Abstract
With over 60 members, the Sterile 20 family of kinases has been implicated in numerous biological processes, including growth, survival, apoptosis and cell migration. Recently, we have shown that, in addition to cell death, the Ste20-like kinase SLK is required for efficient cell migration in fibroblasts. We have observed that SLK is involved in cell motility through its effect on actin reorganization and microtubule-induced focal adhesion turnover. Scratch wounding of confluent monolayers results in SLK activation. The induction of SLK kinase activity requires the scaffold FAK and a MAPK-dependent pathway. However, its recruitment to the leading edge of migrating fibroblasts requires the activity of the Src family kinases. Since SLK is microtubule-associated, it may represent one of the signals delivered to focal contacts that induces adhesions turnover. A speculative model is proposed to illustrate the mechanism of SLK activation and recruitment at the leading edge of migrating cells.
Key words: cell migration, cell adhesion, SLK, microtubules, adhesion turnover
Cell migration is involved in multiple biological processes such as development, tissue regeneration, immune surveillance and tumor metastasis. Numerous studies reported a multitude of cellular and molecular players that take part in the signaling networks that regulate cell migration.1,2 Recently, we reported the participation of a new member, the Ste20 serine/threonine kinase SLK, in the regulation of cell migration. We have shown that SLK is a novel adhesion disassembly signal that is activated and recruited downstream of the FAK/Src complex following scratch wound-induced migration.3 Furthermore, SLK-dependent signals are required to mediate microtubule-dependent focal adhesion tunrnover.3 These findings provide new insights into the mechanisms of cell migration and adhesion dynamics.
Since sterile 20 protein (Ste20p) acts as a MAP4K in yeast, it was suggested that mammalian homologues of Ste20p also function as MAP4K.4 Several members of the Ste20 family of kinases have been identified in mammals and implicated in various biological processes such as stress responses, cell death and cytoskeletal reorganization.5 We and others previously identified a novel Ste20-related kinase termed SLK, which is a part of a signaling pathway mediating c-Jun terminal kinase 1 (JNK1) activation and apoptosis in cultured fibroblasts.6–8 In addition, recent reports showed that SLK is involved in C2C12 myoblast differentiation and plays a role in cell cycle progression.9,10 SLK is ubiquitously expressed, but during embryogenesis it is highly enriched in muscle and neuronal tissues.11 It has been shown that SLK is associated with the microtubule cytoskeleton and we have demonstrated that SLK-induced disassembly of actin stress fibers can be inhibited by dominant negative Rac1.12–14
Recently, SLK was identified as a member of a new signaling pathway that induce vasodilatation in response to angiotensin II type 2 receptor activation.15 It was reported that SLK negatively regulates RhoA-dependent functions by phosphorylation of RhoA at Ser188.15 These findings suggest that SLK represents a novel relaxation signal involved in cytoskeletal remodeling and cell migration.
We have observed that SLK is recruited to the leading edge of migrating fibroblasts by a mechanism involving c-Src signaling.3 The molecular mechanism regulating SLK recruitment is still unclear but is likely to implicate the association of SLK with another protein. The translocation of SLK could involve a microtubule-dependent mechanism leading to its redistribution to peripheral adhesions, using actin stress fibers as tracks. The Rho GTPases have been shown to be important in the targeting of signaling components, such as c-Src, to specific adhesion sites.16,17 Whether SLK recruitment to the leading edge requires the Rho GTPases remains to be investigated. The Rho-mDia pathway regulates polarization and adhesion turnover by aligning microtubules and actin filaments and is responsible for delivering APC/Cdc42 and c-Src to their respective sites of action.18 One attractive possibility is that mDia facilitates SLK-microtubule translocation in a c-Src dependent manner.
