Abstract
Rac1 is a member of the small Rho GTPase family, which controls actin cytoskeleton and focal adhesion dynamics in cellular protrusions. While Rac1 therefore contributes to regulation of endothelial cell-cell and cell-matrix interactions, a detailed understanding of its role in endothelium function is lacking. Recently, the role of Rac1 in development and postnatal regulation of the cardiovascular system has been investigated in murine models lacking Rac1 specifically in endothelium. Homozygous endothelial deletion was lethal, primarily due to defects in angiogenesis. Rac1-deficient endothelial cells were unable to form cellular protrusions/lamellipodia, leading to impaired cell-cell and cell-matrix interactions, and resulting in dysfunctional adhesion, motility, permeability and capillary morphogenesis. Development was normal in the heterozygous model, however a hypertensive phenotype was observed as a result of reduced nitric oxide signalling. Nitric oxide synthase activity was regulated by Rac1 at multiple levels; expression, mRNA stability and uptake of the nitric oxide synthase substrate L-arginine. Therefore, Rac1 activity is essential in regulating developmental and postnatal angiogenesis and cardiovascular function, by controlling nitric oxide production, and formation of endothelial cell protrusions.
Key words: Rac1, angiogenesis, endothelial, motility, lamellipodia, nitric oxide, nitric oxide synthase
Introduction
Rho GTPases play a key role in communicating signals from cell surface receptors to regulate focal adhesion and cytoskeletal dynamics, thereby controlling cell-cell and cell-matrix interactions in response to external signals. Rac1 in particular, regulates focal adhesion and actin-based dynamics in cellular protrusions.1 As an effector of both integrin and growth factor signalling pathways, Rac1 activity is sensitive to a large milleu of extracellular signals thereby allowing rapid cellular responses to alterations in the immediate environment. In endothelium, rapid responses are vital in maintaining barrier function for controlled passage of nutrients, oxygen and waste products, and in immunological responses. In addition, rapid induction of sprouting angiogenesis is required for maintaining tissue homeostasis and function.
To investigate the role of Rac1 in cardiovascular development and homeostasis, Rac1 was deleted specifically from endothelium using the Cre-Flox system, under the control of Tie2 expression.2,3 In this system, exon 1 of Rac1 is flanked by Cre-LoxP sites (Rac1-Flox), resulting in deletion in the presence of Cre recombinase. To delete Rac1 in endothelium (denoted EC-Rac1−/−), homozygous Rac1-Flox mice (on a C57BL/6J background) were crossed with those expressing Cre recombinase under the control of Tie2 expression, which occurs in endothelial cells and their precursor hemangioblasts.2 Endothelial deletion resulted in lethality at E9.5, due to defects in vasculogenesis and sprouting angiogenesis in both the embryo and yolk sac, in addition to defective heart development. While the dorsal aorta developed normally, further development into major arteries and smaller vessels was prevented, thus developmental angiogenesis was particularly affected.2 However, since Tie2 activity is found in hemangioblasts, which are precursors of both endothelial and hematopoietic cell lineages, the phenotype of EC-Rac1−/− mice may also result from Rac1 deletion in haematopoietic cells. To confirm Cre recombinase expression in endothelium, Tie2Cre/EC-Rac1+/− mice were crossed with a reporter line, Rosa26R, where histological staining of β-galactosidase activity confirmed Cre recombinase activity in endothelial and endocardial cells at E9.5. Further, the authors assert that in contrast to their observations, defects in haematopoiesis generally result in lethality between E10.5 and E12.5, with few morphological changes.2 Thus, while an effect of Rac1 deletion on haematopoiesis cannot be ruled out, this is unlikely to play a major role in embryonic death observed in the study.2
In order to understand the lethal phenotype of EC-Rac1−/−-mice, pulmonary endothelial cells were isolated from homozygous Rac1-Flox mice, and Rac1 deleted in vitro, by adenoviral mediated expression of GFP-Cre recombinase.2 Validating this approach, GFP alone did not affect Rac1 expression, nor did GFP or Cre recombinase expression affect Rac1 levels in cells from wildtype mice (C57BL/6J strain). Rac1 deletion also did not affect expression of the related proteins Rac2 and Rac3, which are only minimally expressed in endothelial cells. Interestingly, while proliferation and viability was unaffected by the absence of Rac1, immunofluorescent staining of paxillin and actin revealed that Rac1 deficient cells were unable to form lamellipodia, and that focal adhesion formation was markedly reduced. Cells were also unable to respond properly to the stimulatory factors VEGF and S1P, resulting in deficient attachment to fibronection, impaired capillary morphogenesis on Matrigel, and reduced motility in Boyden chamber assays. In addition, the ability of S1P and VEGF to increase endothelium permeability was impaired, measured by passage of fluorescein isothiocyanate-conjugated dextran through endothelial cell monolayers. Subsequent immunofluorescent analysis of cell-cell contacts revealed that both adherens (VE-cadherin and β-catenin) and tight junctions (zonula occludens-1) were aligned in an abnormal zigzag-like pattern, and further, that reorganization into a characteristic punctate pattern was not induced by VEGF or S1P. Thus Rac1 was suggested to play a role not only in proper development of the cardiovascular system, but in regulating endothelium integrity and angiogenic responses.2
