrieval. Nonspecific antibody binding was blocked by incubating in 10% goat serum, then the matrices and control were exposed to TUJ1 (1:250) or Osteocalcin (1:50) antibodies, followed by appropriate secondary antibodies conjugated to FITC (TUJ1) or TRITC (ostoecalcin). DAPI was used to stain the cell nuclei.
For Alizarin Red S staining, the matrices, controls, and scaffold sections were fixed by the same method and then stained with 40mM Alizarin Red S solution, pH 4.2 at room temperature for 10 min. Thin matrices and controls were then rinsed 5 times in distilled water and washed 3 times in PBS on an orbital shaker at 40 rpm for 5 minutes each to reduce nonspecific binding. Scaffold sections were dehydrated in acetone and rinsed in xylene before mounting with permamount. Scaffold sections were also stained with hematoxylin and eosin-phloxine.
Western Blotting Analysis
Scaffolds were treated with ethanol and PBS as described above for cell culture. Scaffolds were then incubated with differentiation medium or oritinal fetal bovine serum. Scaffolds were quickly washed with PBS for 2 times (1 min each), cut into pieces and transferred to 1.5ml tubes. 600ul PBS was added, and the scaffolds were washed for three times. PBS was removed, and the scaffolds were centrifuged for 1 min at 12,000 rpm for 2 times to remove any liquid remained. 100μl 1% SDS was added and incubated for 1 hr. This process was repeated twice. The 3 samples were pooled to form a total collection of 300 μl sample. For microBCA (Pierce, Rockford, IL), 50 μl of the collection sample was used (n=3), while 30 μl of the collection sample was used for each gel. Western blot analysis was conducted as previously described [19]. Briefly, the recovered serum protein samples were subject to fractionation through 4–12% SDS-polyacrylamide gel electrophoresis (PAGE). The fractionated proteins were transferred to a PVDF membrane (Sigma). The blots were washed with TBST (10 mM Tris-HCl, 150 mM NaCl, 0.05% Tween-20, pH 8.0), and blocked with Blotto (5% nonfat milk in TBST) at room temperature for 1 h. The blots were incubated in anti-bovine fibronectin polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at room temperature for 1 h. After being washed with TBST, the blots were incubated in anti-goat immunoglobulin G-horseradish peroxidase-conjugated antibody (Sigma), and then in chemiluminescence reagent (SuperSignal West Dura; Pierce). The relative densities of the protein bands were analyzed with QualityOne (Biorad).
Mineral Quantification
After 4 weeks of culture, scaffolds for mineralization quantification were washed three times for 5 min each in double-distilled water and then homogenized with a Tissue-Tearor in 1 mL of double-distilled water. Samples were then incubated in 0.5 M acetic acid overnight. The total calcium content of each scaffold was determined by using the o-cresolphthalein-complexone method following the manufacturer’s instructions (Calcium LiquiColor, Stanbio Laboratory, Boerne, Texas).
Collagen Quantification
The collagen content of the scaffolds was determined using a colormetric hydroxyproline quantification method [20]. Briefly, scaffolds for collagen quantification were washed three times for 5 min each in double-distilled water and then homogenized with a Tissue-Tearor in 500 μL of double-distilled water. 600 μL of 12N hydrochloric acid was added and the samples were incubated at 100–110°C for 18–24 hrs. 10 μL of methyl red was added and the samples were neutralized to a PH between 6–7 with sodium hydroxide and hydrochloric acid. 320 μL of Chloramine T assay solution was added to 640 μL of the sample, which was placed on an orbital shaker for 20 min at 100 rpm. 320 μL of dimethylaminobenzaldehyde assay solution was added and the samples placed at 50°C for 30min. After which, the samples were read at 550 nm. Collagen content was estimated assuming a ratio of 1 μg hydroxyproline: 7.46μg collagen [21].
Statistical Analysis
All experiments were conducted at least 3 times. All quantifiable data is reported with the mean and standard deviation. Student t-tests were performed where applicable. Signficance was set at a p-value of less than 0.05.
