Abstract
Antiangiogenic therapies aimed at inhibiting the formation of tumor vasculature hold great promise for cancer therapy, with multiple compounds currently undergoing clinical trials. As with many forms of chemotherapy, antiangiogenic drugs face numerous hurdles in their translation to clinical use. Many such promising agents exhibit a short half-life, low solubility, poor bioavailability and multiple toxic side effects. Furthermore, when targeting malignant brain tumors the blood-brain barrier represents a formidable obstacle, preventing drugs from penetrating into the central nervous system (CNS). In this review, we discuss several preclinical antiangiogenic therapies and describe issues related to the unique conditions in the brain with regard to cancer treatment and neurotoxicity. We focus on the limitations of antiangiogenic drugs in the brain, along with numerous solutions that involve novel biomaterials and nanotechnological approaches. We also discuss an example in which modifying the properties of an antiangiogenic compound enhanced its clinical efficacy in treating tumors while simultaneously mitigating undesirable neurological side-effects.
Key words: angiogenesis, CNS, glioma, drug-delivery, brain, blood-brain-barrier, nanoparticles, lodamin
Introduction
Angiogenesis is the development of new blood vessels from existing vasculature.1 Physiological angiogenesis takes place during development, reproduction and wound healing via a process that is typically focal and limited in time.2 Pathological angiogenesis, however, plays an important role not only in cancer growth, but also in the development of other non-neoplastic diseases such as rheumatoid arthritis, atherosclerosis, haemangiomas, endometriosis, diabetic retinopathy and age-related macular degeneration. Unlike physiological angiogenesis, pathological angiogenesis is persistent and can last for years. Tumor growth and metastasis, for example, are dependent on angiogenesis to supply the neoplastic mass with oxygen and nutrients, thereby permitting growth beyond the limits of oxygen diffusion. Such pathologic angiogenesis is typically disorganized leading to bleeding, vascular leakage and tissue destruction. In addition, vascular irregularities provide a route for the escape of metastatic cells from the confines of the primary tumor into the bloodstream.2
A delicate balance between pro- and anti-angiogenic factors determines whether blood vessels stay quiescent or flourish as angiogenesis is initiated. Various stimuli have been reported to shift this balance.1 Healthy tissue appears to produce balanced levels of these factors, whereas tumors skew the angiogenic balance by secreting more pro-than anti-angiogenic factors into the surrounding environment. This imbalance promotes angiogenesis, frequently contributing to the abnormal growth of tumors.3,4 Controlling this process and inhibiting angiogenesis has been proven useful in the clinic, not only for optimizing current cancer treatments, but also for treatment of other angiogenesis-dependent diseases. In fact, several angiogenesis inhibitors have already been approved by the U.S. Food and Drug Administration (FDA) and many others are currently in clinical trials. The first approved specific antiangiogenesis drugs were the inhibitors of vascular endothelial growth factor (VEGF): bevacizumab (Avastin; by Genentech), ranibizumab (Lucentis; by Genentech) and pegaptanib (Macugen; by OSI Pharmaceuticals). Numerous antiangiogenic inhibitors are currently in the final stages of approval with many already in clinical use in combination with conventional therapies.2
Angiogenesis in Glioma
While angiogenesis plays an important role in embryological brain development, there is almost no new blood vessel formation in the normal adult brain. In contrast, the most common type of primary brain tumor, malignant glioma, exhibits strong angiogenic behavior in the brain.5 Astrocytic tumors are common primary brain tumors that are classified into four categories according to the histological appearance, cellular polymorphism, degree of neovascularization and necrosis: pilocytic astrocytoma (WHO grade I, non-invasive), low grade astrocytoma (WHO grade II), anaplastic (malignant), astrocytoma (WHO grade III) and glioblastoma (WHO grade IV). Formation of the new blood vessels first appears in the low grade astrocytoma and increases as the disease stage advances.6–8 The highly aggressive and malignant grades, anaplastic astrocytoma and glioblastoma, are characterized by rapid proliferation, infiltration and high levels of angiogenesis. Glioblastomas are among the most vascularized tumors and express significantly elevated levels of pro-angiogenic factors in the nervous system.9,10
Malignant gliomas cause significant morbidity and mortality regardless of stage and histological type, largely due to their infiltrative tendencies, high levels of recurrence and central nervous system (CNS) involvement.11 Management of gliomas and related tumors has proven to be particularly challenging.12 The survival rate for patients with these tumors has not changed significantly despite existing treatments that combine surgical resection, radiotherapy and chemotherapy. There is, therefore, a pressing need for the development of new therapeutic approaches to control and treat such malignancies. The high degree of angiogenesis in malignant glioma suggests that, in this context, angiogenesis inhibition can present a promising therapeutic approach.
