Abstract
Cell-to-extracellular matrix (ECM) adhesion plays important roles in various biological events, such as proliferation, differentiation and migration. Distinct from other types of adhesion structures (focal complexes, focal adhesions and so on), podosomes and invadopodia are thought to have additional functions beyond attachment, possibly including invasion into the ECM. For podosomes and invadopodia to invade into the ECM, molecules involved in adhesion, actin polymerization and ECM degradation must be recruited to sites of action. Our recent study demonstrated that podosomes form near newly formed focal adhesions via the minimally expressed phosphoinositide PtdIns(3,4) P2-mediated recruitment of the Tks5-Grb2 scaffold, followed by the accumulation of N-WASP. Although this study demonstrated details of molecular interplay during the transformation of focal adhesion, its regulation in the in vivo invasion process remains to be clarified. Here, we discuss the molecular bases of the transformation of focal adhesions to podosomes/invadopodia based on current understanding.
Key words: podosome; invadopodium; focal adhesion; Tks5; PtdIns(3,4)P2; N-WASP
Extra View
Podosomes/invadopodia are dynamic membrane protrusions found in cells with high motility. They are responsible for focal pericellular proteolysis of the extracellular matrix (ECM), which leads to cellular invasion and metastasis under both physiological and pathological conditions.1 Podosomes were initially discovered in cells transformed with the Rous sarcoma virus2 and in monocyte-derived cells, such as osteoclasts3 and macrophages.4 Research on podosomes gained momentum in the late 1990s when they were found to be associated with the gene responsible for Wiskott-Aldrich syndrome (WAS). The product of the gene (i.e., WASP) and its ectopic analogue, N-WASP, were necessary for actin polymerization at podosomes.5,6 Invadopodia were initially found to be precisely localized at sites of ECM degradation.7 Subsequent discoveries that revealed the localization of a number of matrix metalloproteases (MMPs) at invadopodia/podosomes are consistent with this observation. In addition, interference with podosome/invadopodium formation was found to directly affect ECM degradation.6,8,9 A particularly important finding was that membrane type 1 MMP (MT1-MMP) localizes to podosomes/invadopodia. In fact, MT1-MMP plays a pivotal role in physiological and pathological cell invasion,10 skeletal embryogenesis11 and cell migration.12 On the basis of these facts, podosomes/invadopodia have been recognized as appendages with both physiological and pathological roles for invading substrates. Although podosomes are proposed to be precursor structures that mature into invadopodia under appropriate physiological conditions,9,13 no direct experimental evidence is available to support this hypothesis. Based on current knowledge, podosomes and invadopodia are thought to have nearly indistinguishable structure and function. Nevertheless, genetic/epigenetic mutations might uncontrollably promote gain-of-function alterations for immobile epithelial cells, endowing these cells with properties of highly motile cells such as monocyte-derived cells. Indeed, Tks5, a scaffold protein that is known to play an essential role in podosome/invadopodium formation, has been shown to be upregulated in invasive cancer,14 macrophages and mesenchymal cells compared with relatively less-invasive cancer cells (unpublished data). Thus, Tks5 expression might be one acquisition of epithelial cells that make them highly motile, although the physiological role of Tks5 and its mechanism of regulation are uncertain.
Podosomes/invadopodia are F-actin-rich contact regions that appear as small (diameter, 1–2 µm; depth, 200–400 nm), round plasma membrane extensions when observed under an electron microscope and assemble at an early stage during cell adhesion to a substrate. The core of actin filaments within each podosome/invadopodium is enriched with integrin, vinculin, talin, α-actinin, Src and tyrosine-phosphorylated proteins.13 Because the family of focal adhesion molecules has many members and shares many similarities with podosomes and invadopodia, relationships between them have been extensively discussed. By analyzing the molecular components, architecture and spatio-temporal regulation of them, podosomes and invadopodia are believed to be distinct from normal adhesion regulators, such as focal contacts and focal adhesions based on the following properties. First, podosomes/invadopodia contain proteins that regulate actin polymerization, such as the Arp2/3 complex and N-WASP,3,15 whereas focal adhesions do not. Second, although podosomes/invadopodia exhibit de novo assembly to some extent, they are more frequently formed from rapid and dynamic turnover of preexisting podosomes/invadopodia.16,17 In contrast, focal adhesions are substantially less dynamic, and their formation appears to require ongoing protein synthesis. Finally, podosomes/invadopodia appear to shape and remodel the ventral plasma membrane, which might be related to exocytosis of proteases at these sites.18 This process is considered to occur through the action of membrane-deforming proteins, such as dynamin and cortactin, although the precise mechanism and the physiological significance are yet to be elucidated. In addition, a recent review19 summarizes the similarities and differences between focal adhesions and podosomes/invadopodia. However, identifying the functional relationship between these entities in the context of cell invasion/migration remains a challenge.