Results
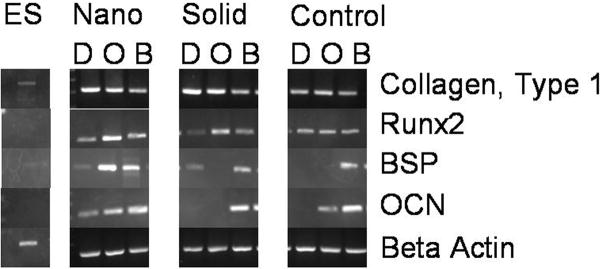
After 3 weeks of culture, ESC cultured on NF matrices in basic differentiation media without the addition of any osteogenic cues expressed osteocalcin and bone sialoprotein (Figure 1). While the addition of osteogenic factors (ascorbic acid, β-glycerolphosphate, and dexamethasone) to the media increased the expression of the bone markers in the ESC on the NF matrices, only the addition of BMP-2 to the osteogenic media lead to the expression of both osteocalcin and bone sialoprotein in the ESC cultured on the flat (solid) films and control substrate (gelatin-coated tissue culture polystyrene). However, bone sialoprotein was expressed by ESC on the solid films on without any osteogenic supplementation. Runx2, an early bone marker, exhibited increased espression in ESC on the NF matrices in all media compared to the ESC on the solid films in all media. ESC on the NF matrices expressed Runx2 at a similar level to ESC on control substrate in differentiation media, while ESC on the NF matrices exhibited increased Runx2 expression compared to ESC on the control substrate in osteogenic and BMP media. These results indicate that the NF matrices promote osteogenic differentiation which can then be enhanced by the addition of biochemical cues. However, the flat (solid) films and controls rely more on biochemical cues to drive the ESC differentiation toward the desired lineage.
Figure 1.
Expression of bone differentiation markers on nanofibrous (Nano) thin matrices, flat films (Solid) and gelatin-coated tissue culture plastic (Control) with various media supplementations ((D) basic differentiation media, (O) osteogenic media, and (B) BMP media) after 3 weeks of culture.
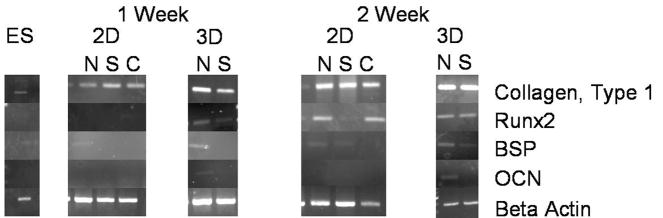
ESC were then cultured on the 2D NF thin matrices or solid films and 3D NF or SW scaffolds in BMP-media, which can lead to the eventual osteogenic differentiation of the ESC. The effects of the different culture conditions on the differentiation of the ESC were examined (Figure 2). After the first week of culture, Runx2, an early osteogenic marker, was expressed by ESC on the 3D scaffolds, but not on any of the 2D NF thin matrices or flat (solid) films until week 2 of culture. Similarly, bone sialoprotein and osteocalcin were expressed by the ESC on the 3D NF scaffold after 1 week of culture, but not by ESC on the 2D thin matrices, 2D flat (solid) films or the 3D SW scaffold until the 2nd week. This indicates that 3D culture promotes osteogenic differentiation compared to 2D culture. The NF architecture also expedites ESC differentiation over smooth surfaces of either 2D flat (solid) films or 3D SW scaffolds.
Figure 2.
Expression of bone differentiation markers on 2D nanofibrous thin matrices (N), flat films (S) and control (C), as well as on 3D nanofibrous (N) and solid-walled (S) scaffolds in BMP media over 2 weeks of culture.
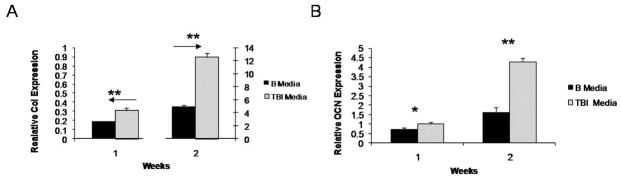
Although BMP media leads to appreciable differentiation of ESC, it does not produce the development of tissue like cellular growth within scaffolds. To increase the production of a more tissue-like ECM, IGF-1 and TGF-β1 were added to the media to create TBI media. After 1 week of culture ESC cultured in TBI media expressed more collagen type I and osteocalcin than cells cultured in BMP media (Figure 3). Increased expression of collagen type I (7 times) and osteocalcin (2.6 times) in ESC cultured in TBI media compared to BMP media continued after two weeks of culture. TBI was therefore used in the remainder of the experiments.
Figure 3.
Expression of bone differentiation markers on nanofibrous scaffolds: (A) type I collagen, and (B) osteocalcin expression in BMP media and TBI media over 2 weeks. * denotes a p<0.05. ** denotes a p<0.01.