Drug Delivery Across the Blood-Brain-Barrier
The brain is a unique organ that is protected physically by the skull, cerebrospinal fluid and three types of meninges. The cerebral vasculature, which is the main conduit for therapeutic agents, consists of endothelial cells embedded in a multicellular structure that is surrounded by pericytes in the basal membrane and enclosed by perivascular astrocytes. This structure forms the blood-brain barrier (BBB).13 Unlike the endothelial cells of non-cerebral capillaries, cerebral endothelial cells have extremely tight junctions. The physiological component of the BBB is critical for maintaining brain homeostasis, as it limits the transport of molecules from the circulation to the brain, serving mostly to protect the brain from exposure to exogenous substances (Fig. 1). Many diseases of the CNS such as stroke, multiple sclerosis, traumatic injury and brain tumors are associated with disruption of the BBB. The association between angiogenesis and the BBB is still under investigation, mainly in pathological conditions.14–16
Figure 1.
Illustration of the blood brain barrier and its disruption in the setting of a tumor. (A) Blood-brain barrier (BBB): cerebral endothelial cells are embedded in multicellular structure surrounded by pericytes in the basal membrane and enclosed by perivascular astrocytes. (B) Unlike the endothelial cells of non-cerebral capillaries, cerebral endothelial cells have extremely tight junctions. These significantly limit the transport of molecules from the circulation into the brain. Only small lipophilic molecules can be delivered through the BBB. In brain tumors, the tumor cells secrete pro-angiogenic factors such as VEGF, thereby increasing vessel permeability, disrupting the BBB and allowing for the penetration of larger molecules through the BBB.
In brain tumors there is an overexpression of pro-angiogenic factors, such as VEGF A, which correlates directly with tumor vascularity and grade and inversely with patients' prognoses.17,18 Several studies have demonstrated that pro-angiogenic factors such as VEGF enhance cerebral microvascular perfusion and increase leakage across the BBB.19,20 For example, it has been shown that the combination of the pro-angiogenic factors VEGF and angiopoietin-2 leads to disruption of BBB by increasing metalloproteinase activity and inhibiting expression of zonula occludens-1, a key regulator of tight-junction formation.21,22 The leakiness of irregular tumor vasculature is known to cause abnormal blood flow and an increase in interstitial fluid pressure. In the brain, this can lead to edema and catastrophic complications as intracranial pressure rise. With regard to drug accessibility, increased interstitial fluid pressure reduces the ability of small molecule drugs to flow down a pressure gradient and towards the site of a tumor.23,24 However, for macromolecules, polymeric drugs and nanoparticles, vessel leakage can actually increase drug accumulation due to a the Enhanced Permeability and Retention (EPR) effect.25–27 EPR is used for the passive targeting of solid tumors and many new cancer drugs rely on this mechanism.28,29
Despite the pathological disruption of the BBB, many anti-cancer drugs are limited by their inability to further penetrate this barrier. Studies comparing a subcutaneous tumor to the same tumor type in its orthotopic brain location demonstrated reduced transvascular transport in the brain. This suggests some barrier functions of the BBB are still intact even in this pathological setting.30,31
As a result of the BBB, treating brain tumors presents one of the most difficult challenges in oncology. The physiochemical properties of anti-cancer drugs, such as their molecular weight and lipophilicity, are the main factors governing passive diffusion through cerebral endothelial cells. Only lipid-soluble low-molecular-weight drugs (<600 Da) have the potential to cross the BBB.32 Many chemotherapy drugs often fail to treat brain tumors because of the cell-resistance that is frequently related to expression of p-glycoprotein.33,34 P-glycoprotein is known to pump anticancer drugs out of the cell once they entered through the outer membrane. For example, the anti-cancer drug doxorubicin accumulates poorly in the brain when given systemically. The low lipophilicity and high molecular weight of doxorubicin prevents penetration across the BBB. In addition, p-glycoprotein in the brain capillaries functions as an efflux pump, restricting the transfer of doxorubicin into the brain.35–37
Due to these limitations of drug delivery, high systemic levels of a drug are often required to achieve therapeutic concentrations within the CNS. However, increasing the anti-cancer drug dosage, frequency and duration is often limited by systemic toxicity and poor patient compliance. Significant efforts have focused on addressing these issues and offering alternatives to existing drugs by modification through polymer-chemistry and nanotechnology.