In a second study, the authors similarly observed that Rac1 homozygous deletion resulted in embryonic lethality. To investigate the role of Rac1 in postnatal endothelium, they chose instead to study the phenotype of heterozygous EC-Rac1+/− mice.3 Heterozygous Rac1-Flox mice that had not been crossed with CreTie2 mice (thus had normal expression of Rac1) were used as controls. In addition, an inducible Rac1 null model was utilised, where CreTie2 mice harbour a tamoxifen responsive element, allowing postnatal deletion of Rac1. EC-Rac1+/− mice were viable and no defects in growth or development were observed. However, blood pressure was modestly elevated. This mildly hypertensive phenotype is a hallmark of reduced production of the vasodilator, NO. NO is generated by eNOS, the activity of which is controlled at the level of mRNA synthesis and stability, in addition to post-transcriptional regulation, through phosphorylation and controlled uptake of the NOS substrate, L-arginine, by CAT-1. Indeed, levels of eNOS mRNA were reduced (analysed by quantitative reverse transcriptase-polymerase chain reaction and Northern blotting) in the aorta and brain of EC-Rac+/− mice, while western blotting showed reduced expression in the heart. In accordance with this observation, eNOS activity, determined by conversion of L-[3H]-arginine to L-[3H]-citrulline, was reduced in aorta. cGMP production (generated by NO-dependent activation of guanylate cyclase) was also reduced, confirming that endothelial NO production was impaired in cardiovascular tissue.3
Vasorelaxation can be induced by stimulating NO production from endothelium with calcium ionophores and acetylcholine, and production followed by analysing levels of the stable NO metabolites, nitrate and nitrite. In line with reduced eNOS activity, NO production by MHECs from EC-Rac+/− mice was reduced.3 Further, acetycholine-mediated relaxation of aortic rings from EC-Rac+/− mice was impaired, and contraction was more intense in response to the vasoconstrictor phenylephrine. Confirming endothelial cell dependent effects, impaired acetylcholine mediated relaxation was also observed in aortic rings from inducible EC-Rac1 null mice. Demonstrating NO dependence in this system, in control aorta, the NOS inhibitor L-NAME increased contraction both by itself, and in response to phenylephrine. Thus reduced Rac1 activity results in reduced NO production and hypertensive responses. NO is also an important regulator of angiogenesis. To investigate the role of Rac1-dependent NO production in angiogenesis, blood flow recovery was monitored following hind limb ischemia, after surgical resection of the left femoral artery. Recovery was severely impaired in EC-Rac1+/− mice, further, NOS inhibition with L-NAME similarly impaired recovery in control mice, supporting a role for Rac1-dependent NO signalling in this system. In addition, aortic explants from EC-Rac+/− mice exhibited reduced capillary sprouting when embedded in Matrigel. Confirming NO dependence in this model, sprouting from control explants was reduced by L-NAME, while either the NO donor S-nitrosoglutathione or NOS substrate L-arginine, were able to at least partially rescued impaired sprouting from EC-Rac+/− aortic explants.3
To investigate how Rac1 regulates NO production, Rac1 activity was inhibited in cultured endothelial cells (human aortic, HAEC, bovine aortic, BAEC, or murine microvascular, bEND.3) by adenovirus-mediated gene transfer of dominant negative Rac1, or use of a pharmacological inhibitor, NSC23766.3 eNOS mRNA and protein levels were reduced by Rac1 inhibition, and a luciferase reporter assay demonstrated that eNOS promoter activity was decreased. In addition, adenoviral-mediated expression of dominant negative PAK1 (a Rac1 effector) reduced eNOS promoter activity. Rac1 was also found to regulate stability of eNOS mRNA, since Rac1 inhibition reduced the half-life of eNOS mRNA when transcription was inhibited (with 5,6-dichlorobenzimidazole 1-β-D-ribofuranoside). Although Rac1 has been shown to decrease NO bioavailability by promoting reactive oxygen species formation through activation of NADPH oxidase, the authors found that NO levels were independent of this function of Rac1, since NADPH oxidase inhibition (with apocynin and N-acetylcysteine) did not reduce eNOS promoter activity. Finally, decreased eNOS activity in MHECs from EC-Rac+/− mice was also associated with reduced uptake of L-arginine. Expression of the L-arginine transporter CAT-1, was not affected, however immunoprecipitation experiments revealed Rac1 dependent association between the Rac1 effector PAK1, and CAT-1. Thus, Rac1 regulates NO production and NO dependent responses in endothelium, through reduced expression and activity of eNOS.