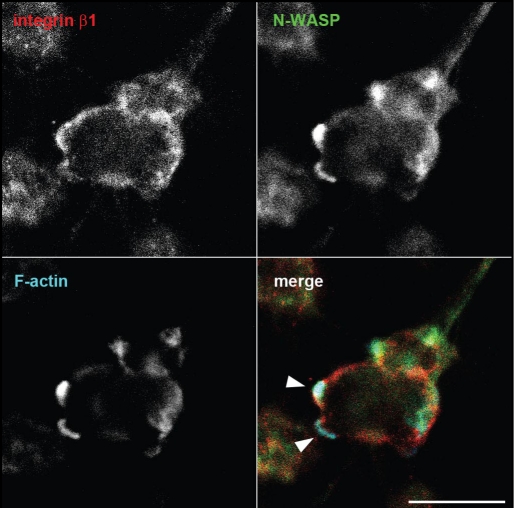
Our study20 demonstrated that a minimally expressed phosphoinositide, PtdIns(3,4)P2, is synthesized by PI3-kinase and synaptojanin2 near focal adhesions and that this phosphoinositide triggers the recruitment of a protein complex that includes Tks5, Grb2 and N-WASP. Tks5 is an adaptor protein originally identified as an Src substrate21 and later was shown to be essential for podosome formation, matrix degradation and tumor growth in vivo.14,22,23 Using phosphoinositide-specific binding domains, we have previously shown that PtdIns(3,4)P2, but not PtdIns(4,5)P2, accumulates at podosomes of NIH-src cells. Furthermore, in NIH-src cells with reduced Tks5 expression, PtdIns(3,4)P2 still localized to adhesion sites. Live-cell imaging and protein interaction analysis revealed that PtdIns(3,4)P2 production and Tks5-Grb2 complex formation at focal adhesions are Src dependent. Thus, these molecules are thought to be responsible for Src-induced podosome formation at focal adhesions (i.e., transformation of adhesions). Although we found that Src stimulated PtdIns(3,4)P2 production at newly formed focal adhesions and subsequent actin polymerization at podosomes, it is unlikely that a pre-existing focal adhesion gradually modified into podosome. Instead, actin dots that assembled de novo were arranged in a circular pattern around the focal adhesion and then fused together, resulting in a mature circular podosome with another adhesion (this structure is also termed rosette). Once formed, the circular podosome exhibits dynamic turnover, as shown in many previous studies. At that point, the original focal adhesion loses integrity. Although the interaction of Tks5 and N-WASP seems to be important in ring-shape mature podosome formation, the precise molecular mechanism underlying the arrangement and fusion of each actin dot remains to be explored. One intriguing possibility is that Tks5 serves as a more potent activator of the N-WASP-Arp2/3 pathway than other adaptors, such as Nck. Our study demonstrated that Nck is not involved in podosome formation per se, although it seems to be necessary for matrix degradation. Nck has previously been shown to be an important adaptor in the EGF-stimulated N-WASP-Arp2/3 pathway, whereas the resultant invadopodia are dot-shaped and appear to be laterally motile.24 In light of this finding, Tks5 could be thought of as an analogue of EspFu, which is found in enterohemorrhagic Escherichia coli (EHEC) and plays a key role in pedestal formation in response to invading pathogens by effectively recruiting and activating N-WASP independent of Nck.25 These differences in the adaptor molecules may explain the appearance and dynamics of podosomes/invadopodia in many cell types. The ring-shape architecture of mature podosomes is consistent with the localization of N-WASP.VCA in NIH-src cells, which was shown to inhibit actin polymerization at podosomes but localize at focal adhesions as rings when moderately expressed. These rings of N-WASPΔVCA co-localized with endogenous Tks5, which implies that these molecules serve as podosome precursors.20 Furthermore, dynamin was found to interact with Tks5, play an essential role in podosome formation, and co-localize with the rings of N-WASPΔVCA (unpublished data). As shown in a previous report, dynamin is involved in microtubule-induced focal adhesion disassembly by interacting with FAK,26 and it is plausible that this precursor complex consisting of Tks5, dynamin and N-WASP is involved in focal adhesion remodeling. Because adhesion molecules, such as FAK and vinculin, were found to be enriched at the inner side of matured podosomes, the original focal adhesions might somehow be utilized for these ring-shape newly formed adhesions in podosomes by a currently unknown mechanism. Recent findings suggest that the dynamics of podosomes27 or the proteolytic activity of invadopodia28 are influenced by the rigidity of the ECM. In fact, NIH-src cells cultured in Matrigel exhibited another distinct morphology, although N-WASP accumulation in F-actin-rich protrusions was commonly observed (Fig. 1, arrowheads). In addition, adhesion molecules were localized adjacent to these protrusions (Fig. 1, arrowheads). Because of the localization of these molecules, the protrusions are thought to represent podosomes in this three-dimensional environment. These results suggest that the types of integrins