Abstract
Neurovascular development in the central nervous system has a rich history and compelling significance. The developing central nervous system (CNS) does not produce vascular progenitor cells, and so ingression of blood vessels is required for continued CNS development and function. Classic studies provide elegant descriptions of formation of the vascular plexus that surrounds the embryonic brain and spinal cord, and the subsequent ingression of blood vessels into the neural tissue. Recent work has focused on the molecular pathways responsible for neurovascular cross-talk and development of the blood-brain barrier. Here we review neurovascular development in the central nervous system, with emphasis on the spinal cord. We discuss the historical work, the current status of our knowledge and unanswered questions. The importance of neurovascular development to diseases of the cerebral vasculature and the neural stem cell niche are discussed.
Key words: neural development, vascular development, neural tube, spinal cord, central nervous system, peri-neural vascular plexus, vessel sprouting, angiogenesis, neural stem cell, vascular niche
Introduction
Neurovascular development is the parallel emergence and patterning of the nervous system and the vascular system during embryogenesis and early life. This symbiosis is particularly important in the central nervous system (CNS) because there are no resident vascular precursor cells so the vessels that invade the developing brain and spinal cord are absolutely essential for CNS growth and maturation. There is a rich history of descriptive studies of neurovascular development in the CNS and compelling medical relevance. Neurovascular cross-talk that initiates early in life sets the stage for a continuing relationship, exemplified by formation of the blood- brain barrier that protects the fragile CNS tissue from metabolic and cellular changes. Neurovascular communication is also the basis for fMRI (functional magnetic resonance imaging), a technique that reveals changes in local blood flow and oxygenation that correlate with immediate and localized neural activity in the brain, thus allowing us to non-invasively watch people “think.”1 Perturbations of neurovascular function are also the hallmarks of diseases such as cerebral cavernous malformations and vascular dementia. Yet little is known regarding the molecular controls and mechanisms that are important in neurovascular development, and how these two complex organs communicate with each other and integrate information. This is beginning to change, as developmental biologists apply modern tools and models to questions of neurovascular development and function. This review is not comprehensive; it will focus on neurovascular development and patterning in the CNS, with emphasis on the spinal cord, since the differentiation and patterning events of both the neural and vascular compartments are accessible to examination and manipulation in the developing spinal cord. We present a brief overview of historical work, and then discuss our current knowledge and future goals.
Historical Overview
There are elegant early descriptions of blood vessel formation and invasion of the CNS.The vascularization of the fetal brain was described by several investigators, primarily through analysis of embryos injected with India ink to visualize patent vessels.2,3 These early investigators realized that blood vessels invaded from the surrounding peri-neural vascular plexus (PNVP) at specific times, and that vessels formed stereotypical patterns once they entered the brain. The patterns were thought to eventually reflect functional domains, but how initial blood vessel patterns were set up within the developing brain was not known. In 1946, Feeney and Watterson elegantly documented the formation of the PNVP around the developing spinal cord in chick embryos, and the stereotypical vessel ingression patterns exhibited over time.2 They concluded, amazingly, that the precise vessel ingression patterns they documented “…would suggest that the points where penetration of capillaries into the cord first occurs are determined by conditions within the cord…”. They did not know what conditions would promote vessel ingression, but they prophetically pointed out that experimental manipulations would be required for further information. A later comprehensive electron microscopic study of spinal cord vascularization in the developing mouse provided important information regarding cellular interactions between endothelial cells and neural cells, and described potential contributions of both cell types to the blood-brain barrier.4 These studies and others set the stage for more recent experiments examining the cellular and molecular basis for co-ordinated neurovascular development.
Neurovascular Development Moves Forward
The “modern era” of neurovascular development began with the generation of an antibody that recognized quail angioblasts and endothelial cells, called QH1.5 QH1 recognizes an epitope that seems unique to quail, since it does not cross-react with endothelial cells of other known species. QH1 reactivity was used to describe the formation of the PNVP and blood vessel ingression into the developing neural tube by Kurz et al.6 They showed that, in addition to ingressing vessel sprouts, single angioblasts also invade the avian neural tube, primarily in the dorsal area. They also confirmed earlier studies that documented a highly stereotypical vessel ingression pattern. Quail-chick chimeras were analyzed using QH1 to identify vessels deriving from grafts, and the source of endothelial cells contributing to the PNVP and vessels invading the neural tube was shown to be primarily lateral plate mesoderm, with contributions from somitic angioblasts.7–9 We extended these studies by analyzing mouse-quail chimeras that had orthotopic grafts of mouse somitic mesoderm in quail hosts.10 These studies showed that mouse angioblasts could also migrate and contribute to the PNVP in the avian embryo, indicating that the neural-derived patterning cues crossed species.
