Abstract
Background
Chronic tic disorders are characterized by motor tics that are often preceded by premonitory urges to “tic.” Functional neuroimaging studies have documented brain activity patterns prior to and during tics, but these studies have not examined whether the activation patterns differ from those seen in normal controls performing similar acts.
Methods
A novel method was used to compare brain patterns during tics and intentional movements. First, the part of motor cortex specific to each patient's tic movement was identified. The brain areas activating prior to, during, and after that part of motor cortex during tics were then identified by temporally cross-correlating the time-course of the localized motor region with activity in other brain areas. Given that motor cortex was active during tic execution, this yielded information regarding the brain areas active prior to, during, and after the movements. The spatiotemporal pattern of co-activation with motor cortex during tics was contrasted with that seen in healthy controls during intentional “tic-like” movements.
Results
Data from 16 adult subjects with tic disorders and 16 matched controls, that performed intentional movements similar to the patients' tics, revealed nearly identical patterns of cross-correlation to motor cortex throughout the brain in the two groups. However, the supplementary motor area showed a significantly broader profile of cross-correlation to motor cortex during tics than during intentional movements.
Conclusions
These findings highlight the importance of the supplementary motor area in tic generation and may point toward novel intervention strategies for individuals suffering with severe tics.
Keywords: Tourette Syndrome, fMRI, supplementary motor area, brain imaging, midbrain, tics
Introduction
The neurophysiological basis of chronic tic disorders has been investigated using a variety of imaging techniques including positron emission tomography (PET)(1-3), single photon emission computed tomography (SPECT)(4-6), and functional magnetic resonance imaging (fMRI)(7). These studies provide converging evidence that Tourette Syndrome (TS) involves aberrant activity in the basal ganglia, midbrain, prefrontal cortex and paralimbic regions, and increased activity in motor cortical regions, including supplementary motor area (SMA). An [150] H20 PET study identified regions activating during tics including the SMA, premotor and primary motor cortices, anterior cingulate, prefrontal cortex, insula, claustrum, putamen and caudate nucleus (8).
Given that many individuals with TS experience premonitions preceding their tics (9, 10), we hypothesize that tics are the result of a chain of neural events that may occur on a time scale slow enough to be examined using current imaging techniques. However, little is known regarding the temporal pattern of brain activity associated with this phenomenon in contrast to voluntary actions. A recent event-related fMRI study investigated brain activity patterns prior to and during tics (11). That study reported activation in several areas prior to tics, including the SMA, insula, and anterior cingulate, that later spread into primary motor cortex. These findings provide our first view of the temporal pattern of brain activity associated with tics, but they fail to address whether this sequence differs from that involved in intentional movements. The aim of this study was to examine the time-course of brain activity that unfolds during motor tics, and to compare that time-course to the pattern of brain activity seen during intentional movements.
Both SMA and the primary somatosensory cortex (S1) are regions that have been implicated in TS, and are candidate loci of the urges patients report preceding, and motivating, their tics. Electrical stimulation of the SMA in humans (using subdural electrodes in patients with epilepsy) produces both movements and urges to move reminiscent of the premonitory urges of TS. The nature of the movements (or corresponding urges) range from simple motor acts to complex movements, paralleling the range of simple to complex tics experienced in TS (12). This suggests the SMA may be involved in premonitory urges. Given that premonitory urges often have a sensory aspect, together with recent magnetoencephalographic data implicating S1 in TS (13), S1 is another promising candidate region for these urges. Therefore, we hypothesized that SMA or S1, due to involvement in premonitory urges, would show more activity prior to tics than prior to intentional movements.
Methods and Materials
Human subjects
Sixteen individuals (thirteen men) with active tic disorders between 18 and 56 years of age were recruited. Only individuals who reported they could execute their tics without moving their heads, and whose repertoire of tics was dominated by one or two specific movements were included. Diagnoses, tic locations and pertinent medication profiles are presented in Table 1. Thirteen of the sixteen patients experienced a bilateral movement as their most frequent tic, thus limiting our ability to investigate laterality issues. Data were obtained regarding the presence of premonitory urges for fourteen of the sixteen patients, all of whom reported experiencing this phenomenon prior to their tics.