involved in adhesion and/or the traction forces may also affect the formation, function and appearance of the adhesion-initiated podosomes/invadopodia. Thus, future studies are required to dissect the dynamics of focal adhesions and podosomes/invadopodia in a more physiological, three-dimensional environment. These studies may provide new insight into podosomes/invadopodia in relation to other protrusions, such as membrane blebbing, which are thought to be important in protease-independent migration in the three-dimensional environment.29,30 Whereas the initiator of podosome formation, PtdIns(3,4)P2, is thought to be produced on the plasma membrane in response to several stimuli, including growth factors, normal NIH3T3 cells with Tks5 expression do not form podosomes. Thus, the localization of Tks5 to focal adhesions followed by PtdIns(3,4)P2 generation, and subsequent interaction with adhesion molecules, such as Grb2, and/or modification of Tks5, including phosphorylation, are likely additional gating factors in allowing podosome assembly. Future works should attempt to validate this hypothesis. Increased knowledge on the mechanisms that underlie the adhesion-mediated formation and function of podosomes/invadopodia will provide insights into the biology and regulation of osteoporosis and cancer metastasis as well as aid in the development of potential therapeutic approaches for managing these diseases.
Figure 1.
NIH-src cells in Matrigel form protrusions enriched with N-WASP and F-actin. NIH-src cells cultured in Matrigel (BD bioscience) were fixed with 2% paraformaldehyde in phosphate buffered saline for 10 min. Cells were then stained with anti-integrinβ1 (Chemicon, MAB1997) (red), anti-N-WASP (green) and Alexa647-phalloidin (Invitrogen) to visualize F-actin (blue). Arrowheads indicate protrusions with N-WASP and F-actin accumulation. The confocal images were sequentially taken between 16.52 µm and 41.46 µm from the glass surface; therefore, the image represents a 29.42 µm section (Olympus, FV1000 confocal microscopy system with 40x objective, NA 0.60). Scale bar, 20 µm.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/7510
References
- 1.Gimona M, Buccione R, Courtneidge SA, Linder S. Assembly and biological role of podosomes and invadopodia. Curr Opin Cell Biol. 2008 doi: 10.1016/j.ceb.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 2.David-Pfeuty T, Singer SJ. Altered distributions of the cytoskeletal proteins vinculin and alpha-actinin in cultured fibroblasts transformed by Rous sarcoma virus. Proc Natl Acad Sci USA. 1980;77:6687–6691. doi: 10.1073/pnas.77.11.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marchisio PC, Cirillo D, Naldini L, Primavera MV, Teti A, Zambonin-Zallone A. Cell-substratum interaction of cultured avian osteoclasts is mediated by specific adhesion structures. J Cell Biol. 1984;99:1696–1705. doi: 10.1083/jcb.99.5.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marchisio PC, Cirillo D, Teti A, Zambonin-Zallone A, Tarone G. Rous sarcoma virus-transformed fibroblasts and cells of monocytic origin display a peculiar dot-like organization of cytoskeletal proteins involved in microfilament-membrane interactions. Exp Cell Res. 1987;169:202–214. doi: 10.1016/0014-4827(87)90238-2. [DOI] [PubMed] [Google Scholar]
- 5.Linder S, Nelson D, Weiss M, Aepfelbacher M. Wiskott-Aldrich syndrome protein regulates podosomes in primary human macrophages. Proc Natl Acad Sci USA. 1999;96:9648–9653. doi: 10.1073/pnas.96.17.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizutani K, Miki H, He H, Maruta H, Takenawa T. Essential role of neural Wiskott-Aldrich syndrome protein in podosome formation and degradation of extracellular matrix in src-transformed fibroblasts. Cancer Res. 2002;62:669–674. [PubMed] [Google Scholar]
- 7.Chen WT. Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. J Exp Zool. 1989;251:167–185. doi: 10.1002/jez.1402510206. [DOI] [PubMed] [Google Scholar]
- 8.Nakahara H, Mueller SC, Nomizu M, Yamada Y, Yeh Y, Chen WT. Activation of beta1 integrin signaling stimulates tyrosine phosphorylation of p190RhoGAP and membrane-protrusive activities at invadopodia. J Biol Chem. 1998;273:9–12. doi: 10.1074/jbc.273.1.9. [DOI] [PubMed] [Google Scholar]