While the PNVP forms and blood vessel sprouts begin to invade the CNS, the neural tube undergoes a complex and elegant developmental program.11,12 The details of neural tube development and patterning are beyond the scope of this review, but relevant to this discussion is that distinctions are established between the luminal (medial) and pial (lateral) surfaces of the neural tube, and domains are also established on the dorsal-ventral axis that contain specific neural progenitor cells and later specific neuronal sub-populations. This dynamic developmental program likely provides important spatial cues to ingressing vessels, but these interactions have not been studied in any detail.
Once vessels invade the CNS, however, they do use cues provided by the developing nervous system to migrate in specific directions. Vessel sprouts migrate along progenitor cells called radial glia that, in the neural tube, form long cell extensions anchored on both the medial and lateral surfaces of the neural tube.13 The radial glia move their nuclei between the two surfaces, and regulate their cell cycle so that mitosis occurs in the medial area. Some daughter cells migrate to the lateral surface and differentiate into neurons and glia, while others remain in the medial area (called the ventricular zone) and continue to cycle. Distinct areas form as a result of this neural progenitor cycling and are recognized by sprouting vessels, because vessel sprouts migrate medially up to the border of the ventricular zone, but do not enter at this time. Instead, they then leave the radial glia there and migrate laterally, to form interconnections with neighbors. In the spinal cord they also migrate along the rostral-caudal axis once they reach this border, to form an endoneural plexus that gives rise to other vessels at later stages.2
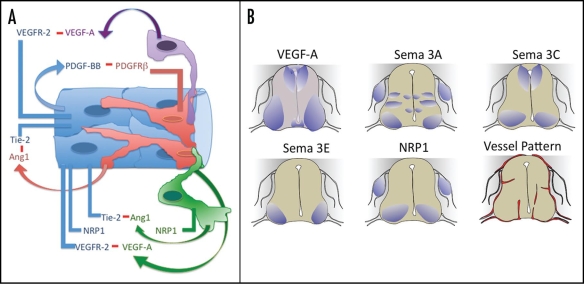
At the cellular level, endothelial cells and surrounding perivascular cells called pericytes begin to form intimate associations with the neurons and astrocytes of the nervous system, and this grouping of cells is called the neurovascular unit.14,15 These cells interact dynamically and communicate to regulate the blood-brain barrier, cerebral blood flow and synaptic activity. The blood-brain barrier is actually made by tight junctions and adherens junctions between endothelial cells. However, this specialized cell-cell adhesion requires signaling from the neural compartment, and molecular cross-talk among all the cell types of the neurovascular unit (Fig. 1A).
Figure 1.
Expression of molecules involved in neurovascular cross-talk. (A) Diagram of the neurovascular unit and some of the molecular interactions between the different cell types. Endothelial cells (blue), pericytes (red), neurons (green) and glia (purple) each secrete ligands and express receptors relevant to neurovascular cross-talk and development of the blood-brain barrier. (B) Expression of relevant molecules in the developing avian neural tube at day 4.5–5 (HH stage 26) of development, when blood vessels ingress into the neural tube. Major areas of expression are noted in purple. Note the shading throughout the neural tube for VEGF-A expression to denote low VEGF-A expression in all sites. Final panel shows the vessel ingression pattern at the same stage. Information from references cited in the text.
