Abstract
Monocyte-extracellular matrix interactions have been implicated in atherosclerosis pathophysiology. Laminin, the main basement membrane protein contains cell binding domains that can be cryptic, presented only after protein modification. In the present study we evaluated monocyte attachment to laminin-1 in the presence of ATP. Monocytes were derived from either healthy volunteers or patients with diabetes mellitus type II. For the estimation of monocyte attachment to laminin the myeloperoxidase assay was used. Monocytes derived from diabetic patients, showed an increased ability to attach to laminin (p = 0.0055). The presence of ATP increased the attachment of control monocytes to laminin (p = 0.0022). On the contrary, the presence of ATP did not affect the attachment of monocytes derived from diabetic patients to laminin. Our results indicate a modified interaction between monocytes and laminin-1 in diabetes mellitus
Key words: monocytes, ATP, laminin-1, diabetes mellitus, attachment
Introduction
Atherosclerosis is a chronic inflammatory disease of the inner tunic of the arteries that is characterized from the formation of the atheromatic lesion. Monocytes play a central role in the pathogenesis of atheromatosis, a common disease in diabetic patients.1 After their attachment to the vascular endothelium, monocytes migrate through the endothelial cells to the sub-endothelial space, triggered by specific surface factors which are present to the endothelial cells after a local dysfunction. In the sub-endothelial space monocytes attach to basement membrane proteins, such as laminin.
Laminins are multidomain and multifunctional cross-shaped glycoproteins that consist of three distinct disulfide linked polypeptide chains (α, β and γ). Laminins are structural components of the basement membrane and play a key role in cell adhesion, migration, differentiation and proliferation of many cell types.2–4
Protein phosphorylation by protein kinases regulates many cellular processes and is mediated by the presence of ATP. Phosphorylation mediated by ectokinases could represent a novel mechanism for the communication and interaction between the cells and the environment. The data of a previous study conducted in our laboratory indicated that laminin-1 is phosphorylated by the ecto-protein kinases of monocytes on serine residues of the β and/or γ chains.5 Furthermore, recent studies indicated that post-translational modifications of the laminin molecule, such as phosphorylation and carbonylation strongly influence its biological properties.5–8
Monocyte attachment and migration have been associated with the formation of the atheromatic lesion, which is a frequent complication of diabetes mellitus.1,9 In an attempt to investigate the biological significance of laminin phosphorylation in the initiation of the atherosclerotic process, we studied monocyte attachment to laminin-1 using monocytes derived from healthy volunteers and diabetic patients.
Results
Monocyte attachment to laminin in the presence of ATP.
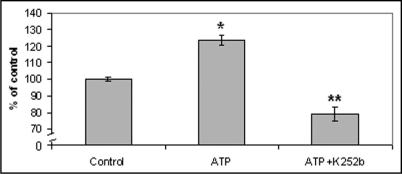
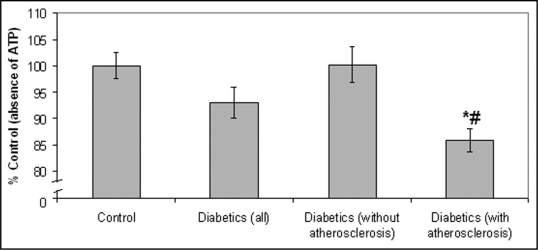
According to our results, healthy volunteers showed an increased attachment to laminin in the presence of ATP (83.2%) (p = 0.0022), as compared to the absence of ATP, while the protein kinase inhibitor, K252b abolished the above effect of ATP (Fig. 1). On the contrary, the presence of ATP did not seem to affect the attachment of diabetic monocytes (Fig. 1). Moreover, all control samples (in the absence of ATP) were treated with the inhibitor K252b and according to our results, K252b didn't affect monocyte attachment to laminin. Our results also indicate that monocyte attachment to laminin was associated with the presence or absence of atheromatic lesions. More precisely, monocytes derived from diabetic patients with atherosclerosis showed a decreased monocyte attachment to laminin in the presence of ATP, as compared to those without atherosclerosis (p = 0.002) and as compared to the absence of ATP (p < 0.0001) (Fig. 2). It is also worth to mention that in some cases, where diabetic monocytes attached to laminin at a higher degree as compared to the absence of ATP (6 from 45 patients), the patients had short duration of diabetes and no apparent atheromatic lesions. Moreover, it should be pointed out that monocyte attachment in the absence of ATP was the same for the three groups studied (all diabetics, diabetics with atherosclerosis and diabetics without atherosclerosis).