Table 1. Patient Characteristics.
| Subject | Diagnoses | Reference tic | Current neuropsychiatric medication |
|---|---|---|---|
| 1 | TS, Bipolar II, Hypothyroidism | eyeblink | aripiprazole (5mg/day), citalopram (10mg/day), clonidine (0.15mg/day), clonazepam (1mg/day) levothyroxine (0.1mg/day), |
| 2 | TS | eyeblink | fluoxetine (70mg/day) |
| 3 | TS, OCD | Abdominal tensing | |
| 4 | TS, ADHD, MDD | Buttock tensing | |
| 5 | TS, ADHD, MDD | Abdominal tensing | atomoxetine (2mg/day), risperidone (30mg/day) |
| 6 | CTD | Left leg | citalopram (20mg/day), clonazepam (1mg/day) |
| 7 | TS | eyeblink | |
| 8 | TS | Eyeblink/eyebrow raise | |
| 9 | TS | Right shoulder/elbow | |
| 10 | TS | Right elbow | guanfacine (0.5 mg/day) |
| 11 | TS | Abdominal tensing | |
| 12 | CTD | Pulling upper lip down | |
| 13 | TS | Eyebrow raise | |
| 14 | TS | Hiccup | escitalopram (20 mg/day) |
| 15 | TS | Cheek puff | escitalopram (10 mg/day) |
| 16 | CTD, OCD | Abdominal tensing/shaking | sertraline (200 mg/day) |
ADHD = Attention deficit hyperactivity disorder; CTD = chronic (motor or vocal) tic disorder; MDD = Major Depressive Disorder; OCD = obsessive-compulsive disorder
A within-subject design requires accurate measurement of the temporal patterns of brain activity associated with imitated tics in the patients. During any time period in which patients are imitating tics, real tics will also be occurring that are difficult to differentiate from the imitated tics. A previous study that attempted to use a within-subject design for an event-related study of tics was unable to collect viable control data for this reason, and because of problematic levels of movement in the patients during tic imitation (11). They also raised concerns regarding the normalcy of voluntary movements in the patient group and recommended that control data be collected in healthy controls instead (11). That is the approach used here.
For each patient, a healthy subject of the same gender, handedness, and sex, and of similar age, was recruited. Socioeconomic status (SES) was estimated using the Hollingshead index and balanced across groups. This matching procedure resulted in populations with very similar age and SES distributions: patient age: mean 30 (standard deviation 9.7), healthy control age: 30(9.0), patient SES: 52(11.4) and healthy control SES 55(12.0). All subjects gave informed consent to a protocol reviewed and approved by the Human Investigations Committee of the Yale School of Medicine.
Experimental Paradigm
A standard approach for obtaining time-courses of brain activity associated with events requires a record of the times of occurrence of the events (in this case, tics) during an imaging session. As accurate records of tic occurrences during a scanning session can be difficult to obtain, a standard event-related protocol was not adopted in this study. A novel paradigm was utilized that does not require knowledge of the time of occurrence of tics during the imaging session. Instead, it depends upon a-priori knowledge concerning a specific brain region that is activated in synchrony with tics. Correlations in activity between that (reference) region and other regions of the brain can then be computed. Assuming the tic events are responsible for a large portion of the variance in the activity level of the reference region, a set of regions that are also activated during the tics will be identified. Correlating to the activity pattern in a brain area known to be involved in events has been shown to produce maps similar to those obtained with a traditional event-related analysis (14).
The region assumed by this study to be active in synchrony with tics was the portion of motor cortex controlling the musculature specific for the selected tic. This region was localized functionally in each subject and then used as a reference region for correlations during scans in which the subject allowed their tics to occur naturally.
Therefore, two different types of functional scans were collected: localizers to identify the motor cortical reference ROI, and scans to be used in the correlation analysis in which patients allowed their tics to occur at a natural rate throughout the scan. The localizers were of block-design: during “on” blocks, patients imitated their most frequent tic at a much higher rate than the tics naturally occurred and during the “off” blocks patients allowed tics to occur at their natural rate. Although this localizer could be used to identify a set of brain areas active during intentional movements in the patient population, it could not provide a temporal sequence of neurophysiological events associated with intentional movements. As discussed in the Subjects subsection above, it is difficult to get a timecourse of brain activity associated specifically with intentional movements in tic patients. For this reason, and because of concerns regarding the normalcy of intentional movements in tic patients (11), the spatiotemporal pattern of brain activity associated with intentional movements was identified separately in a group of healthy controls.