Molecular Aspects of Neurovascular Development
Despite an abundance of descriptive information, relatively little is known regarding the molecular cues that effect communication between neural progenitor cells and the endothelial cells that comprise vessel sprouts during neurovascular development. Risau recognized in 1986 that CNS tissue produced pro-angiogenic factors, and he and colleagues documented expression of FGF1 (acidic FGF) in the developing brain.16,17 Other pro-angiogenic factors, including VEGF-A, are also expressed, and expression of VEGF-A is regulated developmentally in the neural tube.18–22 Genetic ablation of VEGF-A in the developing neural tube reduced vessel density and branching, and neural tube development was also compromised.23,24
The studies analyzing genetic perturbation of neuronal VEGF-A illustrate an important point—that many molecular signals and guidance pathways are utilized by both the developing neural system and the developing vascular system.25–27 Among the pathways that are implicated in both vascular development and neural development are: VEGF/VEGF receptors, semaphorins/plexin receptors, neuropilins (co-receptors of both VEGF and plexin receptors) (see Fig. 1B for expression patterns), netrins/UNC receptors/DCC receptor, and slits/Robo receptors. This congruence brings up intriguing questions about the evolution of organ systems and signaling pathways, but it also presents an experimental dilemma for molecular dissection of neurovascular development. For example, global genetic manipulation of the pathways often leads to effects on both neural development and vascular development, but it is not clear whether this is due to a disruption of neurovascular cross-talk or due to independent sensitivity of each developmental program to the genetic perturbation.
One way around this dilemma is to specifically delete cell-autonomous components of the pathway in developing vessels or in the developing nervous system. In the case of VEGF-A, Haigh et al.23 analyzed a nervous tissue-specific deletion of a VEGF-A receptor, flk-1, in the developing nervous system. They reported no deleterious effects of this deletion on neurovascular development, and concluded that VEGF-A acted directly on endothelial cells to produce the defects seen with the neural-specific VEGF-A deletion. However, others have reported that VEGF signaling is important for neural survival and function.28–30 Another way to dissect differential effects utilizes genetically modified grafts, and that was our approach. First, mouse somitic mesoderm grafts were replaced by embryonic stem cell (ES)-derived embryoid body (EB) grafts in the quail host, and mouse EB grafts contributed angioblasts and endothelial cells to the host PNVP. We then grafted genetically modified EBs into quail hosts, and showed that loss of the VEGF receptor flk-1 in the graft prevented angioblast migration and PNVP contribution, suggesting a role for VEGF signaling in PNVP formation.31 Transplants of mouse neural tube into quail embryos led to formation of quail-derived PNVP around the graft, showing that the neural tube was the source of patterning signals. We developed an explant system to co-culture quail neural tubes with mouse somitic mesoderm, and found that neural-derived VEGF was required for vessel formation from somitic tissue.20 These studies provided a model whereby neural tube-derived VEGF-A was required for the migration and patterning of angioblasts to form the PNVP around the developing neural tube.
VEGF signaling is amplified by Neuropilin (NRP) co-receptors, but as their name implies, the NRPs are also expressed and required in the nervous system. Gu et al. analyzed an endothelial-specific deletion of NRP1 and reported large unbranched vessels in the brain.32 This phenotype is consistent with that of a global NRP1 deletion, since in these embryos vessels ingressed into the neural tube and migrated along radial glia, but they did not form the lateral turns at the border of the ventricular zone that normally occur.33 These studies implicate NRP1 in aspects of neurovascular development, but bring up another issue, since NRP acts as a co-receptor with VEGFR-2 (flk-1) to amplify VEGF signaling, and with plexins to modulate semaphorin signaling. Moreover, endothelial cells express some plexin receptors, so semaphorins could also directly affect vascular development. Thus a recent study by Ruhrberg and colleagues undertook to dissect the potential interactions.34 The surprising conclusion of their work was that, in the developing hindbrain, Sema/NRP signaling was required only in the neural compartment, and it was not utilized in vascular compartment to regulate vessel ingression or branching. This finding suggests that, despite the potential overlap in signaling components, distinct signaling pathways are operative in each cell type during neurovascular development.
The effects of genetic manipulation of other pathways that are likely candidates to impact neurovascular development are even less well-characterized. For example, genetic deletion of Slit ligands or the Robo4 receptor that is expressed in vascular endothelial cells does not compromise vascular development; however, Robo4 deletion compromises vessel permeability in the retina, suggesting effects on blood-retinal barrier function.35 Genetic manipulation of the Netrin/UNC pathway clearly leads to defects of vascular development. However, the effects are controversial since different studies report that loss-of-function leads to either excess vasculature, suggesting a negative role for Netrin/UNC signaling in vascular development, or lack of vasculature, suggesting a positive signaling role for Netrin/UNC.36,37 A recent study suggests that some of these differences may be due to vascular bed specific differences, while another suggests that levels of ligand may determine positive vs. negative effects on vascular development.38,39 Thus several molecular signaling pathways can affect both neural development and vascular development; however, deciphering any unique effects on neurovascular development and function is complex and ongoing.
