Abstract
The mammalian central nervous system (CNS) is comprised of billions of neurons and glia that are intertwined with an elaborate network of blood vessels. These various neural and vascular cell types actively converse with one another to form integrated, multifunctional complexes, termed neurovascular units. Cell-cell communication within neurovascular units promotes normal CNS development and homeostasis, and abnormal regulation of these events leads to a variety of debilitating CNS diseases. This review will summarize (1) cellular and molecular mechanisms that regulate physiological assembly and maintenance of neurovascular units; and (2) signaling events that induce pathological alterations in neurovascular unit formation and function. An emphasis will be placed on neural-vascular cell adhesion events mediated by integrins and their extracellular matrix (ECM) ligands. I will highlight the role of a specific adhesion and signaling axis involving αvβ8 integrin, latent transforming growth factor β's (TGFβ's), and canonical TGFβ receptors. Possible functional links between components of this axis and other signal transduction cascades implicated in neurovascular development and disease will be discussed. Comprehensively understanding the pathways that regulate bidirectional neural-vascular cell contact and communication will provide new insights into the mechanisms of neurovascular unit development, physiology and disease.
Key words: αvβ8 integrin, latent TGFβ, neurovascular unit, brain angiogenesis, cerebral hemorrhage
Neurovascular Units in CNS Development, Physiology and Disease
Neurovascular units are dynamic, multicellular complexes comprised of CNS neurons and glia, vascular endothelial cells and pericytes, as well as a milieu of ECM proteins.1–3 Intimate relationships between neural cells and vascular cells initiate at early stages of vertebrate development, and these associations are maintained throughout post-natal life. For example, vascular patterning in the embryonic CNS is established when angiogenic blood vessels invade the neural parenchyma along a pre-formed latticework of radial glial cells.4,5 Radial glia are embryonic neural stem cells,6 and elegant lineage-tracing analyses have revealed that these cells give rise to neurons and astrocytes in many regions of the CNS.7 In neurogenic regions of the adult brain, neural stem cells retain many radial glial-like characteristics and preferentially cluster around cerebral blood vessels in ‘vascular niches.’8 These associations likely regulate adult neural stem cell growth, survival and fate determination.9–13 Astrocytes, which are the most abundant cell type in the post-natal CNS, and are derived from radial glial cells, maintain long-term functional connections with blood vessels.14,15 These interactions are essential for many CNS physiological processes, including homeostatic regulation of the blood-brain barrier and modulation of cerebral blood flow and metabolism.16–18
Abnormal neural-vascular cell communication is linked to a variety of fetal- and adult-onset diseases.19–21 Intraventricular hemorrhage is a birth defect prevalent in premature infants, and is characterized by vascular hemorrhages in neurogenic regions of the developing brain which often lead to long-term cognitive and motor deficits.22 In the adult brain, vascular occlusions leading to stroke cause acute neurovascular unit perturbations, leading to blood-brain barrier breakdown and neuronal dysfunction.23–25 In cancer, primary and metastatic brain tumors induce pathological angiogenesis, and tumor-associated blood vessels often lack normal neurovascular unit cytoarchitecture and have enhanced blood-brain barrier permeability.26,27 Lastly, age-related dementias such as Alzheimer disease and vascular dementia are linked to chronic abnormalities in blood flow and blood-brain barrier permeability which, in part, contribute to the progressive neurodegeneration that signifies these diseases.28,29
Integrin-Mediated Adhesion and Signaling in Neurovascular Units
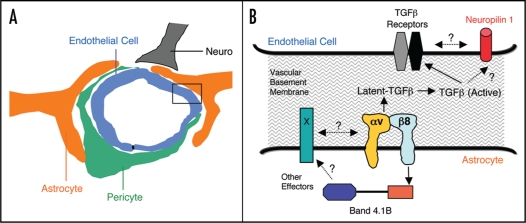
Cell-cell communication within neurovascular units occurs mainly via basement membranes that contain a milieu of ECM proteins (Fig. 1). Perivascular neural cells adhere to ECM proteins primarily via integrins, which are heterodimeric transmembrane receptors consisting of α and β subunits.30 Functions for integrins and their ECM protein ligands are linked to neurovascular unit development, homeostasis and disease. For example, β1 integrin protein expression is downregulated in cerebral endothelial cells following experimentally-induced ischemia31 and this correlates with elevated MMP-mediated degradation of ECM proteins.32 α6β4 integrin, a laminin receptor, is expressed in perivascular astrocytes where it plays important roles in regulating proper cell-ECM adhesion within neurovascular units.33 Expression of α6β4 integrin is rapidly diminished following ischemia in the brain and this correlates with reduced astrocyte-endothelial cell adhesion and increased blood-brain barrier permeability.34 Another laminin receptor, α6β1 integrin, mediates adhesion between adult neural stem cells and basement membranes within brain vascular niches, which likely influences neural stem cell fate determination.11
Figure 1.