Solutions for Improving Drug Delivery to the Brain
Many strategies have been developed to overcome the limitations of drug delivery in the brain. These have included local delivery by polymeric or non-polymeric systems and osmotic opening of the BBB.
One approach to bypass the BBB is to introduce the drug directly into the brain parenchyma by local implantation. The first local drug-delivery technology for chemotherapy in the brain was FDA approved in 1996 and is now in commercial use for cancer treatment. Gliadel® is a wafer composed of polyanhydride polymer (poly[1,3-bis(carb-oxyphenoxy)propane-co-sebacic acid]) (PPCP-SA) releasing carmustine (BCNU) in a slow manner post-brain implantation.38,39 Other non-polymeric systems used for local delivery of chemotherapy agents include minipumps and convection-enhanced delivery (CED). CED, first proposed by the National Institutes of Health (NIH) in the early 1990s, involves the continuous injection of drug solution via a catheter under positive pressure. This delivery technique enables large volume distributions and high drug concentrations.40 Polymeric micro/nano particles and carriers are in wide development for brain tumor therapy. These include micelles, liposomes, dendrimers, nanoparticles and drug conjugation methods. Indeed, several anti-cancer drugs including paclitaxel, 5-fluorouracil, doxorubicin and dexamethasone have successfully been formulated using polymers and nanomaterials for local or systemic administration.41–47
Other approaches that have been utilized for the efficient administration of chemotherapy agents include the use of osmotic pressure to open the tight junctions of the BBB endothelial lining.48 Osmotic opening of the BBB by intracarotid infusion of hypertonic arabinose or mannitol is mediated by dilation of the cerebral blood vessels and shrinkage of the endothelial cells, causing widening of the endothelial tight junctions.49
Antiangiogenesis Inhibitors: Potential Limitations and Solutions
Antiangiogenic agents can be proteins, peptides, low molecular weight molecules or antibodies.50–52 Unlike chemotherapeutics that generally target all rapidly proliferating cells, antiangiogenic drugs target blood vessel development at different stages of formation. Targeting the genetically stable endothelial-cells, antiangiogenic drugs typically generate less resistance and toxicity than existing chemotherapeutics. This difference in cellular targets allows the activity of antiangiogenic drugs to be systemic without having to cross the BBB in order to effectively treat brain tumors.
Despite these advantages of antiangiogenic therapies, they face challenges when used in the clinic similar to anti-cancer drugs. For example, the delivery of antiangiogenic drugs is complex since many of these drugs are insoluble, have short half-lives and circulating time, have low bioavailabilty, and cause severe side-effects in high doses. Multiple approaches are being used to combat these obstacles, many of which come from the field of biomaterials and nanotechnology.