The two studies discussed here, demonstrate that Rac1 activity is required for development and postnatal regulation of the cardiovascular system.2,3 Failure of cells to form lamellipodia and therefore establish a leading edge in the absence of Rac1 abrogates directional motility and alignment into capillary networks.2 Indeed, Rac1 dependent protrusions are crucial for angiogenic morphogenesis.4 Further, a causal link between Rac1 activity and eNOS activation and NO production was demonstrated in EC-Rac1+/− mice.3 In support of this link, the hypertensive phenotype and impaired angiogenesis observed in this model is reminiscent of eNOS deficient mice.5 Also of relevance, the enzyme DDAH I metabolises an L-arginine analogue, ADMA, which competitively inhibits NOS activity. Similar to both eNOS deficient, and EC-Rac1+/− mice, DDAH I heterozygous knockout mice (which have increased ADMA levels and reduced NO production) exhibit hypertension and reduced capillary sprouting from aortic rings.6 Further, inhibition of NO production by ADMA decreases Rac1 activity in endothelial cells, resulting in reduced motility7 and failure to form lamellipodia, leading to impaired angiogenesis in DDAH I heterozygous mice, which is rescued by over-expression of active Rac1 (Fiedler LR, Bachetti T, Leiper J, Zachary I and Wojciak-Stothard B, manuscript in submission). Thus NO production both regulates, and is regulated by, Rac1 activity. On a molecular level, the crucial role played by Rac1 in establishing lamellipodia structures, could spatially control activity of cell surface molecules such as eNOS and CAT-1. eNOS localization in lamellipodia of migrating endothelial cells is dependent on cytoskeletal organization,8 further, eNOS and CAT-1 co-localization and association may facilitate activation of eNOS.9 The absence of cellular protrusions when Rac1 activity is reduced would not be permissive for formation and activation of such complexes. Further, Rac1 appears to play an important role in targeting such activities to the leading edge of endothelial cells.
In summary (Fig. 1), activity of the Rho GTPase Rac1 is required for cardiovascular development and postnatal endothelium function. Rac1 regulates NO production by enhancing expression of eNOS and uptake of the eNOS substrate, L-arginine. Thus inhibition of Rac1 reduces NO production, resulting in hypertension and impaired angiogenesis. In turn, inhibiting NO production decreases Rac1 activity, indicating a reciprocal link between Rac1 activity and NO production. Rac1 deficient endothelial cells fail to form lamellipodia, required for proper cell-cell and cell-matrix interactions, resulting in impaired barrier function and dysregulated motility, and leading to impaired vascular development and angiogenesis. Reduced formation of lamellipodia/protrusions may also spatially regulate activity of cell surface molecules such as CAT-1 and eNOS, which depend on lamellipodial co-localization for activation. Thus Rac1 activity is critically required for both development and postnatal regulation of the cardiovascular system, through control of NO production, cell motility and localization of signalling complexes to the leading edge of the cell.
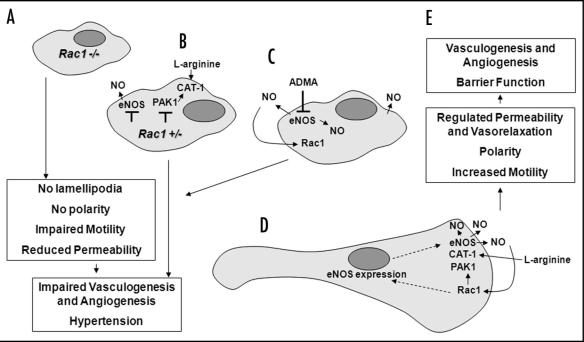
Figure 1.