A Model of αvβ8 integrin-mediated adhesion and signaling pathways in neurovascular units. (A) The multicellular composition of a central nervous system neurovascular unit, comprised of a capillary endothelial cell and pericyte, as well as neurons and astrocytes that adhere to extracellular matrix proteins within intervening vascular basement membranes. (B) A more detailed view of the boxed area in (A) showing the astrocyte-endothelial cell interface that is coupled, in part, by the αvβ8 integrin-TGFβ adhesion and signaling axis. αvβ8 integrin on the astrocyte binds to latent TGFβ's in the vascular basement membrane and induces TGFβ activation, resulting in liberation of TGFβ from the extracellular matrix. TGFβ signaling pathways in endothelial cells are likely regulated by canonical TGFβ receptors, but may also involve cross talk with Neuropilin 1. In astrocytes, αvβ8 integrin also interacts with Band 4.1B, a cytoskeletal adaptor protein, which may link this integrin with other intracellular and cell surface signaling effectors. Various molecular genetic data reveal that perturbation of normal astrocyte-endothelial cell coupling, via ablation of genes encoding the αvβ8 integrin or TGFβ signaling components, leads to abnormal neurovascular development and premature death.
The αv subfamily of integrins is comprised of five members: αvβ1, αvβ3, αvβ5, αvβ6 and αvβ8.35 These integrins bind to RGD peptide motifs present in ECM proteins, including vitronectin,36 collagen IV,37 and the latent forms of TGFβ's.38,39 Molecular genetic studies reveal essential roles for αv integrins in neurovascular unit development. Mouse embryos genetically null for αv integrin expression in all cells, and thus lacking all five αv integrin-containing heterodimers, develop CNS vascular pathologies that include abnormal cerebral blood vessel morphogenesis, endothelial cell hyperplasia and intracerebral hemorrhage.40,41 Complete ablation of the β8 integrin gene also leads to very similar CNS angiogenesis defects;42 in contrast, genetic deletion of the other four αv-associated β subunits does not yield similar CNS phenotypes.39,43–45
In addition to CNS-specific neurovascular phenotypes, αv and β8 integrin mutant mice develop a cleft palate and are unable to feed after birth, which likely accounts for their early neonatal death.40 In order to study integrin functions in the post-natal CNS, Cre/Lox strategies were used to selectively ablate the αv or β8 integrin genes in CNS neural progenitor cells. Mutant animals develop CNS-specific vascular phenotypes, however, they do not develop a cleft palate and survive for several post-natal months. Interestingly, all αv conditional mutants develop progressive neurological phenotypes, including sporadic seizures and a rigid gait, and die by eight months of age.46 Similar phenotypes have been reported for the conditional β8 integrin mutants,47 suggesting that the neurological impairments that develop in the αv mutants are due largely to loss of αvβ8 integrin functions. Interestingly, in these conditional knockouts the severe neurovascular pathologies that are so apparent in embryonic and neonatal periods mostly resolve by adulthood. Resolution of the neurovascular pathologies occurs within a developmental period (three to four post-natal weeks) when CNS blood vessels transition from an angiogenic to a quiescent status,48 suggesting that αvβ8 integrin provides essential roles in modulating active phases of CNS blood vessel development. It remains to be determined if this integrin is also involved in blood vessel homeostasis in the adult CNS, or if pathological angiogenesis in the adult CNS is regulated by αvβ8 integrin.