For control subjects, there were also two types of functional scans. There were localizing scans of block-design in which the “on” blocks involved imitating the tic movement of the patient they were matched to at a high frequency and the “off” blocks were rest periods. For the remaining scans (used for correlation analyses), the control subjects were instructed to imitate the tic movement of the patient whom they were matched to throughout the scan. They were told they would need to produce the movement in clusters, so that some movements occurred close together in time and others further apart, but such that their total frequency over the scanning session should approximate that of the patient. Both the movement and frequency were practiced prior to the scanning session by the control subject, until they reported feeling that they could make the movement easily and with the desired temporal pattern.
Data Acquisition
Subjects were scanned in a Siemens 3T Trio scanner. Each functional scan involved the acquisition of 222 volumes, each composed of 22 4.5 mm axial slices (aligned with the AC-PC line). A T2*-sensitive gradient-recalled, single shot echo-planar pulse sequence was used (TR= 1.5s, TE = 25ms, flip angle = 80, FOV = 202 cm, 642 acquisition matrix).
After the functional data were collected, 22 T1-weighted slices were collected (prescribed in the same locations) for anatomical identification. Immediately prior to the collection of anatomical data, subjects were given a button-box and told to press the box each time they experienced a tic (if they were a patient) or imitated a tic (if they were a control subject). These button-press records were used to estimate movement frequencies during the functional scans. There was no button pressing, or reference to that aspect of the experiment, during or prior to the functional scans. The estimated frequency of tics in patients had a mean of 7.3 tics/minute (standard deviation: 4.7). This was well matched by the control population that had a mean of 7.7 movements/minute (standard deviation: 7.7).
Data Analyses
Aside from motion correction, all other analyses were done using in-house software.
Preprocessing
Data were adjusted for slice acquisition time, motion corrected using the Statistical Parametric Mapping (SPM) algorithm (http://www.fil.ion.ucl.ac.uk/spm/), and spatially smoothed. Pixels with a median value below one-fifth of the maximum median pixel value were set to zero.
Data used in correlational analyses were also low-pass filtered (0.2 Hz). Prior to correlation, the mean signal from each slice was removed from the time-course of activity in each pixel in that slice and from the reference time-course. This is similar to the removal of global mean time course, but is better at reducing noise caused by transient physiologic fluctuations that occur during part of the volume collection and that can lead to elevated correlations between slices collected next to one another in time.
Localizer runs
Linear drift was removed (15), images were assigned to “tic forcing” or “resting” blocks, and a pixel-wise, two-sample t-test was performed. The reference region was defined for each subject as the 30 most activated pixels in primary motor cortex, with no requirement of spatial contiguity.
Correlational analyses
The reference time-course was computed by averaging across pixels in the reference region. The reference time-course was correlated with the time-course of every pixel within each of the scans used in the correlation analysis. The resulting correlations were transformed to a standard normal distribution (16, 17). These z-transformed data were then averaged across scans to yield a map representing the strength of correlations to motor cortex in terms of standardized z-values.
The reference time-course was then shifted one TR earlier and similar computations were performed to produce a map of areas that tended to activate 1.5 seconds before motor cortex. This process was repeated across a range of temporal shifts, resulting in nine maps representing areas activated from 6s prior to motor cortex to 6s after. The nine z-maps of the correlations from each patient were transformed to Talairach coordinates using a piece-wise linear transform.
Group composites
At each Talairach pixel the mean across the 16 patients/controls was computed and compared to a surrogate distribution of 12,000,000 values (each the mean of 16 random numbers from a standard normal distribution). This surrogate distribution represented the distribution of values expected by chance when averaging random data (i.e. with no effect) across 16 subjects. The fraction of surrogates with a greater magnitude than the computed mean value across patients/controls was the p-value for that pixel. Composite maps are shown at a Bonferroni-corrected cutoff (adjusted for total number of nonzero pixels) of p< 0.05.