In this context, a set of genetic studies elegantly highlights the importance of integrin signaling for maturation of the neurovascular unit and the blood-brain barrier. Genetic deletion of either αv or β8 integrins leads to intracerebral hemorrhage and peri-natal lethality, although sprouting and branching are normal.40,41 A subsequent study selectively deleted αv integrin in either the vascular endothelium or the neural progenitors of the CNS, and only the CNS deletion of αv recapitulated the cerebral vascular phenotype.42 Thus McCarty and colleagues concluded that αvβ8 is required in the neural compartment for proper neurovascular communication and maintenance of the neurovascular unit.
Recent Advances in Neurovascular Development
We asked whether neural tube-expressed VEGF-A is required for the invasion of vessel sprouts that is a critical early step in neurovascular development. To test this hypothesis, we locally modified VEGF-A signaling in the quail neural tube, reasoning that local, time-constrained changes in VEGF signaling were less likely to affect neural development than the genetic manipulations previously described. We also wanted to begin to understand how neural-derived cues are spatially regulated to result in the stereotypical vessel ingression patterns noted by ourselves and others.2 VEGF-A is alternatively spliced to produce multiple protein isoforms with differential matrix-binding affinity.43 We selectively expressed the major VEGF-A isoforms in localized places in the developing neural tube via electroporation. The matrix-binding VEGF-A isoforms, VEGF165 and VEGF189, induced ectopic ingression of PNVP vessels only in areas that expressed electroporated VEGF, without significantly affecting neuronal survival or patterning (ref. 44 and Fig. 2A).44 We also showed that localized expression of a VEGF inhibitor, the soluble VEGF receptor sFlt-1, locally blocked vessel ingression (ref. 44 and Fig. 2B).44 These studies indicate that neural tube-derived VEGF-A is involved not only in the initial formation of the PNVP, but in the subsequent stereotypical ingression of vessel sprouts into the developing neural tube.
Figure 2.
Gain and loss of function of neural tube VEGF-A signaling perturbs vessel ingression patterns. Neural tubes of day 3 quail embryos were electroporated in ovo with DNA constructs expressing (A) human VEGF165 or (B) murine sFlt-1. Each cDNA was linked to eGFP via an IRES sequence, so areas of eGFP expression (green) correspond to expression of the cDNA. Embryos were processed at day 5, and sections were stained with QH1(red) to visualize blood vessels. (A) The VEGF165 electroporated (left) side of the neural tube exhibits ectopic vessel ingression points, denoted by arrows. The contralateral side does not express ectopic VEGF165 and shows the normal vessel ingression pattern (arrowheads). (B) The sFlt-1 electroporated (left) side of the neural tube exhibits loss of vessel ingression points, compared to the unmanipulated contralateral side that has a normal vessel ingression pattern (arrowheads).
Recently a role for canonical Wnt signaling in neurovascular development was described by McMahon and colleagues.45 Genetic deletion of both Wnt7a and Wnt7b (Wnt7a/b) in the developing neural tube compromised vessel ingression. Moreover, endothelial-specific deletion of β-catenin also had a profound effect on vessel ingression and maturation of PNVP vessels to express the blood- brain barrier marker Glut-1. Taken together, these results indicate that canonical Wnt signaling in endothelial cells of the PNVP, that is initiated by Wnt7a/b from the neural compartment, is important in vessel ingression and neurovascular development. Given the pace at which genetic loci are being “knocked out” in the mouse, and the relative ease of generating tissue-specific and double knock out embryos, we predict that genetic requirements for neurovascular development will be elucidated in the near future.