The β subunit cytoplasmic domains of most integrins bind to effector proteins and regulate multiple intracellular signaling cascades. Interestingly, the cytoplasmic domain of β8 integrin does not contain signaling motifs that are present in other β subunits, suggesting unique signaling capabilities. Along these lines, members of the Band 4.1 family of cytoskeletal adaptors interact selectively with the β8 integrin cytoplasmic tail, but not with other integrins.49 The functional significance of the αvβ8-Band 4.1 associations have yet to be determined, but it is enticing to speculate that Band 4.1's may serve to ‘bridge’ αvβ8 integrin with other signaling effectors, resulting in the formation of multi-protein complexes in perivascular astrocytes that modulate blood vessel morphegenesis in the CNS (Fig. 1).
Integrin-Mediated TGFβ Activation Regulates CNS Neurovascular Development
TGFβ's are potent cytokines that are secreted from cells as ECM-bound, inactive complexes.50 Dissociation of bioactive TGFβ's from latency-associated peptides (LAPs) leads to release from the ECM, facilitating receptor engagement and activation of intracellular signaling events.51 The LAPs that associate with TGFβ1 and TGFβ3, but not TGFβ2, contain RGD peptide sequences that are classic integrin binding motifs. All five αv-containing integrins can adhere to LAP-TGFβ's via these RGD sequences, however, only αvβ6 and αvβ8 integrin have been reported to activate TGFβ's.38,39 The functional significance of integrin-mediated TGFβ activation has been demonstrated using ‘knock-in’ strategies, in which the RGD binding within the endogenous TGFβ1 gene was mutated to RGE, resulting in abrogation of integrin adhesion. These mutant mice develop phenotypes that are identical to those observed in TGFβ1-null mice, indicating that integrins play central roles in TGFβ activation.52 Mice lacking active TGFβ1 or TGFβ3 gene products do not develop obvious neurovascular pathologies; however, mice deficient for both active TGFβ1 and TGFβ3 develop CNS-specific neurovascular phenotypes that are very similar to those observed in αv and β8 integrin knockout mice.53 These data strongly support a model whereby activation of TGFβ signaling, primarily via αvβ8 integrin in radial glial cells and astrocytes, is critical for proper regulation of CNS angiogenesis and blood-brain barrier formation (Fig. 1). In support of this model, D'Amore and colleagues have shown that astrocyte induction of blood-brain barrier properties in endothelial cells occurs via a two-step process: the first requires cell-cell interactions between astrocytes and endothelial cells, and the second involves activation of TGFβ1-dependent signaling pathways in endothelial cells.54 Similarly, Nishimura and colleagues have shown that TGFβ1, activated by cultured astrocytes, leads to the upregulation of various factors in endothelial cells that regulate angiogenesis and promote blood-brain barrier formation.55
TGFβ's modulate intracellular signal transduction pathways primarily via two canonical receptors.56 TGFβ receptors are expressed in nearly all cell types and are linked to various physiological events; however, gene-targeting strategies in mice reveal that they are essential for normal vascular development.57 For example, ablation of TGFβ receptor signaling selectively in murine vascular endothelial cells leads to defective angiogenesis and lethality by embryonic day 11,58 making it difficult to determine if integrin-activated TGFβ's signal primarily via the canonical TGFβ receptors, or if other non-canonical TGFβ receptors are involved.59 Neuropilin 1, a cell surface receptor that binds secreted semaphorins as well as vascular endothelial growth factors, has also been reported to bind to TGFβ's.60 Interestingly, genetic ablation of Nrp1 leads to CNS neurovascular phenotypes that are similar, although not as severe, as those observed in αvβ8 integrin and TGFβ knockouts.61,62 Based on these data, it is enticing to speculate that αvβ8 integrin, latent TGFβ's, canonical TGFβ receptors, and possibly Nrp1, are components of a signal transduction axis that bridges astrocytes, vascular basement membranes and endothelial cells, and is essential for neurovascular unit development and homeostasis in the CNS.
Functional Links between αvβ8 Integrin and other Signal Transduction Pathways
While αvβ8 integrin-mediated TGFβ activation is critically involved in CNS neurovascular development, it is likely that other signaling cascades also play roles in this process. Indeed, several knockout mouse models develop CNS vascular pathologies that are similar to those reported for αvβ8 integrin and TGFβ1/TGFβ3 mutants. For example, genetic ablation of the transcription factors Id1 and Id3 leads to severe neurovascular pathologies, including abnormal angiogenesis and intracerebral hemorrhage.63 Id1/3 mutant embryos display increased numbers of neurons in the CNS due to premature differentiation of radial glial cells. This raises the interesting possibility that radial glial cells, which serve as guides for angiogenic blood vessels, may be progressively depleted, thus contributing to the abnormal blood vessel morphogenesis and hemorrhage in Id1/Id3 mutant mice. Alternatively, Id1 and Id3 may regulate expression of other gene products that normally function in conjunction with αvβ8 integrin in radial glial cells.