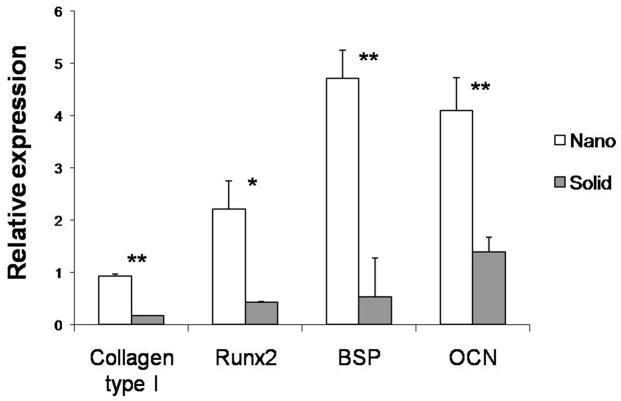
After 4 weeks of culture in TBI media, the expression of bone differentiation markers was examined on scaffolds (Figure 4). ESC growing on NF scaffolds expressed higher levels of type I collagen (5.5 times), Runx2 (5 times), bone sialoprotein (8.5 times), and osteocalcin (2.9 times) compared to the SW scaffolds.
Figure 4.
Expression of bone differentiation markers on nanofibrous (Nano) and solid-walled (Solid) scaffolds after 4 weeks of culture in TBI media. * denotes a p<0.05. ** denotes a p<0.01.
The appearance of these late stage bone markers coincides with the mineralization of the matrix during bone formation. The NF thin matrices and films (Figure 5) were stained for calcium to examine mineral deposition on each of the surfaces. After one week of culture, there was significantly more mineralization on the NF matrices compared to the flat (solid) films. It is only after 3 weeks of culture that the flat (solid) films appear to have a similar amount of mineral to the NF matrices after one week of culture. This result indicates that the NF matrices are more suitable to the promotion of mineralization. NF matrix (control) cultured in media for the same time periods without cells did not show significant staining suggesting that the mineralization on the NF matrix is due to active cellular deposition and not biomimic absorption from the media. This indicates a more mature osteogenic cell population has developed on the NF matrix than on either the flat (solid) films or the control surface and suggests that the NF matrix better promotes the differentiation of ESC toward the osteogenic lineage than the flat (solid) films or control surface.