Integrin molecules which link the extracellular matrix to the intracellular machinery are key players in initiating polarized cell migration into the wound. We investigated SLK activity in a scratch-induced migration model and have been able to decipher various signaling components regulating SLK activation.3 Using knockdown and dominant negative approaches, we showed that SLK is required for microtubule-dependent focal adhesion turnover and cell migration downstream of the FAK/Src complex.3
The molecular mechanisms by which microtubules contribute to cell migration have been intensively studied. Geiger's group provided the first demonstration that cytoskeletal modulation, such as microtubule disruption, triggers integrin-dependent signaling in the absence of external growth factor stimulation.19 The authors suggested that the involvement of microtubules in adhesion dependent signaling is related to microtubule interaction with the contractile actin-myosin system.19 By using a nocodazole washout system, it was shown that FAK and the GTPase dynamin are required for microtubule-induced focal adhesion disassembly.20
Adhesion turnover involves a number of adapters and signaling molecules, most of which are engaged in FAK signaling pathways.21 FAK stimulates adhesion disassembly through a signaling pathway that includes extracellular signal-regulated kinase (ERK) and myosin light chain kinase (MLCK).22 Our data have shown that SLK is activated downstream of FAK/Src/MAPK signaling, suggesting that SLK may be a new target of this pathway that leads to adhesion disassembly. Furthermore, if RhoA is a bona fide substrate for SLK in fibroblasts, then by phosphorylating and inhibiting RhoA, SLK could tilt the Rho/Rac antagonistic interplay toward relaxation and adhesion disassembly. Downstream targets of FAK and Src kinase activity often regulate the recruitment of adapter and structural protein complexes to adhesions.22 The integration of molecules such as zyxin, α-actinin or paxillin into focal contacts can lead to their stabilization and maturation into focal adhesions.22 Interestingly, depending on their phosphorylation state, these components can promote adhesion destabilization and turnover. Therefore, it is tempting to speculate that activated SLK at the leading edge may phosphorylate key signaling components to induce adhesion turnover.
A recent study has shown that the frequency of microtubule catastrophes is higher at focal adhesion sites and this event leads to a local release of microtubule regulatory proteins, such as GEF-H1 and APC.23 Signaling molecules that are released from the microtubules at adhesions could directly associate with molecular factors concentrated at the adhesion plaques, such as Src, PAK and Arp2/3. Furthermore, it was speculated that microtubule catastrophe could be associated with phosphorylated paxillin-dependent protein complexes.23 One possibility is that through the microtubule, SLK is delivered to focal contacts or adhesions where it serves as a scaffold for disassembling signals. Alternatively, SLK may be phsophorylating key signaling molecules, which ultimately leads to adhesion destabilization and turnover.
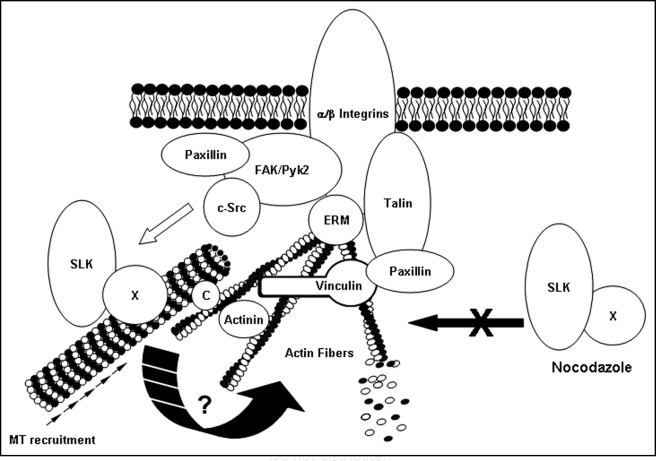
Overall, our recent findings suggest that SLK is novel regulator of focal adhesion turnover and cell migration (Fig. 1). The molecular mechanisms regulating SLK activity and SLK-dependent adhesion turnover remain to be uncovered and await the identification of SLK substrates.
Figure 1.