Primary ROI analyses
Two cortical ROIs (S1 and SMA) were defined in bilaterally in the composite anatomical brain, formed by averaging the Talairach transformed anatomical data from all 32 subjects. These ROIs are shown in Supplemental Figure 1.
Values from the Talairach transformed z-maps of correlation for each subject were averaged across all pixels in each ROI and entered into an ANOVA with three repeated factors: time-point (nine levels), hemisphere (two levels) and group (two levels). Subjects were treated in a paired manner given the one-on-one matching of age, gender, handedness, movement type, and movement frequency. A timepoint-by-group interaction was expected for any region differentially involved in tics as compared to intentional movements. All results were corrected for violations of sphericity using the Greenhouse-Geisser correction. For the SMA, which showed a significant timepoint-by-group interaction, post-hoc paired t-tests were performed at each time-point to identify time-points that differed significantly between groups.
Exploratory ROI analyses
Five subcortical/midbrain ROIs (caudate, lenticular nucleus, thalamus, ventral basal ganglia including substantia nigra and subthalamic nucleus, and the midbrain tegmentum including the periaqueductal grey) that have been repeatedly implicated in TS (1-8, 11, 18-22) were defined bilaterally in the composite brain and analyzed in the same manner as the primary ROIs. These ROIs are also shown in Supplemental Figure 1.
Secondary analyses of the SMA
The difference between groups in the SMA was re-evaluated for the following four subsets of our participants:
the fourteen patients known to have premonitory urges and their matched controls.
the eleven patients with no comorbid conditions and their matched controls.
the thirteen patients with a diagnosis of TS (not chronic tic disorder) and their matched controls.
the eight patients who were not taking neuropsychiatric medication and their matched controls.
In addition, we conducted an ANOVA on the patient data with three repeated factors: medication status (2 levels), timepoint (9 levels), and hemisphere (2 levels). A medication status-by timepoint interaction would provide evidence that the timecourse of SMA activity in the eight patients taking neuropsychiatric medication differed from the timecourse of the SMA in the eight patients not taking medication.
Motion comparisons
For each of the six SPM realignment parameters, the absolute value of the difference in the parameter between each volume and the next was added across all volumes in a given scan. For each subject, these numbers were averaged across scans and a 2-sample t-test was performed to determine whether the total movement along that dimension differed in patients and controls at a p<0.05 level.
Results
The maps of cross-correlations to motor cortex during tics revealed activity throughout the motor circuit (Supplemental Figure 2). Positive correlations with motor cortex were apparent in primary and secondary motor cortex, SMA, somatosensory cortex, superior temporal gyrus, dorsal anterior cingulate cortex, inferior parietal lobule, cerebellum, orbitofrontal cortex, insula, claustrum, substantia nigra, lenticular nuclei, thalami, and red nuclei. Negative correlations were present in ventral anterior cingulate cortex, superior regions of the parietal lobe, middle and superior frontal gyri, parahippocampal regions, and caudate nuclei. Most regions peaked around the same time as motor cortex with a few exceptions. For example, the loci in orbitofrontal cortex (Brodmann's area 47 in the most ventral slice) and the lenticular nuclei appeared to peak earlier.
The maps of cross-correlations to motor cortex during intentional movements were very similar to those seen during the tics (Supplemental Figure 3). Group contrast maps were computed at each time-point, but nothing was significant after Bonferroni correction.