Presenilin 1 is a component of the γ-secretase complex and mutations in the human presenilin 1 gene are linked to familial forms of the age-related dementia, Alzheimer disease.64 Interestingly, genetic ablation of presenilin 1 in mice leads to severe developmental neurovascular pathologies in the CNS,65 and these phenotypes can be rescued by transgenically expressing presenilin 1 in embryonic neural progenitor cells.66 These data reveal that Presenilin 1, like αvβ8 integrin, is a neural cell regulator of angiogenesis in the CNS. Various data also link the amyloid precursor protein, a substrate for the γ-secretase complex, to neurovascular physiology and pathology. Transgenic expression of amyloid precursor protein containing the naturally occurring ‘Dutch’ mutation results in cerebral angiopathy, intracerebral hemorrhage and Alzheimer disease-like phenotypes.67 Various reports have linked amyloid precursor protein to integrin-mediated cell adhesion and signaling.68 Full-length amyloid precursor protein, in complex with its intracellular binding partner FE65, regulates integrin-dependent cell migration. Integrins directly bind to an RHDS tetrapeptide motif within the C-terminus of amyloid precursor protein and Aβ peptides. This RHD peptide sequence is very similar to the RGD motif commonly found in ECM ligands for αvβ8 integrin, including latent TGFβ's. Interestingly, mutations in the TGFβ1 gene are linked to early-onset AD, and elevated TGFβ1 expression in mice and humans correlates with increased Aβ deposition.69 Based on these in vivo and in vitro data, there is likely crosstalk between presenilin 1, amyloid precursor protein and αvβ8 integrin signaling pathways, and these events may cooperatively regulate neurovascular unit development and/or homeostasis.
Wnts are ECM-associated proteins that regulate intracellular signaling pathways via their Frizzled/Wingless and LRP cell surface receptors.70 A recent report by Stenmann et al. has revealed that combined ablation of the Wnt7a and Wnt7b genes in CNS neural stem cells leads to neurovascular pathologies, including CNS-specific hemorrhage.71 Genetic ablation of β-catenin in vascular endothelial cells induces CNS neurovascular pathologies. Similarly, Dejana and colleagues have shown that Wnt-mediated activation of β-catenin induces blood-brain barrier properties in cultured endothelial cells.72 Collectively, these data reveal that canonical Wnt-dependent signaling pathways are required for neurovascular development during mouse embryogenesis. It will be fascinating to determine how Wnt-mediated processes are functionally connected to the other adhesion and signaling pathways, particularly integrin activation of TGFβ's, as summarized above.
Like other organs, microvessels in the CNS are composed of endothelial cells and pericytes that communicate with each other via the ECM and direct cell-cell contacts. In the developing chick embryo, blocking antibodies directed against the homophilic cell adhesion receptor, N-cadherin, causes impaired endothelial-pericyte interactions, leading to abnormal brain angiogenesis and intracerebral hemorrhage.73 Functional links between integrin and cadherin signaling pathways have been reported,74,75 and it will be interesting to determine if integrins also play roles in endothelialpericyte associations in CNS neurovascular units.