The use of biocompatible and biodegradable polymers for the local slow-release of drugs into the brain has been beneficial. Local delivery leads to increased and focused drug concentrations in and around the tumor, thereby minimizing the need for frequent systemic administration. This can be particularly helpful in the case of unstable drugs that require very high and frequent doses in order to achieve therapeutic concentrations at the tumor site. Minocycline, when incorporated into the biodegradable polymer, exemplifies the method of local slow-release antiangiogenic therapy. Minocycline was incorporated into the biodegradable polymer polyanhydride poly[bis(p-carboxyphenoxy) propane-sebacic acid] (pCPP:SA) at a ratio of 50:50 by weight and was slowly released over time at the tumor sites in a rat intracranial 9L gliosarcoma model. While systemic administration did not extend survival time, local treatment with minocycline in this system extended median survival time by 530%.53 The same system was used for the slow release of heparin and was demonstrated to be efficient in inhibiting the growth of rat 9L gliomas.54
In recent studies the successful development of another polymeric delivery system for local implantation was demonstrated in an orthotopic glioma mouse model. Microspheres (30–50 µm) composed of poly lactic co-glycolic acid (PLGA) were used to encapsulate and release two endogenous antiangiogenic peptides in the mouse brain: human recombinant hemopexin like domain of metalloproteinase 2 (PEX) and C-terminal fragment of platelet factor 4 (PF-4/CTF). It was demonstrated that in U87/MG glioblastoma tumors in mice, a single injection of PLGA microspheres loaded with micrograms of PEX or PF4/CTF adjacent to the tumor in subcutaneous mouse models led to 88% and 95% inhibition 30 days post-administration, and 72% tumor inhibition 14 days post PF4/CTF microsphere injection in an orthotropic model.55,56 Significant inhibition of angiogenesis was obtained in these studies with considerably lower doses than those required for systemic administration. Other studies demonstrated the same principle using non-polymeric techniques in which continuous and local delivery of PEX, PF4/CTF and endostatin using microinfusions was more efficient than systemic administration.57,58
Other approaches for the continuous release of angiogenic inhibitors were developed following the discovery of endogenous angiogenesis inhibitors. One such approach involves cell-encapsulation, in which genetically manipulated cells producing angiogenic inhibitors are used. In this technique, cells are encapsulated inside a hydrogel capsule, such as alginate, thereby enabling the sustained release of cellular protein while simultaneously protecting the cells from rejection by the immune system. Endogenous inhibitors such as interferon-□, angiostatin and endostatin were successfully formulated for use in brain tumor models.59–62
Sengupta et al.63 developed a multi-drug delivery system composed of a copolymer PLGA and Phosphatidylcholine:cholesterol (PEG-DSPE) named nanocell, which enables the release of two drugs at different kinetics. The outer PEG-DSPE shell first releases an antiangiogenic agent, combretastatin, which regresses blood vessel growth, while the inner core of the nanoparticle composed of a polymer PLGA-doxorubicin, is trapped inside the tumor and slowly releases the chemotherapy drug.63 This antiangiogenic and anticancer activity of the nanocell was demonstrated on both Lewis lung carcinoma and melanoma tumors, with potential for efficacy in brain tumors as well.
Other polymeric systems are also being developed, including gene therapy64,65 and targeted delivery using angiogenesis elements such as the Arg-Gly-Asp (RGD) peptide. The latter approach is currently being studied for the therapy and imaging of brain tumors.66,67 Table 1 lists polymeric antiangiogenic drugs designed or tested for treating brain tumors.
Table 1.
Examples of polymeric systems designed for brain delivery of antiangiogenesis compounds
| Technique of delivery | Antiangiogenic factor | Polymer | Reference |
| Cell encapsulation | Endostatin, Interferon-□, Angiostatin | Alginate/PLL | 59–62 |
| Non-viral gene therapy | interleukin-12 | PPC | 64, 65 |
| Biodegradable matrices/Nano/micro particles | PEX, PF4/CTF, heparin minocycline, | PLGA, pCPP:SA, PEG | 54–56 |
| Polymer conjugations/ | interferon alpha, | HPMA, | 82, 83, 87 |
| Polymeric micelles | TNP-470 | mPEG-PLA |
This list is partial and includes only direct antiangiogenic factors studied in brain tumors. PLL, Poly L-lysine; PLGA, Poly-(lactic-co-glycolic)acid; pCPP:SA, poly[bis(p-carboxyphenoxy)propane-sebacic acid]; PPC, polyethylenimine—methoxypolyethyleneglycol and cholesterol; PEG, Polyethylene glycol.