Figure 1.
Monocyte attachment to laminin-1 in the presence of ATP. According to our results monocytes derived from healthy volunteers attach to laminin at a higher degree in the presence of ATP as compared to the absence of ATP. Moreover, the inhibitor K252b abolished the above effect of ATP. The results are expressed as % of control. Bars represent mean value ± standard deviations. *indicates significant difference with the control value. **indicates significant difference with both the control and the ATP value.
Figure 2.
Diabetic monocyte attachment to laminin in the presence of ATP. Our results indicate that monocytes derived from diabetic patients with atherosclerosis showed a decreased monocyte attachment to laminin in the presence of ATP, as compared to those without atherosclerosis and as compared to the absence of ATP. Bars represent mean value ± standard deviations. *indicates significant difference with the control value (absence of ATP). #indicates significant difference with the value obtained in monocytes derived from diabetic patients without atherosclerosis.
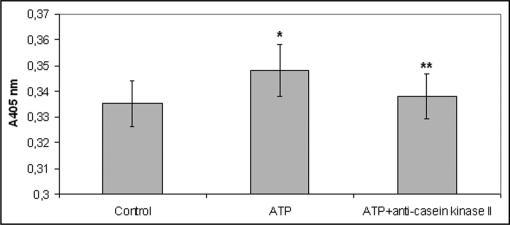
Anti-casein kinase II antibody had a similar effect with K252b. More precisely, monocytes derived from healthy volunteers that preincubated with the anti-casein kinase II antibody showed a decreased attachment to laminin in the presence of ATP, as compared to those incubated in the absence of the antibody (Fig. 3).
Figure 3.
Monocyte attachment to laminin-1 in the presence of ATP after their pre-incubation with the anti-casein kinase II antibody or not. Monocytes derived from healthy volunteers that preincubated with the anti-casein kinase II antibody showed a decreased attachment to laminin in the presence of ATP, as compared to those incubated in the absence of the antibody. Bars represent mean value ± standard deviations. *indicates significant difference with the control value (absence of ATP). **indicates significant difference with the ATP value.
Furthermore, the above differences in the presence of ATP weren't observed in the case that laminin was oxidized (data not shown).
Monocyte attachment to pre-phosphorylated by casein kinase II laminin.
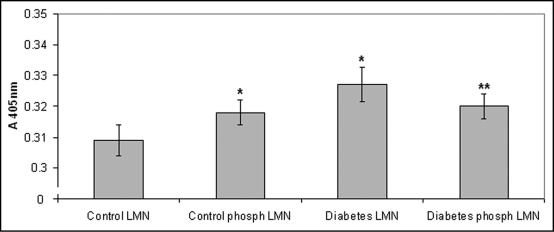
In an attempt to elucidate whether the increased monocyte attachment could be attributed to laminin phosphorylation, monocyte attachment to pre-phosphorylated by casein kinase II laminin was then estimated. Our results showed that control monocytes attached to pre-phosphorylated laminin at a higher degree as compared to the non-phosphorylated molecule (p = 0.0284). On the other hand, diabetic monocytes attached at a lower degree to pre-phosphorylated laminin as compared to the non-phosphorylated molecule (p = 0.0063), while no differences between control and diabetic monocyte in relation to attachment to pre-phosphorylated laminin were observed in these experiments (Fig. 4). Furthermore, according to our results, monocyte attachment to pre-phosphorylated laminin was lower than monocyte attachment to laminin in the presence of ATP (p = 0.05).
Figure 4.
Monocyte attachment to pre-phosphorylated by casein kinase II laminin. Control monocytes attached to pre-phosphorylated laminin at a higher degree as compared to the non-phosphorylated molecule, while diabetic monocytes attached at a lower degree to pre-phosphorylated laminin as compared to the non-phosphorylated molecule. Bars represent mean value ± standard deviations. *indicates significant difference with the control value. **indicates significant difference with both the control and the diabetic value.