Figure 5.
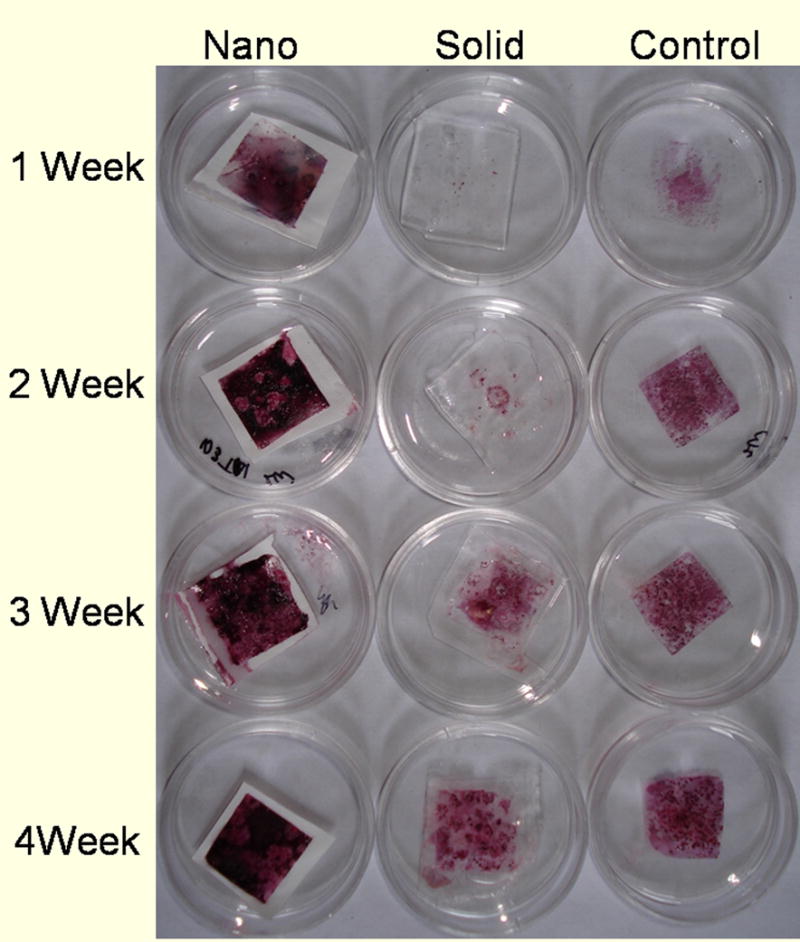
Calcium staining over 4 weeks of culture on nanofibrous matrices (Nano), flat films (Solid) and Control in TBI media
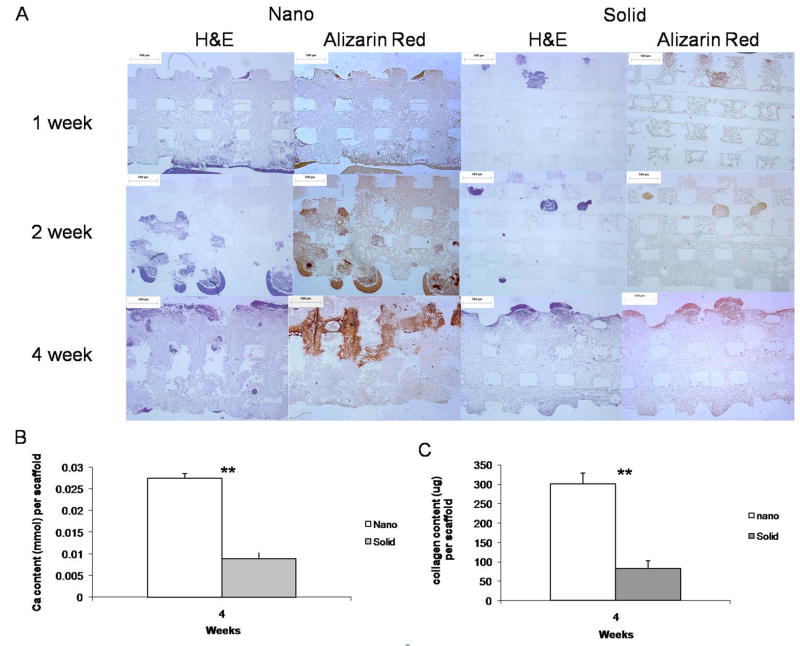
A similar phenomenon was observed in the 3D scaffolds (Figure 6A), where the NF scaffolds mineralized to a greater degree and more quickly than the SW scaffolds. Upon quantification NF scaffolds were found to contain 3 times more calcium than the SW scaffolds after 4 weeks of culture (Figure 6B). NF scaffolds were also found to contain over 3 times more collagen than the SW scaffolds after 4 weeks of culture (Figure 6C).
Figure 6.
(A) Histology of cellular (H&E) and calcium (Alizarin Red) staining over 4 weeks of culture on nanofibrous (Nano) and solid-walled (Solid) scaffolds in TBI media. Scale bar =500μm. (B) Quantification of scaffold calcium content after 4 weeks of culture in TBI media. (C) Quantification of scaffold collagen content after 4 weeks of culture in TBI media. ** denotes a p<0.01.
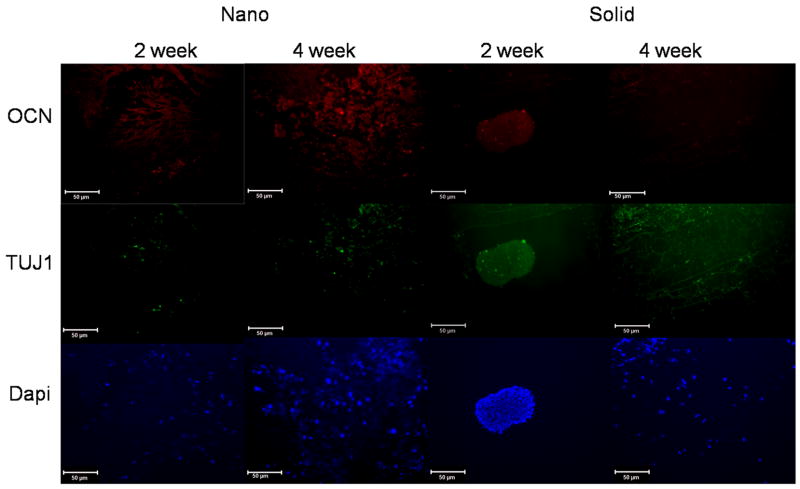
Additionally, after 2 weeks of culture ESC were found to be distributed throughout the NF scaffold while the ESC on the SW scaffold were still primarily in embryoid bodies and not directly interacting with the scaffolds (Figure 7). After 4 weeks of culture the ESC on the SW scaffolds did associate with the scaffolds like the ESC on the NF scaffolds at both 2 and 4 weeks (Figure 7). However, the ESC on the SW scaffold did not express osteocalcin in the center regions of the scaffold as strongly as the ESC on the NF scaffolds at either time point.