An exciting study by Vasudevan et al.46 suggests that certain vessels entering the CNS may use the homeobox genes to integrate spatial information. The vascularization of the telencephalon was shown to result from migration of ventrally invasive angiogenic sprouts into the dorsal area, and the dorsal pial vessels did not contribute to the internal vessel plexus. Manipulation of expression of homeobox transcription factors that were associated with ventral (i.e., Nkx2.1) or dorsal (i.e., Pax6) domains compromised proper migration of endothelial cells, although proliferation was also affected. Thus endothelial cells may contribute to spatial domains in an active way in some instances of neurovascular development.
Future Directions
There are many important and provocative questions that remain to be answered regarding neurovascular development. One question centers around the stereotypical patterning of blood vessel ingression into the developing neural tube—how do the vessels know when and where to enter the neural tube? And how do they know where to go once they enter? We know that VEGF-A and Wnt signaling are likely not the whole story, because neither VEGF-A nor Wnt7 expression is localized only at sites of vessel ingression into the quail neural tube. Thus negative cues produced by the neural tube likely integrate with the positive signals to provide explicit patterning information.44 The nature of these cues remains to be elucidated. However, without knowing the precise molecular nature of neural tube-derived cues important in temporal and spatial regulation of vessel ingression, one can perturb neural development and determine how the perturbation affects vessel ingression. We have begun to do this, and we find that neural tube patterning changes do affect the vessel ingression pattern (James JM and Bautch VL, manuscript in preparation). Thus the ingression of blood vessels into the developing spinal cord intimately depends on the developmental events of neural tube differentiation, providing an excellent example of neurovascular communication at the earliest stages of development.
Another important and exciting set of questions centers around the role of the vasculature in establishing a putative stem cell niche for the neural lineage.28,30,47,48 We now know that neural stem cells persist in the CNS after the initial differentiation events. Recent work localizes neural stem cells to the sub-ventricular zone, and in this location they are intimately associated with the microvasculature.49 Moreover, endothelial-expressed PEDF (pigment epithelium derived factor) is important for the maintenance of neural stem cells.50 These recent studies are just the beginning of investigations into how neurovascular cross-talk sets up and maintains the vascular niche that maintains neural stem cells. A better knowledge of these relationships will obviously impact our ability to treat diseases of and injury to the CNS.
A final set of questions centers around the connection between neurovascular communication during development and the diseases and perturbations that affect the neurovascular unit later in life. For example, aging people are prone to vascular dementia, which is cognitive impairment secondary to vascular deficiencies and/or “mini-strokes” in the CNS. Moreover, there is some evidence that Alzheimer disease may result from neurovascular perturbations, and in any case Alzheimer disease is often exacerbated when present along with vascular defects.25,51 Another compelling example of how neurovascular development may impact disease is a set of mutations that genetically pre-dispose individuals to develop cerebral cavernous malformations (CCMs) later in life.52,53 CCMs are bundles of abnormal vessels that form in the CNS. CCMs promote potentially life-threatening hemorrhages that seem to arise from defects in the tight junctions that form the blood-brain barrier. Many CCMs result from lesions in one of three genes, CCM1 (KRIT1, Rap1 effector, integrin signaling), CCM2 (OSM, a scaffold protein), and CCM3 (PDCD10, associated with apoptosis). It is not clear exactly how the genetic mutations lead to the malformations, although it is interesting that KRIT1 mediates the stabilizing effects of Rap on endothelial junctions,54 and CCM1 and CCM2 may participate in a common scaffold protein complex. One hypothesis is that neurovascular development is somehow compromised, and this leads to subtle deficiencies that manifest only later in life or with a “second hit” somatic mutation. Our ability to treat these and other CNS diseases will be enhanced by more detailed knowledge of how neurovascular communication is set up developmentally and maintained throughout life.
Thus the neurovascular communication that begins as soon as blood vessels and neural tissue form during early embryonic development is crucial for the development and function of the CNS. This early communication sets the stage for a life-long relationship between these two organs that is manifested by the “neurovascular unit” in the CNS, and is susceptible to aging and disease. Neurovascular development reminds us of Rick and Captain Renault walking off together at the end of the movie classic Casablanca, with Rick saying, “Louis, I think this is the beginning of a beautiful friendship.”55
Acknowledgements
We thank members of the Bautch lab for fruitful discussions. This work was supported by grants from the NIH (HL43174 and HL86564) to V.L.B., and T32 (HL69768) NIH Pre-doctoral training grant (J.M.J.).