Nanoparticles and Polymer Conjugations Eliminate Neurotoxicity
Another consideration with regard to chemotherapies and antiangiogenic drugs targeted to the brain is the possibility of adverse neurological effects. Nanotechnology and polymer chemistry now offer new solutions to these clinical limitations, as well. One such example is the modification of the antiangiogenic drug TNP-470. A small molecule drug, TNP-470 is an analogue of fumagillin which was isolated from a fungus68 and is among the most potent and broad spectrum inhibitors of angiogenesis.69 In mice, TNP-470 inhibited growth and reduced the vascularization of various murine tumors and human xenografts including brain tumors.70–73 In 1992, TNP-470 became one of the first antiangiogenic drugs to undergo clinical trials74 and showed encouraging results. It slowed tumor progression, stabilized the disease, and in some cases led to a durable complete regression75–77 when given as a single agent or in combination with other conventional chemotherapeutic drugs such as paclitaxel and carboplatin.75–81 However, the dose-limiting toxicity presented as neural side-effects including dizziness, decreased concentration, short-term memory loss, confusion, depression, weakness and diplopia.77 The neurological symptoms were reversible and dose dependent. Since the plasma half-life of TNP-470 was found to be very short, a prolonged intravenous infusion schedule was suggested.77
Given that the neurological symptoms were caused by a small hydrophobic molecule drug, the strategy to eliminate these symptoms involved enlarging the molecule by attaching it to a water soluble polymer of high molecular weight, thus preventing penetration through the BBB. The first formulation aimed at solving the neurotoxicity problem of TNP-470 was Caplostatin—a 30 kDa conjugate of TNP-470 to Methacrylate-2-hydroxy-propyl (HPMA), a water soluble polymer.82 This polymeric drug proved to have antiangiogenic and anti-cancer effects in mice with no related neurotoxicity. The half-life of Caplostatin was significantly higher than that of the original drug.82 Recently, an oral formulation of TNP-470 named Lodamin™ was developed.83 In this formulation, TNP-470 was conjugated to the di-block co-polymer: monomethoxy Poly (Ethylen glycol)-Poly (lactic acid) (mPEG-PLA). The conjugate mPEG-PLA-TNP-470 forms a multi-chain 3-dimentional micelle-like structure. The size of the nanomicelles is 10–100 nm when introduced to an aqueous solution. The advantage of this structure is that it has a stable solid core which contains the drug, thereby protecting it in the stomach. Moreover, the micelles are small enough to be absorbed in the intestine, but are simultaneously large enough to avoid penetration into the brain. This size modification may be the reason for the elimination of neurotoxocity symptoms as was evaluated in a coordination test in mice.83 Lodamin's anti-cancer activity was demonstrated on primary tumors in mice and was found to be particularly effective in preventing liver metastases due to uptake in the hepatic-portal circulation.83
The promise of an oral, non-toxic, antiangiogenic drug is of great significance. Such formulations can potentially be used in the chronic, long-term treatment of existing tumors and might also present a reasonable regimen for the prevention of tumor recurrence.
Challenges in Developing a Clinically Relevant Antiangiogenesis Delivery System
Clinical translation of antiangiogenic polymeric drug delivery has been hindered by a number of obstacles. Some difficulties are based on the nature of antiangiogenic therapy, namely that efficacy is higher in the early stages of neovascularization. Therapy is thus heavily dependent upon the stage of the tumor, such that the visible effect of antiangiogenic treatment in a patient with advanced vascularized glioblastoma may be minimal. In cases of highly infiltrative malignancies in which tumor cells co-opt and grow on existing blood vessels, antiangiogenic drugs would not necessarily affect this established vasculature. Still other difficulties relate to the polymeric delivery system rather than the drug. For example, local polymer delivery may suffer from a poor drug diffusion rate in the brain tissue as a result of interstitial fluid pressure. Another problem may involve reduction of the EPR effect as a result of antiangiogenic therapy which reduces vessel permeability. As mentioned above, EPR is typically used for the passive targeting of nanoparticles to foci of tumor growth.