ROI analyses
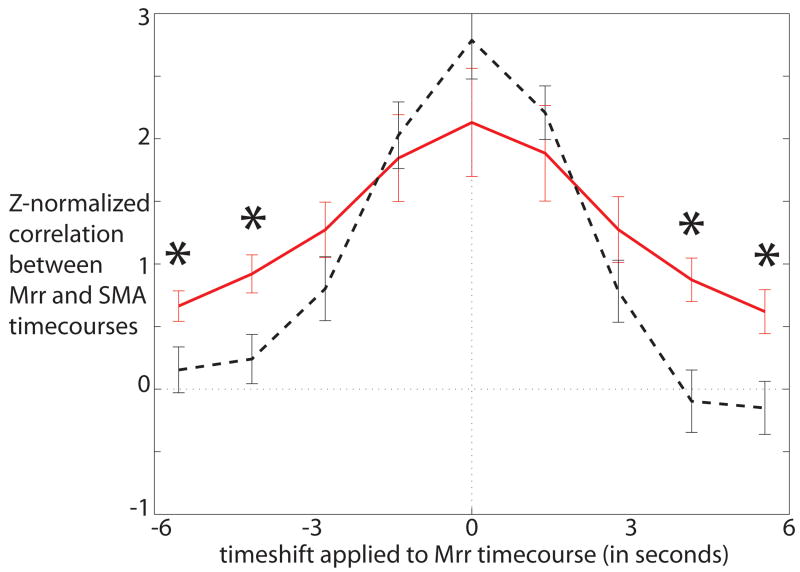
The interaction between time-point and group was significant in the SMA after a Bonferroni correction for the 2 primary ROIs examined (p = 0.003), indicating that this region had a different temporal profile during tics than during intentional movements. The temporal cross-correlations between motor cortex and this area is shown in Figure 1. The temporal cross-correlations for the other regions (with no significant group-by-timepoint interactions) are shown in Supplemental Figure 4.
Figure 1.
Temporal cross-correlation between the functionally defined motor cortical reference region (Mrr) and the SMA during tics (red solid line) and during intentional movements in control subjects (black dashed line). Asterisks indicate differences between patients and controls at specific time-points as identified in the post-hoc t-tests. SMA is more active both prior to and after the motor cortical reference region during tics than it is during intentional movements.
Secondary analyses of the SMA
A significant group-by-timepoint effect (p=0.004) was found using the fourteen patients known to experience premonitory urges and their matched controls.
A significant group-by-timepoint effect was found (p=0.01) using the eleven patients who did not have comorbid conditions and their matched controls.
A significant group-by-timepoint effect was found in the SMA at an uncorrected level, that approached significance at a corrected level (p=0.028) using the thirteen patients with a diagnosis of Tourette Syndrome and their matched controls,
No significant group-by-timepoint effect was found when limiting analysis to the eight patients who were not taking neuropsychiatric medication and their matched controls. However, this may be due to the large reduction in power associated with halving our sample size rather than to drug effects. To further investigate this issue, an ANOVA was conducted on the patients' SMA data with medication status as a factor. This analysis revealed no significant timepoint-by-medication status interaction, suggesting that the time course of activity in the SMA was similar across patients, regardless of whether they were taking neuropsychiatric drugs or not. Furthermore, patients both on and off drugs showed similar patterns of broadening in the timecourse of the SMA when compared with their matched controls, as illustrated in Supplemental Figure 5. Therefore, the differences we have identified in the SMA are unlikely to be due to a medication effect.
Motion comparisons
There was no significant difference between groups in motion along any of the six dimensions.
Discussion
Cross-correlations with the time-course of motor cortex during tics revealed a progression of activity through motor circuitry. The pattern of neural activity during intentional movements in controls was very similar to that obtained during tics. This underscores the importance of collecting control data to differentiate brain activity specific to tics from brain activity generally associated with movement.
ROI analyses revealed differences between patients and controls in the temporal cross-correlation of the motor cortex and the SMA. The SMA region had a broader profile of cross-correlation to motor cortex in the patients than in the control subjects. This suggests that the SMA has abnormally elevated activity in the seconds preceding and following tic execution. These differences are unlikely to be due to head motion, as the groups did not differ significantly in amount of motion. It is possible that greater clustering over time of motor activity in the patients could yield broader patterns of cross-correlation to motor cortex throughout the brain. However, our control subjects were explicitly instructed to produce their movements in a temporally clustered manner. In addition, our data do not suggest that there was global broadening effect in cross-correlation to motor cortex: most areas did not show such broadening. Therefore, the localized pattern of broadening identified in the SMA is unlikely to be due to a global effect such as that arising from different temporal patterns of movement in the two groups.
One possibility is that the difference found in the SMA across groups is related to premonitory urges. The SMA is a promising candidate locus for these urges given that electrical stimulation of the SMA in healthy subjects can induce urges to move that are remarkably similar to the premonitory urges of TS patients (12). If aberrant activity in the SMA does give rise to premonitory urges, there may be a relationship between the intensity of premonitory urges experienced by patients and the activity level in their SMA prior to tics. Unfortunately, we did not collect clinical data relevant to this issue. Further investigation of this issue would be very interesting but should consider the fact that urges increase when subjects inhibit their tics and thus are context dependent as well as subject specific.