Figure 7.
Immunofluorescence staining of late bone differentiation (Osteocalcin-red) and neuronal (TUJ1-green) marker expression over 4 weeks of culture in TBI media on nanofibrous (Nano), solid-walled (Solid) scaffolds. Scale bar =50μm.
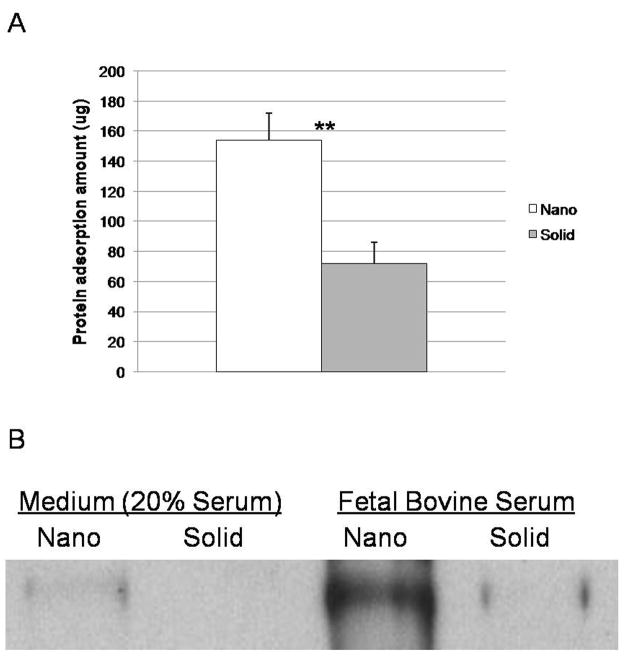
A previous study found that the difference in the amount of protein adsorbed from the media onto the NF matrices compared to that onto the flat (solid) films [22] could contribute to the difference in osteogenic differentiation on substrates with different matrix architectures. The initial microenvironment created by protein adsorption from the medium was examined on the 3D scaffolds to see if a similar trend occurs. The NF scaffolds were found to adsorb significantly more protein from the media than the SW scaffolds (Figure 8A). Western blots for fibronectin on NF scaffolds exposed to serum-containing media (differentiation media) or pure fetal bovine serum were shown to adsorb more fibronectin than similarly treated solid scaffolds (Figure 8B).
Figure 8.
Protein adsorption to materials after exposure to medium containing fetal bovine serum (differentiation medium) or exposure to original fetal bovine serum for 4 hrs: (A) Protein amounts adsorbed onto nanofibrous scaffolds (Nano) and solid-walled scaffolds (Solid) in serum-containing media, determined using the Micro BCA assay; ** denotes a p-value < 0.01. (B) Western blots of fibronectin adsorbed onto nanofibrous scaffolds (Nano) and solid-walled scaffolds (Solid) treated with serum-containing media or original fetal bovine serum.
Discussion
ESC hold great promise as a tissue engineering cell source. However, their ability to form multiple lineages must be contained and the ESC must be directed toward only the lineages needed for the desired tissue [23, 24]. In bone, the development process involves ECM proteins, growth factors, signaling molecules, hormones and transcription factors in a temporary and spatially organized process [17, 25]. In this study we examine the effects of NF materials and biochemical cues on the osteogenic differentiation of ESC in 2D and 3D compared to SW materials.
Through mimicking the ECM, 2D NF matrices without osteogenic supplements are able to induce osteogenic differentiation, while flat (solid) films require both osteogenic supplements and growth factors to achieve the same results. The NF matrices may provide a better niche for ESC osteogenesis due to improved protein adsorption from the serum [22]. Fibronectin, the earliest of the bone matrix proteins synthesized by osteoblasts, is thought to play an important role particularly in early osteogenesis [26, 27]. A previous study found that NF scaffolds adsorb nearly 4 times more fibronectin than their SW counterparts [28], which may contribute to increased differentiation on NF scaffolds without the addition of osteogenic factors.