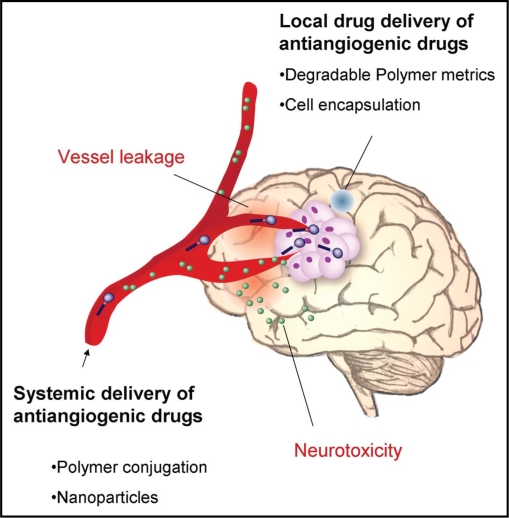
Furthermore, polymeric drugs require the development of robust systems with well defined characteristics such as drug release kinetics, stability and bioavailability. Mechanisms of action may be also somewhat different than in the related non-polymeric drug, given that the introduction of the drug to the target cells and the uptake may be different. Figure 2 summarizes some of the issues discussed above.
Figure 2.
Illustration of the brain and various issues related to drug delivery of antiangiogenic drugs. Brain tumors are characterized by robust angiogenesis. The unique cranial vessels form the blood brain barrier (BBB) limits the delivery of drugs into the brain. This is critical for chemotherapies, but less so for antiangiogenic drugs that can be effective without crossing the BBB. Polymeric drug delivery systems have been developed to improve drug properties (such as: solubility, half-life, stability, toxicity) and treat pathologies within the brain parenchyma. These delivery system can be either local (e.g., wafers, nano/micro particles, cell encapsulated capsules) or systemic (e.g., polymer conjugates, nanoparticles). The BBB is disrupted at tumor site causing high vessel leakage that leads to high interstitial fluid pressure. This results in poor accumulation of small molecule drugs, but enables passive targeting of nanoparticles at the tumor site (known as the EPR effect). Neurotoxicity represents another challenge for antiangiogenic and anticancer drugs, though this may be overcome by enlarging molecular size of the drug by polymer conjugation.
Conclusions, Challenges and Future Directions
Indeed, antiangiogenic therapy has become an important modality of tumor treatment, specifically in brain tumors.84–86 Many angiogenesis inhibitors, including those recently discovered, are limited when their clinical potential is considered. There is, therefore, an increased need for the novel approaches offered by nanotechnology and polymer chemistry. In the future, many antiangiogenic drugs may be reformulated or improved using carriers or polymer conjugation to yield more favorable clinical properties. This will be particularly important in the case of brain tumors, as the brain represents a unique environment with barriers that make drug delivery vastly more challenging. Changing drug solubility, availability in the brain, circulating time, release kinetics or route of administration are frequently as important as drug discovery itself. Although many new challenges await the translation of these drug delivery systems, often without addressing these issues even the most promising drugs cannot be translated to clinical use.
Acknowledgements
We thank Dr. Judah Folkman for overseeing and supporting our work on the development of Lodamin, Dr. Robert D'Amato for his guidance and support, Dr. Donald Ingber, Dr. Marcelle Machluf for sharing her expertise in the field, the Vascular Biology Program, Lauren Bazinet and Lior Braunstein for suggestions and proofreading.