In contrast to the SMA, no differences were found between patients and controls in the temporal pattern of their S1 activity. However, individual subjects are likely to be activating discrete portions of this large region (depending on which part of the sensory homunculus their tic/movement was associated with). Therefore, the spatial averaging inherent in the ROI analyses of this study likely limited its power to identify interesting effects in S1. Thus, we cannot rule out the possibility that this region is implicated in the premonitory urges of TS.
For serious tic disorders that do not respond well to behavioral or pharmacologic intervention, more invasive treatments, such as repetitive transcranial magnetic stimulation (rTMS) and deep brain stimulation (DBS), may be needed. The most effective locus for rTMS is an open question. Trials of rTMS applied to primary motor, premotor, and prefrontal areas have improved tics only modestly or not at all (23-25). Given that SMA activity appears to be abnormally elevated prior to tics, this region may provide a more effective target. Two recent reports that rTMS of the SMA reduced TS symptoms (26, 27) are particularly exciting in this context.
This study adopted a novel method for extracting event-related brain patterns that involved correlating to a component region in the circuit of interest. This method does not require knowledge regarding the time of occurrence of events. In addition, it does not assume that events are instantaneous in nature, or that the hemodynamic responses to closely spaced events sum linearly, as standard event-related analyses do. Therefore the method is well suited for event-related analyses of temporally extended events that occur in clusters, such as tics.
However, validity of this method depends upon the selection of a seed region that is involved in the events of interest. The seed region used in this study was the part of primary motor cortex controlling tic musculature. There are several reasons to believe that tics arise via neural activity in the voluntary motor pathway and involve activation of motor cortex. First, in contrast to other movement disorders, many patients with TS report the subjective perception that their tics are intentionally produced, often in response to an almost undeniable urge (9, 28). Second, abnormalities have been found in the cortical silent period and intracortical inhibition of the motor cortex of individuals with TS(29), suggesting this region behaves in an aberrant manner in patients with TS. Finally, evidence that motor cortex is involved in the generation of motor tics comes from neuroimaging studies implicating this region in TS(1), reporting correlations between activity in motor cortex and tic frequency (8), and identifying primary motor cortical activity occurring at tic onset (11). Thus, the assumption of this study that motor cortex is involved in tics is consistent with the bulk of literature on the neurobiology of TS.
Interpretation of results using this method must always consider the possibility that the events of interest do not sufficiently affect the variance in the reference region to allow just the identification of event-related brain patterns. In our view it is likely that the underlying functional connectivity to the reference region is also revealed by this technique. We maximized the degree to which our results were influenced by event-related activity patterns by correlating with primary motor cortex, an area that is known to activate robustly during motor acts, during time periods in which such motor acts were occurring regularly. However, our results may reflect differences in the functional connectivity of motor cortex between patients and controls, rather than just reflecting different activity patterns during tics and intentional movements. In either case, our findings highlight the importance of the SMA in tic disorders, and suggest that it is influencing primary motor cortex differentially during tics as compared with intentional movements.
There are several limitations to this study. First, clinical data was not collected on all relevant patient characteristics. For example, intensity of premonitory urges, severity of illness, and duration of illness are not characterized in our patient sample. Thus, we are unable to investigate the relationship between SMA activity patterns and these variables. Second, this study used a between-groups design. Therefore, we cannot rule out the possibility that differences in SMA activity patterns may be due more to differences between groups than to differences between tics and intentional movements. However, it should be noted that a prior study that attempted to use a within-subject design to compare tics and intentional movements found it very difficult to collect control data (11). The manuscript reporting that previous study also raised issues regarding the normalcy of intentional movement in tic patients and recommended that future studies employ a between-group design, as we have done. Finally, many of the patients in our study were using neuropsychiatric drugs. However, patients on and off medication had no significant differences in their SMA timecourses and showed similar patterns of broadening in the timecourse of the SMA when compared with their matched controls. Therefore, our findings are unlikely to be due to a medication effect.