Model for SLK activation and recruitment at the leading edge. A proportion of SLK is microtubule-associated, likely through a microtubule-binding protein (X). Following activation of the FAK/c-Src complex, signaling through the MAPK pathway can activate and recruit the microtubule-SLK complex, inducing adhesion turnover by destabilization of the actin network or focal contacts/adhesions through an unknown mechanism. (C) denotes a cargo protein coupling the microtubule to polymerized actin. Nocodazole treatment fails to recruit SLK resulting in stable adhesions.
Acknowledgements
We would like to thank to Paul O'Reilly for critical reading of the manuscript.
Abbreviations
- Ste20p
sterile 20 proteins
- SLK
murine Ste20-like kinase
- FAK
focal adhesion kinase
- c-Src
cellular Src
- MAPK
mitogen-activated protein kinase
- MAP4K
mitogen-activated protein kinase kinase kinase kinase
- JNK1
C-Jun N-terminal kinase 1
- mDia
mouse diaphanous protein
- APC
adenomatous polyposis coli
- GTPase
guanine triphosphat-ase
- ERK
extracellular signal regulated kinase
- MLCK
myosin light chain kinase
- GEF-H1
guanine nucelotide exchange factor H1
- PAK
P21 activated protein
- Arp2/3
actin related proteins 2/3
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/7229
This work was supported by the Canadian Institute for Health Research, Canadian Breast Cancer Foundation, MDAUSA and a Premier's Research for Excellence Award. Simona Wagner was supported by OGSST and NSERC scholarships.
References
- 1.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 2.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 3.Wagner S, Storbeck CJ, Roovers K, Chaar ZY, Kolodziej P, McKay M, et al. FAK/src-family dependent activation of the Ste20-like kinase SLK is required for microtubule-dependent focal adhesion turnover and cell migration. PLoS ONE. 2008;3:1868. doi: 10.1371/journal.pone.0001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dan I, Watanabe NM, Kusumi A. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 2001;11:220–230. doi: 10.1016/s0962-8924(01)01980-8. [DOI] [PubMed] [Google Scholar]
- 5.Ling P, Lu TJ, Yuan CJ, Lai MD. Biosignaling of mammalian Ste20-related kinases. Cell Signal. 2008;20:1237–1247. doi: 10.1016/j.cellsig.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 6.Hao W, Takano T, Guillemette J, Papillon J, Ren G, Cybulsky AV. Induction of apoptosis by the Ste20-like kinase SLK, a germinal center kinase that activates apoptosis signal-regulating kinase and p38. J Biol Chem. 2006;281:3075–3084. doi: 10.1074/jbc.M511744200. [DOI] [PubMed] [Google Scholar]
- 7.Sabourin LA, Rudnicki MA. Induction of apoptosis by SLK, a Ste20-related kinase. Oncogene. 1999;18:7566–7575. doi: 10.1038/sj.onc.1203119. [DOI] [PubMed] [Google Scholar]
- 8.Sabourin LA, Tamai K, Seale P, Wagner J, Rudnicki MA. Caspase 3 cleavage of the Ste20-related kinase SLK releases and activates an apoptosis-inducing kinase domain and an actindisassembling region. Mol Cell Biol. 2000;20:684–696. doi: 10.1128/mcb.20.2.684-696.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Reilly PG, Wagner S, Franks DJ, Cailliau K, Browaeys E, Dissous C, et al. The Ste20-like kinase SLK is required for cell cycle progression through G2. J Biol Chem. 2005;280:42383–42390. doi: 10.1074/jbc.M510763200. [DOI] [PubMed] [Google Scholar]