Discussion
Previous studies performed in our laboratory showed that laminin-1 is phosphorylated by the protein kinase A (PKA) and the protein kinase C (PKC).6,10 The scope of the present study was to investigate the role of extracellular ATP in monocyte attachment to laminin and find possible differences between healthy volunteers and diabetic patients. Laminin is phosphorylated by PKC and this phosphorylation could affect cell binding to the molecule,6 while laminin can be also phosphorylated by the monocyte casein kinase II type ectokinase, affecting functions, including cell binding and proliferation.5 Furthermore, a previous study performed in our laboratory, showed that diabetic monocytes attach at a higher degree to laminin as compared to those from healthy volunteers.7 The data of the present study indicate for the first time that monocyte attachment to laminin is influenced by ATP in control but not in diabetic monocytes.
Laminin-1 has been widely used for the study of laminin interactions with blood cells.5,7,11,12 It is largely limited to the epithelial basement membranes, while is not found in bone marrow and the majority of vessels.13,14 The vascular-specific laminin isoforms that encounter with monocytes during extravasation are laminin-8 and laminin-10.15 Previous studies performed in our laboratory showed that monocytes phosphorylate laminin on its β-1 and/or γ-1 chains.5 Interestingly, although laminin-1, laminin-8 and laminin-10 have different α chains, they share the same beta and gamma isoforms (β1-α1).16 Moreover, a major advantage of laminin-1 is that it can be easily isolated from the Engelbreth-Holm-Swarm (EHS) tumor.17 Therefore, in the present study laminin-1 instead of either laminin-8 or laminin-10 was used.
Our results showed that control monocytes attach at a higher degree to laminin in the presence of ATP while the ecto-protein kinase inhibitor, K252b abolished the above effect of ATP. Monocytes interact with extracellular matrix (ECM) components promoting their biological properties. Reversible attachment of monocytes to ECM promotes their migration from the vascular sheet to regions, where they are differentiated into macrophages.18 It could be assumed, that the mechanism of reversible attachment is connected with the reversible mechanism of phosphorylation (phosphorylation ↔ de-phosphorylation). The presence of ATP decreased monocyte attachment after ectokinase inhibition. Accordingly, it could be conjectured that ATP might inhibit monocyte attachment through its negative charge. The same mechanism could also explain the low diabetic monocyte attachment to laminin in the presence of ATP.
In an attempt to elucidate whether the increased monocyte attachment could be attributed to laminin phosphorylation, monocyte attachment to pre-phosphorylated laminin was also estimated. Our results showed that control monocytes attached to pre-phosphorylated laminin at a higher degree as compared to the non-phosphorylated molecule. Monocyte attachment to pre-phosphorylated laminin was lower than monocyte attachment to laminin in the presence of ATP. Based on the above, it is suggested that normal monocytes may first phosphorylate and then attach to laminin, however an additional direct effect of ATP to monocytes cannot be excluded. On the other hand, diabetic monocytes attached at a lower degree to pre-phosphorylated laminin as compared to the non-phosphorylated molecule. Moreover, no differences between control and diabetic monocyte in relation to attachment to pre-phosphorylated laminin were observed in these experiments. These results indicate a different behaviour of diabetic monocytes considering attachment to laminin.
Previous studies performed in our laboratory showed that monocytes seem first to oxidize and then to attach to laminin.7 Moreover, according to previous experiments performed by our research group, ATP does not significantly influence monocyte attachment to the oxidized laminin. Therefore, the sensitivity of monocytes to ATP at the early stages of diabetes could be attributed to differences in their oxidation capacity. However, previous data of our group7 did not establish a difference between diabetic monocytes with and without atherosclerosis considering their ability to oxidize laminin. Another explanation may be the inhibition of ectoprotein kinase in monocytes with advanced diabetes, as mentioned above. A third explanation may include differences on the expression of monocyte integrin receptors as has been previously reported.7
Monocyte attachment to laminin is mainly mediated through integrin receptors. Integrins that recognise laminin and are present on monocytes are: a2b1, a1b1, a6b1, aLb2 and aMb2. Previous studies performed in our laboratory showed that the integrin α2β1 plays a significant role in the increased diabetic monocyte attachment to laminin-1 as compared to that of healthy volunteers. Moreover, our results showed that diabetic monocytes contain higher levels of the alpha2 integrin subunit as compared to monocytes derived from healthy volunteers.7 An increased level of alpha2 integrin subunit expression was also found in tissues with the presence or risk of developing diabetic nephropathy as compared to the control tissue, while alpha2 is also known to be involved in atherosclerosis.19–21 The role of other integrin molecules in the above phenomena cannot be excluded. The aMb2 integrin levels are also increased in patients with diabetes mellitus and are related to ROS production after monocyte attachment to oxidized lipoproteins.22 It is also suggested that phosphorylation could modify apart from laminin, the integrin structure, promoting increased attachment to phosphorylated or non-phosphorylated laminin. Thus, the elucidation of the involvement of other integrin and non integrin cell surface molecules remains within the scope of our future studies.
Other data suggest that extracellular phosphorylation promotes cellular attachment. This mechanism could be supported by the fact that the protein kinase inhibitor K252b abolished the increased monocyte attachment to laminin observed in the presence of ATP. When monocytes differentiate into macrophages the activity of their surface ectokinases is increased.23 Moreover, during inflammation, endothelial cells, T-helpers and T-cytotoxic cells, release increased amounts of ATP, which are necessary for the activation of ectokinases.24 On the other hand, direct action of ATP through adenosine receptors cannot be excluded.
Monocyte-laminin interactions are crucial, not only under physiological, but also under pathological conditions and specifically, during atherogenesis, which is a frequent complication of diabetes mellitus.1,9,25 Therefore, in the present study, monocyte samples derived from diabetic patients with macro or microvascular complications were also included. Our data indicate that monocytes derived from patients with diabetes mellitus showed an increased attachment to laminin as compared to controls. Moreover, the presence of ATP did not influence monocyte binding to LMN in most diabetic patients. This phenomenon was not observed in diabetic patients with short duration of diabetes and without atheromatic lesions.
It is possible that in diabetes mellitus are activated different mechanisms of monocyte attachment to laminin, in relation to physiological circumstances. Those mechanisms are probably independent from the presence of ATP and result to a wider affinity of monocytes to laminin. The lower ATP synthesis found in type II diabetes could also support the above hypothesis.26 The data of the present study document for the first time that the presence of ATP affects lamininmonocyte interactions and suggest that laminin phosphorylation by activated monocytes may be an additional puzzle piece towards the solution of atherosclerosis pathophysiology.
Materials and Methods
Materials.
Laminin-1 was purchased from Invitrogen. Percoll and RPMI-1640 were from Biochrom. ATP, hexadecyltrimethylammonium bromide and dianisidinedihydrochloride tablets were from Sigma. K252b was from Calbiochem and mouse anti-human casein kinase 2a monoclonal antibody was from Chemicon international MAB 8695.
Participating subjects.
Monocytes were prepared by differential centrifugation and solid face attachment from 90 blood samples taken either from 45 patients with diabetes mellitus II or from 34 age matched healthy volunteers (46.9 ± 10.89 years) (Table 1). The average diabetes duration of the patients was 15 ± 3 years. The presence of atherosclerotic plaques was ascertained by an ultrasonic examination in 23 diabetic patients. The presence of atherosclerotic plaques wasn't ascertained in any of the 34 healthy volunteers studied. Moreover, neither the healthy donors nor the diabetic patients participated in our study took any specific medication such as aspirin or statins, which are known to affect monocyte adhesion. All participants were informed about the aims of the study. The reported investigations have been carried out in accordance with the principles of the Declaration of Helsinki as revised in 2000.
Table 1.
Clinical parameters of healthy and diabetic participants
| Age (years) | BMI | Glucose levels (mg/dl) | Glycated haemoglobin | Prevalence of atherosclerosis | Medication | |
| Healthy volunteers | 46.9 ± 10.89 | 22.98 ± 2.11 | 89 ± 6* | 4.9 ± 0.3%* | - | - |
| Diabetic patients | 47.13 ± 12.65 | 23.4 ± 1.53 | 149 ± 12 | 7.7 ± 0.8% | 25 from 56 patients | - |
indicates statistically significant difference to the respective diabetic value.
Monocyte preparation.
Monocytes were prepared by differential centrifugation and solid face attachment.
Twenty ml of blood were placed in a 50 ml falcon that contained 1 ml 200 mM ethylenodiaminotetracetic acid (EDTA) in normal saline. An equal quantity of cell culture medium RPMI 1640 containing 1% of L-gloutamine, 1% penicillin/streptomycin, 1% 1M N-(2-hydroxymethyl) piperazine-N'-(2-ethanesulfonicacid) (HEPES) and 10% fetal calf serum. The blood was then layered over 6 ml Percoll in two falcon tubes and centrifuged for 30 min at 1,500 rpm and room temperature in a swinging head centrifuge with minimum acceleration and no brake. The leukocyte layer formed between the Percoll and serum layer was then carefully aspirated by a Pasteur pipette and placed in a separate tube. The leukocytes were then centrifuged and washed three times with cell culture medium (1,500 rpm for 10 min). The cell suspension was then placed in a cell culture flask and incubated for 1 h at 37°C. After incubation, the culture medium was removed and the monocytes were washed for two times with 10 ml of cell culture medium. After the addition of 10 ml of cell culture medium, the cells were incubated at 37°C overnight. The cell medium was then removed and attached cells were washed twice with culture medium. Monocytes were then obtained using a cell scraper. Monocytes were washed once and re-suspended in 2 ml of culture medium without serum. The number of monocytes isolated was estimated by measurement on a Neubauer plate. A total of 1–2 million cells were isolated each time with a viability of at least 98%.
Monocyte adhesion assays.
For the evaluation of monocyte attachment to laminin-1 two different assays were used.
Two hundred microliters of cell suspension that contained 80,000 cells were placed in each well of polystyrene plates that have been precoated with 37.5 µg/ml laminin-1. Cell suspension (cell culture in RPMI 1640, 10% FCS) contained 200 µM Nα2MoO4, 200 µM Nα3VO4 and 1 mM Mn2+. 5 µM ATP and 3.6 µg/ml K252b an ectokinase inhibitor, were also added when necessary. Control samples contained K252b in all the experiments performed in order to exclude any possible toxic effects of the inhibitor. In some experiments monocytes were pre-incubated with 2 µg/ml of a specific mouse anti-human casein kinase 2a monoclonal antibody or with 20 µl of 250 µg/ml anti-human IgG in order to block nonspecific binding, for 10 min in eppendorf tubes at 37°C. Cells were then incubated for 30 min at 37°C. After incubation, non-adherent cells were discarded by aspiration and the wells were rinsed three times with 100 µl sterile phosphate buffered saline (PBS).
Monocyte attachment to laminin was quantified using the myeloperoxidase (MPO) assay. In brief, the cells were lysed using 0.5% (w/v) hexadecyltrimethylammonium bromide in PBS (pH 6.0) for 30 min/37°C. After lysis, the substrates of the MPO enzyme (50 µl 0.2 mg/ml dianisidinedihydrochloride in PBS (pH 6.0) that also contained 0.4 mM H2O2) were added and the cells were then incubated under gentle stirring for 15 min at 37°C. The MPO activity of the lysate was measured spectrophotometrically at 405 nm, using an ELISA reader.
In the above described conditions monocytes are known to phosphorylate laminin. In particular, previous studies performed in our laboratory showed that incubation of monocytes with laminin in the presence of ATP for 10 min at 37°C resulted to the phosphorylation of the molecule.5 In the present study monocytes were incubated with laminin in the presence of ATP for more than 10 min (30 min) in order to be able not only to phosphorylate laminin, but also to attach to the protein.