Supplementary Material
Acknowledgments
This work was supported by the Tourette Syndrome Association (MH) and NIMH:K05MH076273 (JFL). We thank V. Rekkas, E. Hanrahan, R. Fulbright, and R. Hoffman for their time and expertise, K. Martin and H. Sarofin for their technical assistance, and the subjects in the study for their participation.
Footnotes
Financial Disclosures: All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Braun AR, Stoetter B, Randolph C, Hsiao JK, Vladar K, Gernert J, et al. The functional neuroanatomy of Tourette's syndrome: an FDG-PET study. I. Regional changes in cerebral glucose metabolism differentiating patients and controls. Neuropsychopharmacology. 1993;9:277–291. doi: 10.1038/npp.1993.64. [DOI] [PubMed] [Google Scholar]
- 2.Chase TN, Geoffrey V, Gillespie M, Burrows GH. Structural and functional studies of Gilles de la Tourette syndrome. Rev Neurol. 1986;142:851–855. [PubMed] [Google Scholar]
- 3.Eidelberg D, Moeller JR, Antonini A, Kazumata K, Dhawan V, Budman C, et al. The metabolic anatomy of Tourette's syndrome. Neurology. 1997;48:927–934. doi: 10.1212/wnl.48.4.927. [DOI] [PubMed] [Google Scholar]
- 4.Klieger PS, Fett KA, Dimitsopulos T, Kurlan R. Asymmetry of basal ganglia perfusion in Tourette's syndrome shown by technetium-99m-HMPAO SPECT. The Journal of Nuclear Medicine. 1997;38:188–191. [PubMed] [Google Scholar]
- 5.Malison RT, McDougle CJ, Dyck CHv, Scahill L, Baldwin RM, Seibyl JP, et al. [123I] B-CIT SPECT imaging of striatal dopamine transporter binding in Tourette's disorder. American Journal of Psychiatry. 1995;152:1359–1361. doi: 10.1176/ajp.152.9.1359. [DOI] [PubMed] [Google Scholar]
- 6.Moriarty J, Costa DC, Schmitz B, Trimble MR, Ell PJ, Robertson MM. Brain perfusion abnormalities in Gilles de la Tourette's syndrome. British Journal of Psychiatry. 1995;167:249–254. doi: 10.1192/bjp.167.2.249. [DOI] [PubMed] [Google Scholar]
- 7.Biswal B, Ulmer JL, Krippendorf RL, Harsch HH, Daniels DL, Hyde JS, et al. Abnormal cerebral activation associated with a motor task in Tourette syndrome. American Journal of Neuroradiology. 1998;19:1509–1512. [PMC free article] [PubMed] [Google Scholar]
- 8.Stern E, Silbersweig DA, Chee KY, Holmes A, Robertson MM, Trimble M, et al. A functional neuroanatomy of tics in Tourette Syndrome. Archives of General Psychiatry. 2000;57:741–748. doi: 10.1001/archpsyc.57.8.741. [DOI] [PubMed] [Google Scholar]
- 9.Bliss J. Sensory experiences of Gilles de la Tourette syndrome. Archives of General Psychiatry. 1980;37:1343–1347. doi: 10.1001/archpsyc.1980.01780250029002. [DOI] [PubMed] [Google Scholar]
- 10.Leckman JF, Walker DE, Cohen DJ. Premonitory urges in Tourette's syndrome. American Journal of Psychiatry. 1993;150:98–102. doi: 10.1176/ajp.150.1.98. [DOI] [PubMed] [Google Scholar]
- 11.Bohlhalter S, Goldfine A, Matteson S, Garraux G, Hanakawa T, Kansaku K, et al. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006;129:2029–2037. doi: 10.1093/brain/awl050. [DOI] [PubMed] [Google Scholar]
- 12.Fried I, Katz A, McCarthy G, Sass KJ, Williamson P, Spencer SS, et al. Functional organization of human supplementary motor cortex studied by electrical stimulation. The Journal of Neuroscience. 1991;11:3656–3666. doi: 10.1523/JNEUROSCI.11-11-03656.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moran KA, Leckman JF, Vaccarino FM, Walton KD, Llinas RR. Neuromagnetic correlates of Gilles de la Tourette syndrome. 35th Annual Meeting of the Society for Neuroscience; Washington, DC. 2005. [Google Scholar]