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/8397
References
- 1.Brown G, Perthen J, Liu T, Buxton R. A primer on functional magnetic resonance imaging. Neuropsychol Rev. 2007;17:107–125. doi: 10.1007/s11065-007-9028-8. [DOI] [PubMed] [Google Scholar]
- 2.Feeney J, Watterson R. The development of the vascular pattern within the walls of the central nervous system of the chick embryo. J Morphol. 1946;78:231–304. doi: 10.1002/jmor.1050780205. [DOI] [PubMed] [Google Scholar]
- 3.Strong L. The early embryonic pattern of internal vascularization of the mammalian cerebral cortex. J Comp Neurol. 1964;123:121–138. doi: 10.1002/cne.901230111. [DOI] [PubMed] [Google Scholar]
- 4.Nakao T, Ishizawa A, Ogawa R. Observations of vascularization in the spinal cord of mouse embryos, with special reference to development of boundary membranes and perivascular spaces. Anatomical Rec. 1988;221:663–677. doi: 10.1002/ar.1092210212. [DOI] [PubMed] [Google Scholar]
- 5.Pardanaud L, Altmann C, Kitos P, Dieterlen-Lievre F, Buck CA. Vasculogenesis in the early quail blastodisc as studied with a monoclonal antibody recognizing endothelial cells. Development. 1987;100:339–349. doi: 10.1242/dev.100.2.339. [DOI] [PubMed] [Google Scholar]
- 6.Kurz H, Gartner T, Eggli PS, Christ B. First blood vessels in the avian neural tube are formed by a combination of dorsal angioblast immigration and ventral sprouting of endothelial cells. Dev Biol. 1996;173:133–147. doi: 10.1006/dbio.1996.0012. [DOI] [PubMed] [Google Scholar]
- 7.Pardanaud L, Luton D, Prigent M, Bourcheix L-M, Catala M, Dieterlen-Lievre F. Two distinct endothelial lineages in ontogeny, one of them related to hemopoiesis. Development. 1996;122:1363–1371. doi: 10.1242/dev.122.5.1363. [DOI] [PubMed] [Google Scholar]
- 8.Pardanaud L, Yassine F, Dieterlen-Lievre F. Relationship between vasculogenesis, angiogenesis and haemopoiesis during avian ontogeny. Development. 1989;105:473–485. doi: 10.1242/dev.105.3.473. [DOI] [PubMed] [Google Scholar]
- 9.Wilting J, Brand-Saberi B, Huang R, Zhi Q, Kontges G, Ordahl CP, et al. Angiogenic potential of the avian somite. Dev Dyn. 1995;202:165–171. doi: 10.1002/aja.1002020208. [DOI] [PubMed] [Google Scholar]
- 10.Ambler CA, Nowicki JL, Burke AC, Bautch VL. Assembly of trunk and limb blood vessels involves extensive migration and vasculogenesis of somite-derived angioblasts. Dev Biol. 2001;234:352–364. doi: 10.1006/dbio.2001.0267. [DOI] [PubMed] [Google Scholar]
- 11.Dessaud E, McMahon AP, Briscoe J. Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development. 2008;135:2489–2503. doi: 10.1242/dev.009324. [DOI] [PubMed] [Google Scholar]
- 12.Gotz M, Huttner W. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- 13.Virgintino D, Maiorano E, Errede M, Vimercati A, Greco P, Selvaggi L, et al. Astrogliamicrovessel relationship in the developing human telencephalon. Int J Dev Biol. 1998;42:1165–1168. [PubMed] [Google Scholar]
- 14.Lok J, Gupta P, Guo S, Kim WJ, Whalen MJ, van Leyen K, Lo EH. Cell-cell signaling in the neurovascular unit. Neurochem Res. 2007;32:2032–2045. doi: 10.1007/s11064-007-9342-9. [DOI] [PubMed] [Google Scholar]
- 15.McCarty JH. Cell biology of the neurovascular unit: implications for drug delivery across the blood-brain barrier. Assay Drug Dev Technol. 2005;3:89–95. doi: 10.1089/adt.2005.3.89. [DOI] [PubMed] [Google Scholar]
- 16.Risau W. Developing brain produces an angiogenesis factor. Proc Natl Acad Sci USA. 1986;83:3855–3859. doi: 10.1073/pnas.83.11.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Risau W, Gautschi-Sova P, Bohlen P. Endothelial cell growth factors in embryonic and adult chick brain are related to human acidic fibroblast growth factor. EMBO J. 1988;7:959–962. doi: 10.1002/j.1460-2075.1988.tb02901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eichmann A, Marcelle C, Breant C, Le Douarin NM. Two molecules related to the VEGF receptor are expressed in early endothelial cells during avian embryonic development. Mech Dev. 1995;42:33–48. doi: 10.1016/0925-4773(93)90096-g. [DOI] [PubMed] [Google Scholar]