- 10.Storbeck CJ, Daniel K, Zhang YH, Lunde J, Scime A, Asakura A, et al. Ste20-like kinase SLK displays myofiber type specificity and is involved in C2C12 myoblast differentiation. Muscle Nerve. 2004;29:553–564. doi: 10.1002/mus.20000. [DOI] [PubMed] [Google Scholar]
- 11.Zhang YH, Hume K, Cadonic R, Thompson C, Hakim A, Staines W, Sabourin LA. Expression of the Ste20-like kinase SLK during embryonic development and in the murine adult central nervous system. Brain Res Dev Brain Res. 2002;139:205–215. doi: 10.1016/s0165-3806(02)00551-5. [DOI] [PubMed] [Google Scholar]
- 12.Burakov AV, Kovalenko OV, Potekhina ES, Nadezhdina ES, Zinovkina LA. LOSK (SLK) protein kinase activity is necessary for microtubule organization in the interphase cell centrosome. Dokl Biol Sci. 2005;403:317–319. doi: 10.1007/s10630-005-0123-9. [DOI] [PubMed] [Google Scholar]
- 13.Burakov AV, Zhapparova ON, Kovalenko OV, Zinovkina LA, Potekhina ES, Shanina NA, et al. Ste20-related protein kinase LOSK (SLK) controls microtubule radial array in interphase. Mol Biol Cell. 2008;19:1952–1961. doi: 10.1091/mbc.E06-12-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner S, Flood TA, O'Reilly P, Hume K, Sabourin LA. Association of the Ste20-like kinase (SLK) with the microtubule. Role in Rac1-mediated regulation of actin dynamics during cell adhesion and spreading. J Biol Chem. 2002;277:37685–37692. doi: 10.1074/jbc.M205899200. [DOI] [PubMed] [Google Scholar]
- 15.Guilluy C, Rolli-Derkinderen M, Loufrani L, Bourge A, Henrion D, Sabourin L, et al. Ste20-related kinase SLK phosphorylates Ser188 of RhoA to induce vasodilation in response to angiotensin II Type 2 receptor activation. Circ Res. 2008;102:1265–1274. doi: 10.1161/CIRCRESAHA.107.164764. [DOI] [PubMed] [Google Scholar]
- 16.Sandilands E, Frame MC. Endosomal trafficking of Src tyrosine kinase. Trends Cell Biol. 2008;18:322–329. doi: 10.1016/j.tcb.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Timpson P, Jones GE, Frame MC, Brunton VG. Coordination of cell polarization and migration by the Rho family GTPases requires Src tyrosine kinase activity. Curr Biol. 2001;11:1836–1846. doi: 10.1016/s0960-9822(01)00583-8. [DOI] [PubMed] [Google Scholar]
- 18.Yamana N, Arakawa Y, Nishino T, Kurokawa K, Tanji M, Itoh RE, et al. The Rho-mDia1 pathway regulates cell polarity and focal adhesion turnover in migrating cells through mobilizing Apc and c-Src. Mol Cell Biol. 2006;26:6844–6858. doi: 10.1128/MCB.00283-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bershadsky A, Chausovsky A, Becker E, Lyubimova A, Geiger B. Involvement of microtubules in the control of adhesion-dependent signal transduction. Curr Biol. 1996;6:1279–1289. doi: 10.1016/s0960-9822(02)70714-8. [DOI] [PubMed] [Google Scholar]
- 20.Ezratty EJ, Partridge MA, Gundersen GG. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat Cell Biol. 2005;7:581–590. doi: 10.1038/ncb1262. [DOI] [PubMed] [Google Scholar]
- 21.Broussard JA, Webb DJ, Kaverina I. Asymmetric focal adhesion disassembly in motile cells. Curr Opin Cell Biol. 2008;20:85–90. doi: 10.1016/j.ceb.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, et al. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- 23.Efimov A, Schiefermeier N, Grigoriev I, Ohi R, Brown MC, Turner CE, et al. Paxillin-dependent stimulation of microtubule catastrophes at focal adhesion sites. J Cell Sci. 2008;121:196–204. doi: 10.1242/jcs.012666. [DOI] [PMC free article] [PubMed] [Google Scholar]