Abbreviations
- FDA
food and drug administration
- CNS
central nervous system
- BBB
blood-brain barrier
- VEGF
vascular endothelial growth factor
- NIH
national institutes of health
- CED
convection-enhanced delivery
- PLGA
poly-(lactic-co-glycolic) acid
- EPR
enhanced permeability and retention effect
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/7766
References
- 1.Folkman J. Tumor angiogenesis: therapeutic implication. N angl J Med. 1971;4:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J, Hanahan D. Switch to the angiogenic phenotype during tumorigenesis. Princess Takamatsu Symp. 1991;22:339–347. [PubMed] [Google Scholar]
- 5.Louis DN, Pomeroy JG, Cairncross JG. Focus on central nervous system neoplasia. Cancer cell. 2002;1:125–128. doi: 10.1016/s1535-6108(02)00040-5. [DOI] [PubMed] [Google Scholar]
- 6.Kleihues P, Soylemezoglu F, Schäuble B, Scheithauer B, Burger P. histopathology, classification and grading of gliomas. Glia. 1995;15:211–221. doi: 10.1002/glia.440150303. [DOI] [PubMed] [Google Scholar]
- 7.Plate K, Risau W. Angiogenesis in malignant gliomas. Glia. 1995;15:339–347. doi: 10.1002/glia.440150313. [DOI] [PubMed] [Google Scholar]
- 8.Brem S, Cotran R, Folkman J. Tumor angiogenesis: a quantitative method for histologic grading. J Natl Cancer Inst. 1972;48:347–356. [PubMed] [Google Scholar]
- 9.Kaur B, Tan C, Brat DJ, Post DE, Van Meir EG. Genetic and hypoxic regulation of angiogenesis in gliomas. J Neurooncol. 2004;70:229–243. doi: 10.1007/s11060-004-2752-5. [DOI] [PubMed] [Google Scholar]
- 10.Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin HG. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer Res. 2000;60:1388–1393. [PubMed] [Google Scholar]
- 11.Jansen M, de Witt Hamer P, Witmer A, Troost D, van Noorden C. Current perspectives on antiangiogenesis strategies in the treatment of malignant gliomas. Brain Res Brain Res Rev. 2004;45:143–163. doi: 10.1016/j.brainresrev.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Senger D, Cairncross JG, Forsyth PA. Long-term survivors of glioblastoma: statistical aberration or important unrecognized molecular subtype? Cancer J. 2003;9:214–221. doi: 10.1097/00130404-200305000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Ramsauer M, Krause D, Dermietzel R. Angiogenesis of the blood-brain barrier in vitro and the function of cerebral pericytes. FASEB J. 2002;16:1274–1276. doi: 10.1096/fj.01-0814fje. [DOI] [PubMed] [Google Scholar]
- 14.Lo E, Singhal A, Torchilin V, Abbott N. Drug delivery to damaged brain. Brain Res Rev. 2001;38:140–148. doi: 10.1016/s0165-0173(01)00083-2. [DOI] [PubMed] [Google Scholar]
- 15.Deane R, Zlokovic B. Role of the blood-brain barrier in the pathogenesis of Alzheimer's disease. Curr Alzheimer Res. 2007;4:191–197. doi: 10.2174/156720507780362245. [DOI] [PubMed] [Google Scholar]
- 16.Rigau V, Morin M, Rousset M, de Bock F, Lebrun A, Coubes P, et al. Angiogenesis is associated with blood-brain barrier permeability in temporal lobe epilepsy. Brain. 2007;130:1942–1956. doi: 10.1093/brain/awm118. [DOI] [PubMed] [Google Scholar]
- 17.Chaudhry I, O'Donovan D, Brenchley P, Reid H, Rpberts I. Vascular endothelial growth factor expression correlates with tumour grade and vascularity in gliomas. Histopathology. 2001;39:409–415. doi: 10.1046/j.1365-2559.2001.01230.x. [DOI] [PubMed] [Google Scholar]
- 18.Ke L, Shi Y, Im S, Chen X, Yung Y. The relevance of cell proliferation, vascular endothelial growth factor and basic fibroblast growth factor production to angiogenesis and tumorigenicity in human glioma cell lines. Clin Cancer Res. 2000;6:2562–2572. [PubMed] [Google Scholar]
- 19.Zhang Z, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Argaw A, Zhang Y, Snyder B, Zhao M, Kopp N, Lee S, et al. IL-1beta regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J Immunol. 2006;177:5574–5584. doi: 10.4049/jimmunol.177.8.5574. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Y, Lee C, Shen F, Du R, Young W, Yang G. Angiopoietin-2 facilitates vascular endothelial growth factor-induced angiogenesis in the mature mouse brain. Stroke. 2005;36:1533–1537. doi: 10.1161/01.STR.0000170712.46106.2e. [DOI] [PubMed] [Google Scholar]