- 19.Flamme I, Breier G, Risau W. Vascular endothelial growth factor (VEGF) and VEGF receptor 2 (flk-1) are expressed during vasculogenesis and vascular differentiation in the quail embryo. Dev Biol. 1995;169:699–712. doi: 10.1006/dbio.1995.1180. [DOI] [PubMed] [Google Scholar]
- 20.Hogan KA, Ambler CA, Chapman DL, Bautch VL. The neural tube patterns vessels developmentally using the VEGF signaling pathway. Development. 2004;131:1503–1513. doi: 10.1242/dev.01039. [DOI] [PubMed] [Google Scholar]
- 21.Ogunshola OO, Stewart WB, Mihalcik V, Solli T, Madri JA, Ment LR. Neuronal VEGF expression correlates with angiogenesis in postnatal developing rat brain. Dev Brain Res. 2000;119:139–153. doi: 10.1016/s0165-3806(99)00125-x. [DOI] [PubMed] [Google Scholar]
- 22.Wilting J, Eichmann A, Christ B. Expression of the avian VEGF receptor homologues Quek1 and Quek2 in blood-vascular and lymphatic endothelial and non-endothelial cells during quail embryonic development. Cell Tissue Res. 1997;288:207–223. doi: 10.1007/s004410050807. [DOI] [PubMed] [Google Scholar]
- 23.Haigh JJ, Morelli PI, Gerhardt H, Haigh K, Tsien J, Damert A, et al. Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Dev Biol. 2003;262:225–241. doi: 10.1016/s0012-1606(03)00356-7. [DOI] [PubMed] [Google Scholar]
- 24.Raab S, Beck H, Gaumann A, Yuce A, Gerber HP, Plate K, et al. Impaired brain angiogenesis and neuronal apoptosis induced by conditional homozygous inactivation of vascular endothelial growth factor. Thromb Haemost. 2004;91:595–605. doi: 10.1160/TH03-09-0582. [DOI] [PubMed] [Google Scholar]
- 25.Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 26.Eichmann A, Makinen T, Alitalo K. Neural guidance molecules regulate vascular remodeling and vessel navigation. Genes & Dev. 2005;19:1013–1021. doi: 10.1101/gad.1305405. [DOI] [PubMed] [Google Scholar]
- 27.Hogan KA, Bautch VL. Blood vessel patterning at the embryonic midline. Curr Top Dev Biol. 2004;62:55–85. doi: 10.1016/S0070-2153(04)62003-5. [DOI] [PubMed] [Google Scholar]
- 28.Carmeliet P. Blood vessels and nerves: common signals, pathways and diseases. Nat Rev Genet. 2003;4:710–720. doi: 10.1038/nrg1158. [DOI] [PubMed] [Google Scholar]
- 29.Lambrechts D, Carmeliet P. VEGF at the neurovascular interface: Therapeutic implications for motor neuron disease. Biochim Biophys Acta. 2006;1762:1109–1121. doi: 10.1016/j.bbadis.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Ward N, LaManna J. The neurovascular unit and its growth factors: coordinated response in the vascular and nervous systems. Neurol Res. 2004;26:870–883. doi: 10.1179/016164104X3798. [DOI] [PubMed] [Google Scholar]
- 31.Ambler CA, Schmunk GA, Bautch VL. Stem cell-derived endothelial cells/progenitors migrate and pattern in the embryo using the VEGF signaling pathway. Dev Biol. 2003;257:205–219. doi: 10.1016/s0012-1606(03)00042-3. [DOI] [PubMed] [Google Scholar]
- 32.Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, et al. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerhardt H, Ruhrberg C, Abramsson A, Fujisawa H, Shima D, Betsholtz C. Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Dev Dyn. 2004;231:503–509. doi: 10.1002/dvdy.20148. [DOI] [PubMed] [Google Scholar]