Rac1 is required for cardiovascular development and postnatal endothelium function. Rac1 deficient endothelial cells fail to form lamellipodia, leading to reduced motility, permeability and impaired vascular development and angiogenesis (A). Reduced Rac1 activity inhibits NO production by decreasing eNOS expression, reducing uptake of the eNOS substrate L-arginine, and preventing formation of a CAT-1/eNOS complex, resulting in hypertension and impaired angiogenesis (B). Conversely, inhibiting NO production (using the NOS inhibitor ADMA) reduces Rac1 activity, similarly resulting in failure of cells to establish a leading edge, and leading to hypertension and impaired angiogenesis (C). Rac1 therefore regulates NO production by upregulating eNOS and increasing L-arginine uptake through CAT-1. CAT-1 and eNOS co-localise in lamellipodia, in association with PAK1, supporting eNOS activity and NO production at the leading edge of the cell (D). Formation of Rac1 dependent lamellipodia supports directional motility, angiogenesis and proper barrier and vasorelaxation functions of endothelium (E).
Abbreviations
- NO
nitric oxide
- eNOS
endothelial nitric oxide synthase
- E
embryonic day
- EC
endothelial cell
- S1P
sphingosine 1 phosphate
- VEGF
vascular endothelial growth factor
- GFP
green fluorescent protein
- CAT-1
cationic amino acid transporter 1
- L-NAME
N-Nitro-L-arginine methyl ester
- cGMP
cyclic guanosine monophosphate
- MHEC
mouse heart endothelial cells
- ADMA
asymmetric dimethylarginine
- DDAH I
dimethylarginine dimethylaminohydrolase 1
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: www.landesbioscience.com/journals/celladhesion/article/8279
References
- 1.Ridley AJ. Rho GTPases and cell migration. J Cell Sci. 2001;114:2713–2722. doi: 10.1242/jcs.114.15.2713. [DOI] [PubMed] [Google Scholar]
- 2.Tan W, Palmby TR, Gavard J, Amornphimoltham P, Zheng Y, Gutkind JS. An essential role for Rac1 in endothelial cell function and vascular development. FASEB J. 2008;22:1829–1838. doi: 10.1096/fj.07-096438. [DOI] [PubMed] [Google Scholar]
- 3.Sawada N, Salomone S, Kim H-H, Kwiatkowski DJ, Liao JK. Regulation of endothelial nitric oxide synthase and postnatal angiogenesis by Rac1. Circ Res. 2008;103:360–368. doi: 10.1161/CIRCRESAHA.108.178897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connolly JO, Simpson N, Hewlett L, Hall A. Rac Regulates Endothelial Morphogenesis and Capillary Assembly. Mol Biol Cell. 2002;13:2474–2485. doi: 10.1091/mbc.E02-01-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas SR, Witting PK, Drummond GR. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2008;10:1713–1765. doi: 10.1089/ars.2008.2027. [DOI] [PubMed] [Google Scholar]
- 6.Leiper J, Nandi M, Torondel B, Murray-Rust J, Malaki M, O'Hara B, et al. Disruption of methylarginine metabolism impairs vascular homeostasis. Nat Med. 2007;13:198–203. doi: 10.1038/nm1543. [DOI] [PubMed] [Google Scholar]
- 7.Wojciak-Stothard B, Torondel B, Tsang LY, Fleming I, Fisslthaler B, Leiper JM, et al. The ADMA/DDAH pathway is a critical regulator of endothelial cell motility. J Cell Sci. 2007;120:929–942. doi: 10.1242/jcs.002212. [DOI] [PubMed] [Google Scholar]
- 8.Bulotta S, Cerullo A, Barsacchi R, Palma CD, Rotiroti D, Clementi E, et al. Endothelial nitric oxide synthase is segregated from caveolin-1 and localizes to the leading edge of migrating cells. Exp Cell Res. 2006;312:877–889. doi: 10.1016/j.yexcr.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Li C, Huang W, Harris MB, Goolsby JM, Venema RC. Interaction of the endothelial nitric oxide synthase with the CAT-1 arginine transporter enhances NO release by a mechanism not involving arginine transport. Biochem J. 2005;386:567–574. doi: 10.1042/BJ20041005. [DOI] [PMC free article] [PubMed] [Google Scholar]