In conclusion, neurovascular units are the cellular and molecular interfaces between the vascular system and central nervous system. We are just beginning to grasp the complex pathways underlying regulation of physiological and pathological cell-cell communication within neurovascular units. A comprehensive understanding of how these various signaling pathways are interconnected will not only increase our understanding of mechanisms that promote neurovascular development and physiology, but will also provide new insights into the causes of debilitating neurovascular pathologies, such as birth defects, stroke, cancer and age-related dementias.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/7767
References
- 1.Lok J, Gupta P, Guo S, Kim WJ, Whalen MJ, van Leyen K, et al. Cell-cell signaling in the neurovascular unit. Neurochem Res. 2007;32:2032–2045. doi: 10.1007/s11064-007-9342-9. [DOI] [PubMed] [Google Scholar]
- 2.McCarty JH. Cell biology of the neurovascular unit: implications for drug delivery across the blood-brain barrier. Assay Drug Dev Technol. 2005;3:89–95. doi: 10.1089/adt.2005.3.89. [DOI] [PubMed] [Google Scholar]
- 3.Pardridge WM. Drug and gene delivery to the brain: the vascular route. Neuron. 2002;36:555–558. doi: 10.1016/s0896-6273(02)01054-1. [DOI] [PubMed] [Google Scholar]
- 4.Marin-Padilla M. Early vascularization of the embryonic cerebral cortex: Golgi and electron microscopic studies. J Comp Neurol. 1985;241:237–249. doi: 10.1002/cne.902410210. [DOI] [PubMed] [Google Scholar]
- 5.Virgintino D, Maiorano E, Errede M, Vimercati A, Greco P, Selvaggi L, et al. Astrogliamicrovessel relationship in the developing human telencephalon. Int J Dev Biol. 1998;42:1165–1168. [PubMed] [Google Scholar]
- 6.Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 7.Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. doi: 10.1016/s0896-6273(04)00140-0. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- 9.Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, Arikawa-Hirasawa E, et al. Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells. 2007;25:2146–2157. doi: 10.1634/stemcells.2007-0082. [DOI] [PubMed] [Google Scholar]
- 10.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wurmser AE, Palmer TD, Gage FH. Neuroscience. Cellular interactions in the stem cell niche. Science. 2004;304:1253–1255. doi: 10.1126/science.1099344. [DOI] [PubMed] [Google Scholar]
- 14.Gordon GR, Mulligan SJ, MacVicar BA. Astrocyte control of the cerebrovasculature. Glia. 2007;55:1214–1221. doi: 10.1002/glia.20543. [DOI] [PubMed] [Google Scholar]
- 15.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 16.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 17.Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- 19.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 21.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Roland EH, Hill A. Germinal matrix-intraventricular hemorrhage in the premature newborn: management and outcome. Neurol Clin. 2003;21:833–851. doi: 10.1016/s0733-8619(03)00067-7. [DOI] [PubMed] [Google Scholar]
- 23.del Zoppo GJ, Hallenbeck JM. Advances in the vascular pathophysiology of ischemic stroke. Thromb Res. 2000;98:73–81. doi: 10.1016/s0049-3848(00)00218-8. [DOI] [PubMed] [Google Scholar]
- 24.Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008;7:476–484. doi: 10.1016/j.cmet.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 26.Fidler IJ, Yano S, Zhang RD, Fujimaki T, Bucana CD. The seed and soil hypothesis: vascularisation and brain metastases. Lancet Oncol. 2002;3:53–57. doi: 10.1016/s1470-2045(01)00622-2. [DOI] [PubMed] [Google Scholar]
- 27.Gilbertson RJ, Rich JN. Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 28.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 29.Zlokovic BV. Neurovascular mechanisms of Alzheimer's neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 31.Tagaya M, Haring HP, Stuiver I, Wagner S, Abumiya T, Lucero J, et al. Rapid loss of microvascular integrin expression during focal brain ischemia reflects neuron injury. J Cereb Blood Flow Metab. 2001;21:835–846. doi: 10.1097/00004647-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Lee SR, Lo EH. Induction of caspase-mediated cell death by matrix metalloproteinases in cerebral endothelial cells after hypoxia-reoxygenation. J Cereb Blood Flow Metab. 2004;24:720–727. doi: 10.1097/01.WCB.0000122747.72175.47. [DOI] [PubMed] [Google Scholar]