- 34.Vieira JM, Schwarz Q, Ruhrberg C. Selective requirements for NRP1 ligands during neurovascular patterning. Development. 2007;134:1833–1843. doi: 10.1242/dev.002402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones CA, London NR, Chen H, Park KW, Sauvaget D, Stockton RA, et al. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nat Med. 2008;14:448–453. doi: 10.1038/nm1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu X, le Noble F, Yuan L, Jiang Q, de Lafarge B, Sugiyama D, et al. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004;432:179–186. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- 37.Wilson BD, Ii M, Park KW, Suli A, Sorensen LK, Larrieu-Lahargue F, et al. Netrins Promote Developmental and Therapeutic Angiogenesis. Science. 2006;313:640–644. doi: 10.1126/science.1124704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouvree K, Larrivee B, Lv X, Yuan L, DeLafarge B, Freitas C, et al. Netrin-1 inhibits sprouting angiogenesis in developing avian embryos. Dev Biol. 2008;318:172–183. doi: 10.1016/j.ydbio.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 39.Navankasattusas S, Whitehead KJ, Suli A, Sorensen LK, Lim AH, Zhao J, et al. The netrin receptor UNC5B promotes angiogenesis in specific vascular beds. Development. 2008;135:659–667. doi: 10.1242/dev.013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCarty JH, Monahan-Earley RA, Brown LF, Keller M, Gerhardt H, Rubin K, et al. Defective associations between blood vessels and brain parenchyma lead to cerebral hemorrhage in mice lacking {alpha}v integrins. Mol Cell Biol. 2002;22:7667–7677. doi: 10.1128/MCB.22.21.7667-7677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu J, Motejlek K, Wang D, Zang K, Schmidt A, Reichardt LF. {beta}8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129:2891–2903. doi: 10.1242/dev.129.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCarty JH, Lacy-Hulbert A, Charest A, Bronson RT, Crowley D, Housman D, et al. Selective ablation of {alpha}v integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 2005;132:165–176. doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- 43.Park JE, Keller GA, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993;4:1317–1326. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.James J, Gewolb C, Bautch VL. Neurovascular development uses VEGF-A signaling to regulate blood vessel ingression into the neural tube. Development. 2009;136:833–841. doi: 10.1242/dev.028845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- 46.Vasudevan A, Long J, Crandell J, Rubenstein J, Bhide P. Compartment-specific transcription factors orchestrate angiogenesis gradients in the embryonic brain. Nat Neurosci. 2008;11:429–431. doi: 10.1038/nn2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riquelme PA, Drapeau E, Doetsch F. Brain micro-ecologies: neural stem cell niches in the adult mammalian brain. Philosoph Trans Royal Society B: Biol Sci. 2008;363:123–137. doi: 10.1098/rstb.2006.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 49.Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramirez-Castillejo C, Sanchez-Sanchez F, Andreu-Agullo C, Ferron SR, Aroca-Aguilar JD, Sanchez P, et al. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci. 2006;9:331–339. doi: 10.1038/nn1657. [DOI] [PubMed] [Google Scholar]
- 51.de la Torre JC. Is Alzheimer's disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004;3:184–190. doi: 10.1016/S1474-4422(04)00683-0. [DOI] [PubMed] [Google Scholar]
- 52.Plummer N, Zawistowski J, Marchuk D. Genetics of cerebral cavernous malformations. Curr Neurol Neurosci. 2005;5:391–396. doi: 10.1007/s11910-005-0063-7. [DOI] [PubMed] [Google Scholar]
- 53.Revencu N, Vikkula M. Cerebral cavernous malformation: new molecular and clinical insights. J Med Genet. 2006;43:716–721. doi: 10.1136/jmg.2006.041079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glading A, Han J, Stockton RA, Ginsberg MH. KRIT-1/CCM1 is a Rap1 effector that regulates endothelial cell cell junctions. J Cell Biol. 2007;179:247–254. doi: 10.1083/jcb.200705175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Curtiz M. Casablanca. USA: Warner Bros; 1942. [Google Scholar]