The addition of osteogenic supplements and growth factors enhanced the osteogenic differentiation on all materials tested illustrating that signaling molecules play an important role in the lineage selection process. This differentiation was further enhanced on NF scaffolds (Figure 3) by crudely mimicking the temporal expression of growth factors (TGF-β1, BMP-2 and IGF-1) during development. During skeletogenesis, TGF-β1 induces cells to migrate along ECM molecules such as fibronectin to bone formation sites [29]. After the migration, TGF-β1 then promotes cellular proliferation and ECM production during development [30]. While BMP-2 is essential in limb patterning and is thought to induce commitment to osteoprogenitors [31]. Following this, IGF-1 plays a pivotal role in longitudinal bone growth during development [32] and has been shown to increase ECM collagen type I production [33]. Although these additional growth factors have been shown to enhance the osteogenic differentiation, additional study is needed to find the optimal combinations of growth factors for the directed osteogenic differentiation of ESC.
During embryogenesis, cell-cell interactions are thought to contribute to cell lineage differentiation [34]. The stimulation of these cellular communication pathways through 3D culture on the scaffolds may explain the enhanced differentiation in 3D culture compared to 2D culture. However, for functional bone formation further work is needed to determine the optimal 3D culture conditions.
Previous studies with pre-osteoblasts showed enhanced differentiation and tissue formation on NF scaffolds compared to SW scaffolds [8, 35]. As the NF architecture was designed to mimic collagen type I, which modulates osteoblast behavior through integrin interactions [36, 37] and adsorbs more protein from the media (Figure 8); additional stimulation of integrin signalling could be occurring on the NF scaffolds compared to the SW scaffolds. A previous study with NF materials found α2 and β1 integrin expression to be up-regulated compared to their SW counterparts even when collagen fibril formation was inhibited, implying a direct interaction between the NF material and the cells [35]. This increased α2β1expression particularly could enhance the osteoblast differentiation of the ESC since increased α2 integrin expression is associated with increased differentiation of ESC [38] and is considered to be necessary for osteogenic differentiation [39, 40], while increased β1 integrin expression has been linked to enhanced ESC mesodermal lineage commitment of ESC [41]. Additional integrins associated with collagen and fibronectin binding have also been up-regulated on NF materials compared to SW materials [35], which could further lead to enhancement of the osteogenic phenotype due to their importance in osteogenesis.
Conclusions
ESC grown on NF architecture exhibited enhanced osteogenic differentiation and mineralization compared to SW architecture. 3D culture and supplementation of media with osteogenic growth factors further enhanced the effects of NF architecture over SW architecture on ESC differentiation. NF scaffolds with osteogenic growth factors provided the best environment for ESC osteogenic differentiation and mineralization.
Acknowledgments
The authors would like to thank Dr. K. Sue O’Shea for her gift of the D3 cells, Dr. Renny Franceschi for critical discussions, Jiang Hu for his technical assistance. This research was partially supported by Michigan Center for hES Cell Research (NIH P20GM069985, Pilot PI: PXM), the NIH (NIDCR DE017689 & DE015384: PXM) and LAS was supported by NIH training grant in tissue engineering and regeneration (DE07057-26).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of Primary and Revision Total Hip and Knee Arthroplasty in the United States From 1990 Through 2002. J Bone Joint Surg. 2005;87:1487–1497. doi: 10.2106/JBJS.D.02441. [DOI] [PubMed] [Google Scholar]
- 2.Liu X, Ma PX. Polymeric Scaffolds for Bone Tissue Engineering. Annals of Biomedical Engineering. 2004;32(3):477–486. doi: 10.1023/b:abme.0000017544.36001.8e. [DOI] [PubMed] [Google Scholar]
- 3.Christenson EM, Anseth KS, vanden Beucken JJJP, Chan CK, Ercan B, Janson JA, et al. Nanobiomaterial Applications in Orthopedics. J Orthop Res. 2007;25:11–22. doi: 10.1002/jor.20305. [DOI] [PubMed] [Google Scholar]