- 33.del Zoppo GJ, Milner R. Integrin-matrix interactions in the cerebral microvasculature. Arterioscler Thromb Vasc Biol. 2006;26:1966–1975. doi: 10.1161/01.ATV.0000232525.65682.a2. [DOI] [PubMed] [Google Scholar]
- 34.Wagner S, Tagaya M, Koziol JA, Quaranta V, del Zoppo GJ. Rapid disruption of an astrocyte interaction with the extracellular matrix mediated by integrin alpha6beta4 during focal cerebral ischemia/reperfusion. Stroke. 1997;28:858–865. doi: 10.1161/01.str.28.4.858. [DOI] [PubMed] [Google Scholar]
- 35.Hynes RO, Lively JC, McCarty JH, Taverna D, Francis SE, Hodivala-Dilke K, et al. The diverse roles of integrins and their ligands in angiogenesis. Cold Spring Harb Symp Quant Biol. 2002;67:143–153. doi: 10.1101/sqb.2002.67.143. [DOI] [PubMed] [Google Scholar]
- 36.Nishimura SL, Sheppard D, Pytela R. Integrin alphavbeta8. Interaction with vitronectin and functional divergence of the beta8 cytoplasmic domain. J Biol Chem. 1994;269:28708–28715. [PubMed] [Google Scholar]
- 37.Venstrom K, Reichardt L. Beta8 integrins mediate interactions of chick sensory neurons with laminin-1, collagen IV and fibronectin. Mol Biol Cell. 1995;6:419–431. doi: 10.1091/mbc.6.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, et al. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGFbeta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, et al. The integrin alphavbeta6 binds and activates latent TGFbeta1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 40.Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis and organogenesis precede lethality in mice lacking all alphav integrins. Cell. 1998;95:507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- 41.McCarty JH, Monahan-Earley RA, Brown LF, Keller M, Gerhardt H, Dvorak HF, et al. Defective associations between blood vessels and brain parenchyma lead to cerebral hemorrhage in mice lacking alphav integrins. Mol Cell Biol. 2002;22:7667–7677. doi: 10.1128/MCB.22.21.7667-7677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu J, Motejlek K, Wang D, Zang K, Schmidt A, Reichardt LF. beta8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129:2891–2903. doi: 10.1242/dev.129.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graus-Porta D, Blaess S, Senften M, Littlewood-Evans A, Damsky C, Huang Z, et al. Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron. 2001;31:367–379. doi: 10.1016/s0896-6273(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 44.Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, Ross FP, Coller BS, Teitelbaum S, Hynes RO. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang X, Griffiths M, Wu J, Farese RV, Jr, Sheppard D. Normal development, wound healing and adenovirus susceptibility in beta5-deficient mice. Mol Cell Biol. 2000;20:755–759. doi: 10.1128/mcb.20.3.755-759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCarty JH, Lacy-Hulbert A, Charest A, Bronson RT, Crowley D, Housman D, et al. Selective ablation of {alpha}v integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 2004 doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- 47.Proctor JM, Zang K, Wang D, Wang R, Reichardt LF. Vascular development of the brain requires beta8 integrin expression in the neuroepithelium. J Neurosci. 2005;25:9940–9948. doi: 10.1523/JNEUROSCI.3467-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Plate KH. Mechanisms of angiogenesis in the brain. J Neuropathol Exp Neurol. 1999;58:313–320. doi: 10.1097/00005072-199904000-00001. [DOI] [PubMed] [Google Scholar]
- 49.McCarty JH, Cook AA, Hynes RO. An interaction between {alpha}v{beta}8 integrin and Band 4.1B via a highly conserved region of the Band 4.1 C-terminal domain. Proc Natl Acad Sci USA. 2005;102:13479–13483. doi: 10.1073/pnas.0506068102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 51.Massague J, Gomis RR. The logic of TGFbeta signaling. FEBS Lett. 2006;580:2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 52.Yang Z, Mu Z, Dabovic B, Jurukovski V, Yu D, Sung J, et al. Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J Cell Biol. 2007;176:787–793. doi: 10.1083/jcb.200611044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mu Z, Yang Z, Yu D, Zhao Z, Munger JS. TGFbeta1 and TGFbeta3 are partially redundant effectors in brain vascular morphogenesis. Mech Dev. 2008;125:508–516. doi: 10.1016/j.mod.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 54.Garcia CM, Darland DC, Massingham LJ, D'Amore PA. Endothelial cell-astrocyte interactions and TGFbeta are required for induction of blood-neural barrier properties. Brain Res Dev Brain Res. 2004;152:25–38. doi: 10.1016/j.devbrainres.