- 9.Baldassarre M, Pompeo A, Beznoussenko G, Castaldi C, Cortellino S, McNiven MA, et al. Dynamin participates in focal extracellular matrix degradation by invasive cells. Mol Biol Cell. 2003;14:1074–1084. doi: 10.1091/mbc.E02-05-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seiki M. Membrane-type 1 matrix metalloproteinase: a key enzyme for tumor invasion. Cancer Lett. 2003;194:1–11. doi: 10.1016/s0304-3835(02)00699-7. [DOI] [PubMed] [Google Scholar]
- 11.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 12.Hotary K, Allen E, Punturieri A, Yana I, Weiss SJ. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2 and 3. J Cell Biol. 2000;149:1309–1323. doi: 10.1083/jcb.149.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13:376–385. doi: 10.1016/s0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 14.Seals DF, Azucena EF, Jr, Pass I, Tesfay L, Gordon R, Woodrow M, et al. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell. 2005;7:155–165. doi: 10.1016/j.ccr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Marchisio PC, Bergui L, Corbascio GC, Cremona O, D'Urso N, Schena M, et al. Vinculin, talin and integrins are localized at specific adhesion sites of malignant B lymphocytes. Blood. 1988;72:830–833. [PubMed] [Google Scholar]
- 16.Stickel SK, Wang YL. Alpha-actinin-containing aggregates in transformed cells are highly dynamic structures. J Cell Biol. 1987;104:1521–1526. doi: 10.1083/jcb.104.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ochoa GC, Slepnev VI, Neff L, Ringstad N, Takei K, Daniell L, et al. A functional link between dynamin and the actin cytoskeleton at podosomes. J Cell Biol. 2000;150:377–389. doi: 10.1083/jcb.150.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakurai-Yageta M, Recchi C, Le Dez G, Sibarita JB, Daviet L, Camonis J, et al. The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. J Cell Biol. 2008;181:985–998. doi: 10.1083/jcb.200709076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Block MR, Badowski C, Millon-Fremillon A, Bouvard D, Bouin AP, Faurobert E, et al. Podosome-type adhesions and focal adhesions, so alike yet so different. Eur J Cell Biol. 2008;87:491–506. doi: 10.1016/j.ejcb.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Oikawa T, Itoh T, Takenawa T. Sequential signals toward podosome formation in NIH-src cells. J Cell Biol. 2008;182:157–169. doi: 10.1083/jcb.200801042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lock P, Abram CL, Gibson T, Courtneidge SA. A new method for isolating tyrosine kinase substrates used to identify fish, an SH3 and PX domain-containing protein, and Src substrate. Embo J. 1998;17:4346–4357. doi: 10.1093/emboj/17.15.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abram CL, Seals DF, Pass I, Salinsky D, Maurer L, Roth TM, et al. The adaptor protein fish associates with members of the ADAMs family and localizes to podosomes of Src-transformed cells. J Biol Chem. 2003;278:16844–16851. doi: 10.1074/jbc.M300267200. [DOI] [PubMed] [Google Scholar]
- 23.Blouw B, Seals DF, Pass I, Diaz B, Courtneidge SA. A role for the podosome/invadopodia scaffold protein Tks5 in tumor growth in vivo. Eur J Cell Biol. 2008;87:555–567. doi: 10.1016/j.ejcb.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, et al. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol. 2005;168:441–452. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campellone KG, Robbins D, Leong JM. EspFU is a translocated EHEC effector that interacts with Tir and N-WASP and promotes Nck-independent actin assembly. Dev Cell. 2004;7:217–228. doi: 10.1016/j.devcel.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Ezratty EJ, Partridge MA, Gundersen GG. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat Cell Biol. 2005;7:581–590. doi: 10.1038/ncb1262. [DOI] [PubMed] [Google Scholar]
- 27.Collin O, Na S, Chowdhury F, Hong M, Shin ME, Wang F, Wang N. Self-Organized Podosomes Are Dynamic Mechanosensors. Curr Biol. 2008 doi: 10.1016/j.cub.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexander NR, Branch KM, Parekh A, Clark ES, Iwueke IC, Guelcher SA, et al. Extracellular Matrix Rigidity Promotes Invadopodia Activity. Curr Biol. 2008 doi: 10.1016/j.cub.2008.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charras G, Paluch E. Blebs lead the way: how to migrate without lamellipodia. Nat Rev Mol Cell Biol. 2008;9:730–736. doi: 10.1038/nrm2453. [DOI] [PubMed] [Google Scholar]
- 30.Fackler OT, Grosse R. Cell motility through plasma membrane blebbing. J Cell Biol. 2008;181:879–884. doi: 10.1083/jcb.200802081. [DOI] [PMC free article] [PubMed] [Google Scholar]