- 22.Valable S, Montaner J, Bellail A, Berezowski V, Brillault J, Cecchelli R, et al. VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: both effects decreased by Ang-1. J Cereb Blood Flow Metab. 2005;25:1491–1504. doi: 10.1038/sj.jcbfm.9600148. [DOI] [PubMed] [Google Scholar]
- 23.Boucher Y, Baxter L, Jain R. Interstitial pressure gradients in tissue-isolated and subcutaneous tumors: implications for therapy. Cancer Res. 1990;50:4478–4484. [PubMed] [Google Scholar]
- 24.Jain R. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 25.Greish K. Enhanced permeability and retention of macromolecular drugs in solid tumors: a royal gate for targeted anticancer nanomedicines. J Drug Target. 2007;15:457–464. doi: 10.1080/10611860701539584. [DOI] [PubMed] [Google Scholar]
- 26.Maeda H, Fang J, Inutsuka T, Kitamoto Y. Vascular permeability enhancement in solid tumor: various factors, mechanisms involved and its implications. Int Immunopharmacol. 2003;3:319–328. doi: 10.1016/S1567-5769(02)00271-0. [DOI] [PubMed] [Google Scholar]
- 27.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 28.Vicent M, Duncan R. Polymer conjugates: nanosized medicines for treating cancer. Trends Biotechnol. 2006;24:39–47. doi: 10.1016/j.tibtech.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Greco F, Vicent M. Polymer-drug conjugates: current status and future trends. Front Biosci. 2008;13:2744–2756. doi: 10.2741/2882. [DOI] [PubMed] [Google Scholar]
- 30.Jain R, di Tomaso E, Duda D, Loeffler J, Sorensen A, Batchelor T. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 31.Hobbs S, Monsky W, Yuan F, Roberts W, Griffith L, Torchilin V, et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci USA. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pardridge W. Molecular Trojan horses for blood-brain barrier drug delivery. Curr Opin Pharmacol. 2006;6:494–500. doi: 10.1016/j.coph.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Schinkel A, Wagenaar E, Mol C, van Deemter L. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest. 1996;97:2517–2524. doi: 10.1172/JCI118699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cordon-Cardo C, O'Brien J, Casals D, Rittman-Grauer L, Biedler J, Melamed M, et al. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci USA. 1989;86:695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohnishi T, Tamai I, Sakanaka K, Sakata A, Yamashima T, Yamashita J, et al. In vivo and in vitro evidence for ATP-dependency of P-glycoprotein-mediated efflux of doxorubicin at the blood-brain barrier. Biochem Pharmacol. 1995;49:1541–1544. doi: 10.1016/0006-2952(95)00082-b. [DOI] [PubMed] [Google Scholar]
- 36.Lesniak M, Upadhyay U, Goodwin R, Tyler B, Brem H. Local delivery of doxorubicin for the treatment of malignant brain tumors in rats. Anticancer Res. 2005;25:3825–3831. [PMC free article] [PubMed] [Google Scholar]
- 37.Neuwelt E, Pagel M, Barnett P, Glassberg M, Frenkel E. Pharmacology and toxicity of intracarotid doxorubicin administration following osmotic blood-brain barrier modification. Cancer Res. 1981;41:4466–4470. [PubMed] [Google Scholar]
- 38.Brem H, Mahaley MJ, Vick N, Black K, Schold SJ, Burger P, et al. Interstitial chemotherapy with drug polymer implants for the treatment of recurrent gliomas. J Neurosurg. 1991;74:441–446. doi: 10.3171/jns.1991.74.3.0441. [DOI] [PubMed] [Google Scholar]
- 39.Grossman S, Reinhard C, Colvin O, Chasin M, Brundrett R, Tamargo R, et al. The intracerebral distribution of BCNU delivered by surgically implanted biodegradable polymers. J Neurosurg. 1992;76:640–647. doi: 10.3171/jns.1992.76.4.0640. [DOI] [PubMed] [Google Scholar]
- 40.Raghavan R, Brady M, Rodríguez-Ponce M, Hartlep A, Pedain C, