Abstract
Cathepsin X is a lysosomal cysteine protease, found predominantly in cells of monocyte/macrophage lineage. It acts as a monocarboxypepidase and has a strict positional and narrower substrate specificity relative to the other human cathepsins. In our recent studies we identified—β2 subunit of integrin receptors and α and γ enolase as possible substrates for cathepsin X carboxypeptidase activity. In both cases cathepsin X is capable to cleave regulatory motifs at C-terminus affecting the function of targeted molecules. We demonstrated that via activation of β2 integrin receptor Mac-1 (CD11b/CD18) active cathepsin X enhances adhesion of monocytes/macrophages to fibrinogen and regulates the phagocytosis. By activation of Mac-1 receptor cathepsin X may regulate also the maturation of dendritic cells, a process, which is crucial in the initiation of adaptive immunity. Cathepsin X activates also the other β2 integrin receptor, LFA-1 (CD11a/CD18) which is involved in the proliferation of T lymphocytes. By modulating the activity of LFA-1 cathepsin X causes cytoskeletal rearrangements and morphological changes of T lymphocytes enhancing ameboid-like migration in 2-D and 3-D barriers and increasing homotypic aggregation. The cleavage of C-terminal amino acids of α and γ enolase by cathepsin X abolishes their neurotrophic activity affecting neuronal cell survival and neuritogenesis.
Key words: cathepsin X, integrin, enolase, T lymphocyte, macrophage, dendritic cell, adhesion, migration, neuritogenesis
Proteases comprise a group of enzymes that catalyse the cleavage of a peptide bond in a polypeptide chain by nucleophilic attack on the carbonyl carbon. The proteases are either exopeptidases cleaving one or a few amino acids at the N- or C-terminus of polypeptide chain or endopeptidases that cleave the peptide bond internally. According to the catalytic mechanism the endopeptidases are divided into aspartic, cysteine, serine, threonine and metallo endoproteases—see MEROPS database.1 To date, 561 genes encoding for proteases have been identified in human genome. Among them 148 genes encode for cysteine proteases including a group of eleven lysosomal cysteine proteases (members of C1 family) also called cathepsins. They exhibit different expression patterns, levels and specificities, all of which contribute to their differential physiological roles. Some of them, like cathepsins B, H, L and C are ubiquitously present in tissues, whereas others (cathepsins S, V, X, O, K, F and W) are expressed by specific cell types. Cysteine cathepsins were long believed to be responsible for the terminal protein degradation in the lysosomes, however, this view has changed dramatically when they have been found to be involved in a number of important cellular processes and pathologies.2,3
In contrast to other cathepsins, cathepsin X was discovered only recently. Its gene,4,5 structure6,7 and activity properties8,9 show several unique features that distinguish it clearly from other human cysteine proteases. It has a very short pro-region7 and a three residue insertion motif which forms a characteristic “mini loop.”6 Cathepsin X exhibits carboxypeptidase activity6 and, in contrast to cathepsin B, the other carboxypeptidase, it does not act as an endopeptidase. Contrary to the first reports,4 cathepsin X is not widely expressed in cells and tissues, but is restricted to the cells of the immune system, predominantly monocytes, macrophages and dendritic cells.10 Higher levels of cathepsin X were also found in tumor and immune cells of prostate11 and gastric12 carcinomas and in macrophages of gastric mucosa, especially after infection by Helicobacter pylori.13 Recently it was shown that cathepsin X is abundantly expressed in mouse brain cells, in particular glial cells. Its upregulation was also detected in the brains of patients with Alzheimer disease.14
The involvement of cathepsin X in signal transduction is implied by the integrin-binding motifs, present in its pro-form (RGD: Arg-Gly-Asp) and mature form (ECD: Glu-Cys-Asp).4,5 Moreover, cathepsin X binds cell surface heparan sulfate proteoglycans15 which are also involved in integrin regulation. A strong co-localization of pro-cathepsin X with β3 integrin subunit was demonstrated in our study in pro-monocytic U-937 cells.16 Further, it was reported that the pro-form of cathepsin X interacts with αvβ3 integrin through the RGD motif in lamellipodia of human umbilical vein endothelial cells (HUVECs).17 However, we showed that the active form of cathepsin X co-localized predominantly with β2 integrin subunit in various cells of monocytes/macrophage lineage. Active cathepsin X was shown to regulate β2 integrin-dependent adhesion, phagocytosis and T lymphocyte activation by interaction with macrophage antigen-1 (CD11b/CD18, Mac-1). We showed that inhibitors and monoclonal antibodies, capable to impair cathepsin X enzymatic activity, reduced the binding of differentiated U-937 cells to fibrinogen and polystyrene surface in a dose dependent manner. The co-localization of active cathepsin X with β2 integrin chain was particularly enhanced in interactions of monocyte/macrophages with endothelial and tumor cells.
Besides in monocytes and macrophages the active cathepsin X plays a role in β2 integrin activation also in dendritic cells (DC), which are crucial for effective antigen presentation and initiation of T cell dependent immune response. Maturation of dendritic cells is accompanied by a range of morphological and cytoskeleton structure changes. In response to maturation stimuli in vitro, DCs rapidly adhere, develop polarity and assemble actin rich structures at the leading edge, known as podosomes.18 The adhesion of immature DCs to the extracellular matrix, is accompanied by recruitment of Mac-1 integrin receptor, which can be activated by cathepsin X. We have shown that, during maturation, cathepsin X translocates to the plasma membrane of maturing DCs, enabling Mac-1 activation and, consequently, cell adhesion.19 In mature DCs cathepsin X redistributes from the membrane to the perinuclear region, which coincides with the de-adhesion of DCs, formation of cell clusters and acquisition of the mature phenotype. Again, the inhibition of cathepsin X activity during DC differentiation and maturation reduced the capacity of DCs to stimulate T lymphocytes.
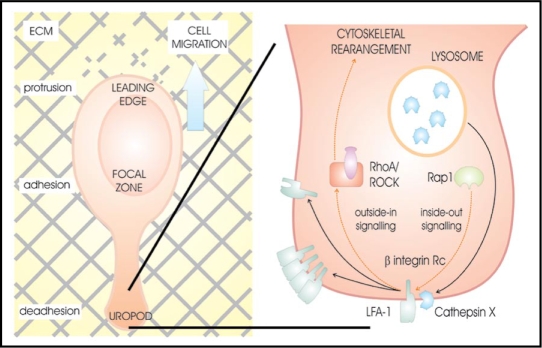
β2 integrin receptors are important also in T lymphocyte functions, such as migration and invasion across the endothelium and tissues. Lymphocyte function-associated antigen-1 (CD11a/CD18, LFA-1), the predominant β2 integrin receptor in T lymphocytes enables cell-cell interactions and homotypic aggregation via LFA-1-ICAM-1 (intracellular adhesion molecule-1) interactions. LFA-1 can act also as a true signaling receptor, causing F-actin reorganization that leads to cytoskeletal changes of the cell20 and a switch from a spherical to a polarized shape.21 Although the concentration of cathepsin X in T lymphocytes is lower compared to monocytes and macrophages, we showed that it interacts with LFA-1 promoting cytoskeleton-dependent morphological changes and migration across 2D and 3D models of ICAM-1 and Matrigel.22,23 Its co-localization with LFA-1 was particularly evident at the trailing edge protrusion, the uropod, which plays an important role in T lymphocyte migration and cell-cell interactions (Fig. 1). Uropodal active cathepsin X cleaves C-terminal amino acids of β chain in LFA-1 promoting its high affinity conformation and the binding of the cytoskeletal protein talin. This interaction stabilizes the uropod and promotes its elongation (Jevnikar, et al. submitted).
Figure 1.
Activation of LFA-1 integrin receptor by cathepsin X at the uropod of T lymphocyte promotes cytoskeleton-dependent morphological changes and cell migration.30
We demonstrated that uropods of cathepsin X upregulated T lymphocytes elongate to extreme length and form cell-to-cell connections, the nanotubes (Obermajer, et al. in press). Membrane (or tunneling) nanotubes were recently found as a new principle of cell-to-cell communication enabling transmission of complex and specific messages to distant cells through a physically connected network. Calcium fluxes, vesicles and cell-surface components can all traffic between cells connected by nanotubes. In immune system nanotubes integrate communities of cells for a better coordination of their action in various stages of immune response. We showed that nanotubes of cathepsin X upregulated T lymphocytes could readily transfer cellular organelles such as mitochondria and lysosomes and proposed that nanotube mediated transfer makes possible T lymphocyte activation without the need for direct contact with antigen presenting cells.
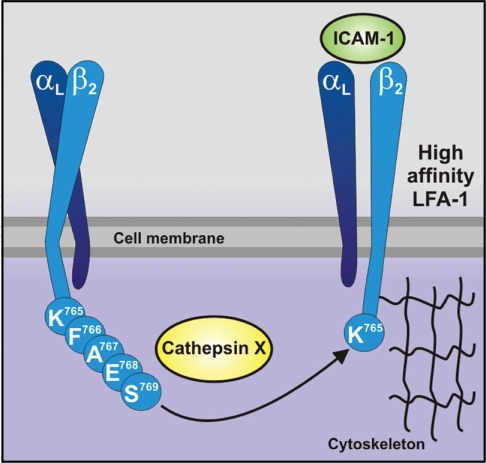
The exact mechanism of cathepsin X translocation towards plasma membrane and degradation of C-terminal amino acids of β chain remains unclear. In lysosomes cathepsin X can be found as a pro- and active form. After cell activation cathepsin X containing vesicles translocate towards the plasma membrane,16 as observed also for some other lysosomal proteases.24 During this process it is possible that pro-cathepsin X is activated by the other cysteine protease cathepsin L, as shown already in vitro.8 Both proteases were strongly co-localized with β2 integrin chain at plasma membrane of activated monocytes/macrophages and at uropodes of T lymphocytes. Simultaneous co-localization with the lysosomal markers demonstrates that at least the initial translocation of cathepsin X towards cytoplasmic tail of β2 integrin chain is vesicular. The interaction of cathepsin X with β2 integrin subunit was confirmed by immunoprecipitation and FRET.22 According to in vitro experiments we propose that cathepsin X cleaves sequentially C-terminal aminoacids F766, A767, E768 and S769 of β2 integrin subunit (Fig. 2) until reaching proline in penultimate position, confirming previous observation that the proline in S2 position leads to resistance to cathepsin X proteolysis.25 Also, our results are in agreement with the previously mentioned monocarboxypeptidase activity of cathepsin X.26,27 Since the signaling to and from the integrins is mainly regulated by the short cytoplasmic tail of β2 subunit,28 cathepsin X mediated β2 integrin truncation leads to regulation of the receptor signaling. The interaction of cytoplasmic tail with different cytoskeletal and regulatory proteins, such as talin, filamin, radixin and α-actinin is crucial for signal transduction and modulation of cytoskeleton.29
Figure 2.
Cathepsin X activates LFA-1 by sequential cleavage of C-terminal amino acids of β2 integrin subunit.
Besides β2 integrin chain we recently identified isozymes α and γ enolases as another molecular target for cathepsin X carboxypeptidase activity (Obermajer, et al. submitted). We demonstrated that cathepsin X sequentially cleaves C-terminal amino acids of both isozymes, abolishing their neurotrophic activity. On this way the neuronal cell survival and neuritogenesis can be regulated. Inhibition of cathepsin X activity increases the generation of plasmin, essential for neuronal differentiation and changes the length distribution of neurites, especially in the early phase of neurite outgrowth. Moreover, cathepsin X inhibition increases neuronal survival and reduces serum deprivation induced apoptosis, particularly in the absence of nerve growth factor.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/7403
References
- 1.Barrett AJ, Rawlings ND, Woessner JF. The Handbook of Proteolytic Enzymes. 2nd ed. Amsterdam: Elsevier; 2004. [Google Scholar]
- 2.Kos J, Lah T. Cystatins in Cancer. In: Žerovnik E, Kopitar Jerala N, Uversky V, editors. Human stefins and cystatins. New York: Nova Science Publisher; 2006. pp. 153–165. [Google Scholar]
- 3.Turk V, Kos J, Turk B. Cysteine cathepsins (proteases)—On the main stage of cancer. Cancer Cell. 2004;5:409–410. doi: 10.1016/s1535-6108(04)00117-5. [DOI] [PubMed] [Google Scholar]
- 4.Santamaria I, Velasco G, Pendas AM, Fueyo A, Lopez-Otin C. Cathepsin Z, a novel human cysteine proteinase with a short propeptide domain and a unique chromosomal location. J Biol Chem. 1998;273:16816–16823. doi: 10.1074/jbc.273.27.16816. [DOI] [PubMed] [Google Scholar]
- 5.Nagler DK, Menard R. Human cathepsin X: a novel cysteine protease of the papain family with a very short proregion and unique insertions. FEBS Lett. 1998;434:135–139. doi: 10.1016/s0014-5793(98)00964-8. [DOI] [PubMed] [Google Scholar]
- 6.Guncar G, Klemencic I, Turk B, Turk V, Karaoglanovic-Carmona A, Juliano L, et al. Crystal structure of cathepsin X: a flip-flop of the ring of His23 allows carboxy-monopeptidase and carboxy-dipeptidase activity of the protease. Structure. 2000;8:305–313. doi: 10.1016/s0969-2126(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 7.Sivaraman J, Nagler DK, Zhang R, Menard R, Cygler M. Crystal structure of human procathepsin X: a cysteine protease with the proregion covalently linked to the active site cysteine. J Mol Biol. 2000;295:939–951. doi: 10.1006/jmbi.1999.3410. [DOI] [PubMed] [Google Scholar]
- 8.Nagler DK, Zhang R, Tam W, Sulea T, Purisima EO, Menard R. Human cathepsin X: A cysteine protease with unique carboxypeptidase activity. Biochemistry. 1999;38:12648–12654. doi: 10.1021/bi991371z. [DOI] [PubMed] [Google Scholar]
- 9.Klemencic I, Carmona AK, Cezari MH, Juliano MA, Juliano L, Guncar G, et al. Biochemical characterization of human cathepsin X revealed that the enzyme is an exopeptidase, acting as carboxymonopeptidase or carboxydipeptidase. Eur J Biochem. 2000;267:5404–5412. doi: 10.1046/j.1432-1327.2000.01592.x. [DOI] [PubMed] [Google Scholar]
- 10.Kos J, Sekirnik A, Premzl A, Zavasnik-Bergant V, Langerholc T, Turk B, et al. Carboxypeptidases cathepsins X and B display distinct protein profile in human cells and tissues. Exp Cell Res. 2005;306:103–113. doi: 10.1016/j.yexcr.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Nagler DK, Kruger S, Kellner A, Ziomek E, Menard R, Buhtz P, et al. Upregulation of cathepsin X in prostate cancer and prostatic intraepithelial neoplasia. Prostate. 2004;60:109–119. doi: 10.1002/pros.20046. [DOI] [PubMed] [Google Scholar]
- 12.Buhling F, Peitz U, Kruger S, Kuster D, Vieth M, Gebert I, et al. Cathepsins K, L, B, X and W are differentially expressed in normal and chronically inflamed gastric mucosa. Biol Chem. 2004;385:439–445. doi: 10.1515/BC.2004.051. [DOI] [PubMed] [Google Scholar]
- 13.Krueger S, Kalinski T, Hundertmark T, Wex T, Kuster D, Peitz U, et al. Upregulation of cathepsin X in Helicobacter pylori gastritis and gastric cancer. J Pathol. 2005;207:32–42. doi: 10.1002/path.1820. [DOI] [PubMed] [Google Scholar]
- 14.Wendt W, Zhu XR, Lubbert H, Stichel CC. Differential expression of cathepsin X in aging and pathological central nervous system of mice. Exp Neurol. 2007;204:525–540. doi: 10.1016/j.expneurol.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Nascimento FD, Rizzi CC, Nantes IL, Stefe I, Turk B, Carmona AK, et al. Cathepsin X binds to cell surface heparan sulfate proteoglycans. Arch Biochem Biophys. 2005;436:323–332. doi: 10.1016/j.abb.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Obermajer N, Premzl A, Zavasnik-Bergant T, Turk B, Kos J. Carboxypeptidase cathepsin X mediates beta2-integrin-dependent adhesion of differentiated U-937 cells. Exp Cell Res. 2006;312:2515–2527. doi: 10.1016/j.yexcr.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Lechner AM, Assfalg-Machleidt I, Zahler S, Stoeckelhuber M, Machleidt W, Jochum M, et al. RGD-dependent binding of procathepsin X to integrin alphavbeta3 mediates cell-adhesive properties. J Biol Chem. 2006;281:39588–39597. doi: 10.1074/jbc.M513439200. [DOI] [PubMed] [Google Scholar]
- 18.Burns S, Hardy SJ, Buddle J, Yong KL, Jones GE, Trasher AJ. Maturation of DC is associated with changes in motile characteristics and adherence. Cell Motil Cytoskeleton. 2004;57:118–132. doi: 10.1002/cm.10163. [DOI] [PubMed] [Google Scholar]
- 19.Obermajer N, Švajger U, Jeras M, Bogyo M, Kos J. Maturation of dendritic cells depends on proteolytic cleavage by cathepsin X. J Leukocyte Biol. 2008;84:1306–1315. doi: 10.1189/jlb.0508285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porter JC, Bracke M, Smith A, Davies D, Hogg N. Relationship of F-actin distribution to development of polar shape in human polymorphonuclear neutrophils. J Immunol. 2002;168:6330–6335. [Google Scholar]
- 21.Coates TD, Watts RG, Hartman R, Howard TH. Relationship of F-actin distribution to development of polar shape in human polymorphonuclear neutrophils. J Cell Biol. 1992;117:765–774. doi: 10.1083/jcb.117.4.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obermajer N, Repnik U, Jevnikar Z, Kreft M, Kos J. Immunosupressive activity of cysteine protease cathepsin X via activation of beta-2 integrins. Immunology. 2008;124:76–88. doi: 10.1111/j.1365-2567.2007.02740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jevnikar Z, Obermajer N, Bogyo M, Kos J. The role of cathepsin X in the aggregation, migration and invasiveness of T-lymphocytes. J Cell Science. 2008;121:2652–2661. doi: 10.1242/jcs.023721. [DOI] [PubMed] [Google Scholar]
- 24.Victor BC, Sloane BF. Cysteine cathepsin non-inhibitory binding partners: modulating intracellular trafficking and function. Biol Chem. 2007;388:1131–1140. doi: 10.1515/BC.2007.150. [DOI] [PubMed] [Google Scholar]
- 25.Devanathan G, Turnbull JL, Ziomek E, Purisima EO, Ménard R, Sulea T. Carboxy-monopeptidase substrate specificity of human cathepsin X. Biochem Biophys Res Commun. 2005;329:445–452. doi: 10.1016/j.bbrc.2005.01.150. [DOI] [PubMed] [Google Scholar]
- 26.Therrien C, Lachance P, Sulea T, Purisima EO, Qi H, Ziomek E, et al. Cathepsins X and B can be differentiated through their respective mono- and dipeptidyl carboxypeptidase activities. Biochemistry. 2001;40:2702–2711. doi: 10.1021/bi002460a. [DOI] [PubMed] [Google Scholar]
- 27.Puzer L, Cotrin SS, Cezari MH, Hirata IY, Juliano MA, Stefe I, et al. Recombinant human cathepsin X is a carboxymonopeptidase only: a comparison with cathepsins B and L. Biol Chem. 2005;386:1191–1195. doi: 10.1515/BC.2005.136. [DOI] [PubMed] [Google Scholar]
- 28.Wiedemann A, Patel JC, Lim J, Tsun A, van Kooyk Y, Caron E. Two distinct cytoplasmic regions of the beta2 integrin chain regulate RhoA function during phagocytosis. J Cell Biol. 2006;172:1069–1079. doi: 10.1083/jcb.200508075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu S, Calderwood DA, Ginsberg MH. Integrin cytoplasmic domain-binding proteins. J Cell Sci. 2000;113:3563–3571. doi: 10.1242/jcs.113.20.3563. [DOI] [PubMed] [Google Scholar]
- 30.Obermajer N, Jevnikar Z, Doljak B, Kos J. Role of cysteine cathepsins in matrix degradation and cell signaling. Connective Tissue Res. 2008;49:193–196. doi: 10.1080/03008200802143158. [DOI] [PubMed] [Google Scholar]