Monocyte attachment to pre-phosphorylated laminin was also estimated using the above described adhesion assay. Laminin-1 was phosphorylated with non-radioactive ATP. The phosphorylation mixture contained 20 mM Tris pH 7.5, 16 mM NaCl, 0.57 mM CaCl2, 5 mM Magnesium acetate, 8 µg/ml phosphatidylserine, 0.8 µg/ml diacylglycerol, 330 µg/ml laminin-1, 9 µM ATP and 2.5 mU protein kinase C in a total volume of 100 µl (in pilot experiments 5 µl endoplasmic reticulum preparation was used instead of protein kinase C). Phosphorylation was performed by incubation at 37°C for 10 min and the reaction was stopped by chilling in ice.6 In some experiments monocyte attachment to oxidized laminin in the presence of ATP was also estimated. Laminin-1 was oxidized using 1 nM 2.2′methylpropionamidine-dihydrochloride (ABAP).
Statistical analysis.
For the statistical evaluation, the statistical software GraphPad InStat version 3.00 for Windows was used (GraphPad Software Inc., San Diego, CA, USA). Values are expressed as means ± standard error of means (S.E.M). The statistical significance of the differences between the sets of data was estimated by the Student's t-test (paired or un-paired). The two-tailed p < 0.05 was used as the significance level.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/8207
References
- 1.Blaha M, Krejsek J, Blaha V, Andrys C, Vokurkova D, et al. Selectins and monocyte chemotactic peptide as the markers of atherosclerosis activity. Physiol Res. 2004;53:273–278. [PubMed] [Google Scholar]
- 2.Castronovo V. Laminin receptors and laminin-binding proteins during tumor invasion and metastasis. Invasion Metastasis. 1993;13:1–30. [PubMed] [Google Scholar]
- 3.De Arcangelis A, Neuville P, Boukamel R, Lefebvre O, Kedinger M, Simon-Assmann P. Inhibition of laminin alpha1-chain expression leads to alteration of basement membrane assembly and cell differentiation. J Cell Biol. 1996;133:417–430. doi: 10.1083/jcb.133.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aumailley M, Smith N. The role of laminins in basement membrane function. J Anat. 1998;193:1–21. doi: 10.1046/j.1469-7580.1998.19310001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trachana V, Christophorides E, Kouzi-Koliakos K, Koliakos G. Laminin-1 is phosphorylated by ecto-protein kinases of monocytes. Int J Biochem. 2005;37:478–492. doi: 10.1016/j.biocel.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Koliakos G, Trachana V, Gaitatzi M, Dimitriadou A. Phosphorylation of laminin-1 by protein kinase C. Mol Cells. 2001;11:179–185. [PubMed] [Google Scholar]
- 7.Kostidou E, Koliakos G, Alamdari DH, Paletas K, Tsapas A, Kaloyianni M. Enhanced laminin carbonylation by monocytes in diabetes mellitus. Clin Biochem. 2007;40:671–679. doi: 10.1016/j.clinbiochem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Kostidou E, Koliakos G, Paletas K, Sarigianni M, Kaloyianni M. Monocyte attachment to native and MGO-treated laminin. Differences between healthy volunteers and diabetic patients. J Adhes. 2008;84:1023–1032. [Google Scholar]
- 9.Takahashi K, Takeya M, Sakashita N. Multifunctional roles of macrophages in the development and progression of atherosclerosis in humans and experimental animals. Med Electron Microsc. 2002;35:179–203. doi: 10.1007/s007950200023. [DOI] [PubMed] [Google Scholar]
- 10.Koliakos G, Kouzi-Koliakou K, Triantos A, Trachana V, Kavoukopoulos E, Gaitatzi M, et al. Laminin-1 phosphorylation by protein kinase A: Effect on self assembly and heparin binding. J Biochem Mol Biol. 2000;5:370–378. [Google Scholar]
- 11.Perri RT, Vercelotti G, McCarthy J, Vessella RL, Furcht LT. Laminin selectively enhances monocyte-macrophage-mediated tumoricidal activity. J Lab Clin Med. 1985;105:30–35. [PubMed] [Google Scholar]