- 14.Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magn Reson Med. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- 15.Skudlarski P, Constable RT, Gore JC. ROC analysis of statistical methods used in functional MRI: individual subjects. Neuroimage. 1999;9:311–329. doi: 10.1006/nimg.1999.0402. [DOI] [PubMed] [Google Scholar]
- 16.Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Human Brain Mapping. 2002;15:247–262. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. NeuroImage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- 18.Devinsky O. Neuroanatomy of Gilles de la Tourette's Syndrome: possible midbrain involvement. Archives of Neurology. 1983;40:508–514. doi: 10.1001/archneur.1983.04210070048013. [DOI] [PubMed] [Google Scholar]
- 19.Garraux G, Goldfine A, Bohlhalter S, Lerner A, Hanakawa T, Hallett M. Increased midbrain gray matter in Tourette's Syndrome. Annals of Neurology. 2006;59:381–385. doi: 10.1002/ana.20765. [DOI] [PubMed] [Google Scholar]
- 20.Peterson BS, Thomas P, Kane MJ, Scahill L, Zhang H, Bronen R, et al. Basal ganglia volumes in patients with Gilles de la Tourette Syndrome. Archives of General Psychiatry. 2003;60:415–424. doi: 10.1001/archpsyc.60.4.415. [DOI] [PubMed] [Google Scholar]
- 21.Leckman JF. Tourette's syndrome. Lancet. 2002;360:1577–1586. doi: 10.1016/S0140-6736(02)11526-1. [DOI] [PubMed] [Google Scholar]
- 22.Leckman JF, Vaccarino FM, Kalanithi PSA, Rothenberger A. A: Tourette syndrome: a relentless drumbeat - driven by misguided brain oscillations. Journal of Child Psychology and Psychiatry. 2006;47:537–550. doi: 10.1111/j.1469-7610.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- 23.Chae JH, Nahas Z, Wassermann E, Li X, Sethuraman G, Gilbert D, et al. A pilot safety study of repetitive transcranial magnetic stimulation (rTMS) in Tourette's syndrome. Cognitive and Behavioral Neurology: Official Journal of the Society for Behavioral and Cognitive Neurology. 2004;17:109–117. doi: 10.1097/01.wnn.0000116253.78804.3a. [DOI] [PubMed] [Google Scholar]
- 24.Münchau A, Bloem BR, Thilo KV, Trimble MR, Rothwell JC, Robertson MM. Repetitive transcranial magnetic stimulation for Tourette syndrome. Neurology. 2002;59:1789–1791. doi: 10.1212/01.wnl.0000036615.25044.50. [DOI] [PubMed] [Google Scholar]
- 25.Orth M, Kirby R, Richardson MP, Snijders AH, Rothwell JC, Trimble MR, et al. Subthreshold rTMS over pre-motor cortex has no effect on tics in patiens with Gilles de la Tourette syndrome. Clinical Neurophysiology. 2005;116:764–768. doi: 10.1016/j.clinph.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Mantovani A, Lisanby SH, Pieraccini F, Ulivelli M, Castrogiovanni P, Rossi S. Repetitive transcranial magnetic stimulation (rTMS) in the treatment of obsessive-compulsive disorder (OCD) and Tourette's syndrome (TS) International Journal of Neuropsychopharmacology. 2006;9:95–100. doi: 10.1017/S1461145705005729. [DOI] [PubMed] [Google Scholar]
- 27.Mantovani A, Leckman JF, Grantz H, King RA, Sporn AL, Lisanby SH. Repetitive transcranial magnetic stimulation of the supplementary motor area in the treatment of Tourette Syndrome: report of two cases. Clinical Neurophysiology. 2007;118:2314–2315. doi: 10.1016/j.clinph.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Lang A. Patient perception of tics and other movement disorders. Neurology. 1990;41:223–228. doi: 10.1212/wnl.41.2_part_1.223. [DOI] [PubMed] [Google Scholar]
- 29.Ziemann U, Paulus W, Rothenberger A. Decreased motor inhibition in Tourette's disorder: Evidence from transcranial magnetic stimulation. American Journal of Psychiatry. 1997;154:1277–1284. doi: 10.1176/ajp.154.9.1277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.