- 4.Ma PX. Scaffolds for tissue fabrication. Materials Today. 2004;7:30–40. [Google Scholar]
- 5.Langer R, Vacanti J. Tissue Engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 6.Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev. 2008;60:184–189. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith LA, Beck JA, Ma PX. Fabrication and Tissue formation with Nano-Fibrous Scaffolds. In: Kumar C, editor. Nanotechnologies for Tissue, Cell and Organ Engineering. Weinheim: Wiley-VCH; 2007. pp. 188–215. [Google Scholar]
- 8.Chen VJ, Smith LA, Ma PX. Bone regeneration on computer-designed nano-fibrous scaffolds. Biomaterials. 2006;27:3973–3979. doi: 10.1016/j.biomaterials.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 9.Atala A. Recent developments in tissue engineering and regenerative medicine. Current Opinion in Pediatrics. 2006;18:167–171. doi: 10.1097/01.mop.0000193294.94646.be. [DOI] [PubMed] [Google Scholar]
- 10.Fleming RG, Murphy CJ, Abrams GA, Goodman SL, Nealey PF. Effects of synthetic micro-and nano-structured surfaces on cell behavior. Biomaterials. 1999;20:573–588. doi: 10.1016/s0142-9612(98)00209-9. [DOI] [PubMed] [Google Scholar]
- 11.Gerecht S, Bettinger CJ, Zhang Z, Borenstein JT, Vunjak-Novakovic G, Langer R. The effect of actin disrupting agents on contact guidance of humand embryonic stem cells. Biomaterials. 2007;28:4068–4077. doi: 10.1016/j.biomaterials.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nur-E-Kamal A, Ahmed I, Kamal J, Schindler M, Meiners S. Three-dimensional nanofibrillar surfaces promote self-renewal in mouse embryonic stem cells. Stem Cells. 2006;24:426–433. doi: 10.1634/stemcells.2005-0170. [DOI] [PubMed] [Google Scholar]
- 13.Hwang YS, Randle WL, Bielby RC, Polak JM, Mantalaris A. Enhanced derivation of osteogenic cells from murine embryonic stem cells after treatment with HepG2-conditioned medium and modulation of the embryoid body formation period: application to skeletal tissue engineering. Tissue Eng. 2006;12:835–843. doi: 10.1089/ten.2006.12.1381. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhry GR, Yao D, Smith A, Hussain A. Osteogenic Cells Derived from Embryonic Stem Cells Produced Bone Nodules in Three-Dimensional Scaffolds. Journal of Biomedicine and Biotechnology. 2004;4:203–310. doi: 10.1155/S111072430431003X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang Y, Varghese S, Theprunsirikul P, Canver A, Elisseeff J. Enhanced chondrogenic differentiation of murine embryonic stem cells in hydrogels with glucosamine. Biomaterials. 2006;27:6015–6023. doi: 10.1016/j.biomaterials.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 16.McCloskey KE, Gilroy ME, Nerem RM. Use of Embryonic Stem Cell-Derived Endothelial Cells as a Cell Source to Generate Vessel Structures in Vitro. Tissue engineering. 2005;11:497–505. doi: 10.1089/ten.2005.11.497. [DOI] [PubMed] [Google Scholar]
- 17.Aszodi A, Bateman JF, Gustafsson E, Boot-Handford R, Fassler R. Mammalian Skeletogenesis and Extracellular Matrix: What can We Learn from Knockout Mice? Cell Struct Funct. 2000;25:73–84. doi: 10.1247/csf.25.73. [DOI] [PubMed] [Google Scholar]
- 18.Doetschman T, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood island and myocardium. J Embryol Exp Mophol. 1985;87:27–45. [PubMed] [Google Scholar]
- 19.Woo KM, Chen VJ, Ma PX. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J Biomed Mater Res. 2003;67A:531–537. doi: 10.1002/jbm.a.10098. [DOI] [PubMed] [Google Scholar]
- 20.Woessner JF. The Determination of Hydroxyproline in Tissue and Protein Samples Containing Small proportions of this Imino Acid. Achivers of Biochemistry and Biophysics. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 21.Neuman R, Logan M. The determination of collagen and elastin in tissues. J Biol Chem. 1950;186:549–556. [PubMed] [Google Scholar]
- 22.Smith LA, Liu X, Wang P, Hu J, Ma PX. Enhancing Osteogenic Differentiation of Mouse Embryonic Stem Cells by Nanofibers. doi: 10.1089/ten.tea.2008.0227. In preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan DL, Moon RT, Vunjak-Novakovic G. It takes a village to grow a tissue. Nat Biotechnol. 2005;23:1237–1239. doi: 10.1038/nbt1005-1237. [DOI] [PubMed] [Google Scholar]