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 55.Cambier S, Gline S, Mu D, Collins R, Araya J, Dolganov G, et al. Integrin alpha(v) beta8-mediated activation of transforming growth factor-beta by perivascular astrocytes: an angiogenic control switch. Am J Pathol. 2005;166:1883–1894. doi: 10.1016/s0002-9440(10)62497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Massague J. TGFbeta in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.ten Dijke P, Arthur HM. Extracellular control of TGFbeta signalling in vascular development and disease. Nat Rev Mol Cell Biol. 2007;8:857–869. doi: 10.1038/nrm2262. [DOI] [PubMed] [Google Scholar]
- 58.Carvalho RL, Itoh F, Goumans MJ, Lebrin F, Kato M, Takahashi S, et al. Compensatory signalling induced in the yolk sac vasculature by deletion of TGFbeta receptors in mice. J Cell Sci. 2007;120:4269–4277. doi: 10.1242/jcs.013169. [DOI] [PubMed] [Google Scholar]
- 59.Park SO, Lee YJ, Seki T, Hong KH, Fliess N, Jiang Z, et al. ALK5- and TGFBR2-independent role of ALK1 in the pathogenesis of hereditary hemorrhagic telangiectasia type 2. Blood. 2008;111:633–642. doi: 10.1182/blood-2007-08-107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glinka Y, Prud'homme GJ. Neuropilin-1 is a receptor for transforming growth factor {beta}-1, activates its latent form and promotes regulatory T cell activity. J Leukoc Biol. 2008 doi: 10.1189/jlb.0208090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gerhardt H, Ruhrberg C, Abramsson A, Fujisawa H, Shima D, Betsholtz C. Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Dev Dyn. 2004;231:503–509. doi: 10.1002/dvdy.20148. [DOI] [PubMed] [Google Scholar]
- 62.Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, et al. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O'Reilly R, et al. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature. 1999;401:670–677. doi: 10.1038/44334. [DOI] [PubMed] [Google Scholar]
- 64.Shen J, Kelleher RJ., 3rd The presenilin hypothesis of Alzheimer's disease: evidence for a loss-of-function pathogenic mechanism. Proc Natl Acad Sci USA. 2007;104:403–409. doi: 10.1073/pnas.0608332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S. Skeletal and CNS defects in Presenilin-1-deficient mice. Cell. 1997;89:629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- 66.Wen PH, De Gasperi R, Sosa MA, Rocher AB, Friedrich VL, Jr, et al. Selective expression of presenilin 1 in neural progenitor cells rescues the cerebral hemorrhages and cortical lamination defects in presenilin 1-null mutant mice. Development. 2005;132:3873–3883. doi: 10.1242/dev.01946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herzig MC, Winkler DT, Burgermeister P, Pfeifer M, Kohler E, Schmidt SD, et al. Abeta is targeted to the vasculature in a mouse model of hereditary cerebral hemorrhage with amyloidosis. Nat Neurosci. 2004;7:954–960. doi: 10.1038/nn1302. [DOI] [PubMed] [Google Scholar]
- 68.Sabo SL, Ikin AF, Buxbaum JD, Greengard P. The Alzheimer amyloid precursor protein (APP) and FE65, an APP-binding protein, regulate cell movement. J Cell Biol. 2001;153:1403–1414. doi: 10.1083/jcb.153.7.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim Y, Lee C. The gene encoding transforming growth factor {beta}1 confers risk of ischemic stroke and vascular dementia. Stroke. 2006 doi: 10.1161/01.STR.0000244782.76917.87. [DOI] [PubMed] [Google Scholar]
- 70.Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 71.Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- 72.Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gerhardt H, Wolburg H, Redies C. N-cadherin mediates pericytic-endothelial interaction during brain angiogenesis in the chicken. Dev Dyn. 2000;218:472–479. doi: 10.1002/1097-0177(200007)218:3<472::AID-DVDY1008>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 74.Shintani Y, Fukumoto Y, Chaika N, Svoboda R, Wheelock MJ, Johnson KR. Collagen I-mediated upregulation of N-cadherin requires cooperative signals from integrins and discoidin domain receptor 1. J Cell Biol. 2008;180:1277–1289. doi: 10.1083/jcb.200708137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.von Schlippe M, Marshall JF, Perry P, Stone M, Zhu AJ, Hart IR. Functional interaction between E-cadherin and alphav-containing integrins in carcinoma cells. J Cell Sci. 2000;113:425–437. doi: 10.1242/jcs.113.3.425. [DOI] [PubMed] [Google Scholar]