- 12.Penberthy T, Jiang Y, Luscinskas F, Graves D. MCP-1-stimulated monocytes preferentially utilize beta-2 integrins to migrate on laminin and fibronectin. Am J Physiol. 1995;269:60–68. doi: 10.1152/ajpcell.1995.269.1.C60. [DOI] [PubMed] [Google Scholar]
- 13.Falk M, Ferletta M, Forsberg E, Ekblom P. Restricted distribution of laminin alpha chain in normal adult mouse tissues. Matrix Biol. 1999;18:557–568. doi: 10.1016/s0945-053x(99)00047-5. [DOI] [PubMed] [Google Scholar]
- 14.Gu Y, Sorokin L, Durbeej M, Hjalt T, Jonsson J, et al. Characterization of bone marrow laminins and identification of alpha5-containing laminins as adhesive proteins for multipotent hematopoietic FDCP-Mix cells. Blood. 1999;93:2533–2542. [PubMed] [Google Scholar]
- 15.Pedraza C, Geberhiwot T, Ingerpuu S, Assefa D, Wondimu, et al. Monocytic cells synthesize, adhere to, and migrate on laminin-8 (alpha4beta1gamma1) J Immunol. 2000;165:5831–5838. doi: 10.4049/jimmunol.165.10.5831. [DOI] [PubMed] [Google Scholar]
- 16.Miner J, Patton B, Lentz S, Gilbert D, Snider W, et al. The Laminin a Chains: Expression, Developmental Transitions, and Chromosomal Locations of α1–5, Identification of Heterotrimeric Laminins 8–11, and Cloning of a Novel a3 Isoform. J Cell Biol. 1997;137:685–701. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, et al. Laminin: A glycoprotein from basement membranes. J Biol Chem. 1979;254:9933–9937. [PubMed] [Google Scholar]
- 18.Owen C, Campbell E, Stockley R. Monocyte adherence to fibronectin: role of CD11/CD18 integrins and relationship to other monocyte functions. J Leukoc Biol. 1992;51:400–408. doi: 10.1002/jlb.51.4.400. [DOI] [PubMed] [Google Scholar]
- 19.Jin DK, Fish AJ, Wayner EA, Mauer M, Setty S, Tsilibary E, et al. Distribution of integrin subunits in human diabetic kidneys. J Am Soc Nephrol. 1996;7:2636–2645. doi: 10.1681/ASN.V7122636. [DOI] [PubMed] [Google Scholar]
- 20.Tsilibary PE, Charonis A, Setty S, Mauer M. Analysis of alpha integrins for the diagnosis of diabetic nephropathy. United States Patent. 2004 6780603.
- 21.Grenache D, Coleman T, Semenkovich C, Santoro S, Zutter M. α2β1 integrin and development of atherosclerosis in a mouse model. Assessment of risk. Arterioscler Thromb Vasc Biol. 2003;23:2104–2149. doi: 10.1161/01.ATV.0000097282.22923.EF. [DOI] [PubMed] [Google Scholar]
- 22.Husemann J, Obstfeld A, Febbraio M, Kodama T, Silverstein S. CD11b/CD18 mediates production of reactive oxygen species by mouse and human macrophages adherent to matrixes containing oxidized LDL. Artioscler Thromb Vasc Biol. 2001;21:1301–1305. doi: 10.1161/hq0801.095150. [DOI] [PubMed] [Google Scholar]
- 23.Geberhiwot T, Skoglund G. Ectoprotein kinase activities on non-differentiated and differentiated U-937 cells. Cell Signal. 1995;7:423–429. doi: 10.1016/0898-6568(94)00093-q. [DOI] [PubMed] [Google Scholar]
- 24.Chiang T, Wojcikiewicz R, Kang A, Fain J. Phosphorylation of the outer surface of platelets enhances the effects of collagen on aggregation, ATP release, calcium translocation and phosphoinositide hydrolysis. Thromb Res. 1988;50:719–731. doi: 10.1016/0049-3848(88)90330-1. [DOI] [PubMed] [Google Scholar]
- 25.Schulz E, Keaney J. Diabetes mellitus and endothelial dysfunction: a central role for oxidative stress. Curr Opin Endocrinol Diabetes. 2003;10:237–244. [Google Scholar]
- 26.Szendroedi J, Schmid AI, Chmelik M, Toth C, Brehm A, et al. Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLOS Medicine. 2007;4:154. doi: 10.1371/journal.pmed.0040154. [DOI] [PMC free article] [PubMed] [Google Scholar]