- 24.Vats A, Tolley NS, Bishop AE, Polak JM. Embryonic stem cells and tissue engineering: delivering stem cells to the clinic. J R Soc Med. 2005;98(8):346–350. doi: 10.1258/jrsm.98.8.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karaplis A. Embryonic Development of Bone and the Molecular Regulation of Intramembranous and Endochondral Bone Formations. In: Bilezikian J, Raisz L, Rodan G, editors. Principles of Bone Biology. San Diego: Academic Press; 2002. pp. 33–58. [Google Scholar]
- 26.Cowles EA, DeRome ME, Pastizzo G, Brailey LL, Gronowicz GA. Mineralization and the Expression of the Matrix Proteins During In Vivo Bone Development. Calcif Tissue Int. 1998;62:74–82. doi: 10.1007/s002239900397. [DOI] [PubMed] [Google Scholar]
- 27.Weiss RE, Reddi AH. Role of Fibronectin in collagenous matrix-induced mesenchymal cell proleration and differentiation in vivo. Exp Cell Res. 1981;133:247–254. doi: 10.1016/0014-4827(81)90316-5. [DOI] [PubMed] [Google Scholar]
- 28.Woo KM, Chen VJ, Ma PX. Nano-fibrous scaffolding architecture selectively enhances protein adsoption contributing to cell attachement. J Biomed Mater Res. 2003;67A:531–537. doi: 10.1002/jbm.a.10098. [DOI] [PubMed] [Google Scholar]
- 29.Atchley WR, Hall BK. A model for development and evolution of complex morphological structures. Biol Rev Camb Philos So. 1991;66:101–157. doi: 10.1111/j.1469-185x.1991.tb01138.x. [DOI] [PubMed] [Google Scholar]
- 30.Kanaan RA, Kanaan LA. Transforming growth factor B1, bone connection. Med Sci Monit. 2006;12:RA164–169. [PubMed] [Google Scholar]
- 31.Rosen V, Wozney JM. Bone Morphogenetic Proteins. In: Bilezikian J, Raisz L, Rodan G, editors. Principles of Bone Biology. San Diego: Academic Press; 2002. pp. 919–928. [Google Scholar]
- 32.Karaplis AC. Embryonic Development of Bone and the Molecular Regulation of Intramembranous and Endochondral Bone Formation. In: Bilezikian J, Raisz L, Rodan G, editors. Principles of Bone Biology. San Diego: Academic Press; 2002. pp. 33–58. [Google Scholar]
- 33.Rossert J, de Crombugghe B. Type I Collagen. In: Bilezikian J, Raisz L, Rodan G, editors. Principles of Bone Biology. San Diego: Academic Press; 2002. pp. 189–210. [Google Scholar]
- 34.Stains JP, Civitelli R. Cell-Cell Interactions in Regulatin Osteogenesis and Osteoblast Function. Birth Defects Research (Part C) 2005;75:72–80. doi: 10.1002/bdrc.20034. [DOI] [PubMed] [Google Scholar]
- 35.Woo KM, Jun J-H, Chen VJ, Seo J, Baek J-H, Ryoo H-M, et al. Nano-fibrous scaffolding promotes osteoblast differentiation and biomineralization. Biomaterials. 2007;28(2):335–343. doi: 10.1016/j.biomaterials.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 36.Lynch MP, Stein JL, Stein GS, Lian JB. The influence of type I collagen on the development and maintenance of the osteoblast phenotype in primary and passaged rat calvarial osteoblasts: modifcation of expression of genes supporting cell growth, adhesion, and extracellular matrix mineralization. Exp Cell Res. 1995;216:35–45. doi: 10.1006/excr.1995.1005. [DOI] [PubMed] [Google Scholar]
- 37.Mizuno M, Fujisawa R, Kuboki Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-alpha2beta1 integrin interaction. J Cell Physiolo. 2000;184:207–213. doi: 10.1002/1097-4652(200008)184:2<207::AID-JCP8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi Y, Furue MK, Okamoto T, Myoishi Y, Fukuhara Y, Abe T, et al. Integrins Regulate Mouse Embryonic Stem Cell Self-Renewal. Stem Cells. 2007;25:3005–3015. doi: 10.1634/stemcells.2007-0103. [DOI] [PubMed] [Google Scholar]
- 39.Mizuno M, Fujisawa R, Kuboki Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-alpha2beta1 integrin interaction. J Cell Physiol. 2000;184:207–213. doi: 10.1002/1097-4652(200008)184:2<207::AID-JCP8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 40.Xiao G, Wang D, Benson D, Karsenty G, Franceschi RT. Role of the a2-Integrin Osteoblast-specific Gene Expression and Activation of the OSF2 Transcription Factor. J Biol Chem. 1998;273:32988–32994. doi: 10.1074/jbc.273.49.32988. [DOI] [PubMed] [Google Scholar]
- 41.Rohwedel J, Guan K, Zuschratter W. Loss of Beta 1 integrin function results in a retardation of myogenic, but an acceleration of neuronal, differentiation of embryonic stem cells in vitro. Del Biol. 1998;201:167–184. doi: 10.1006/dbio.1998.9002. [DOI] [PubMed] [Google Scholar]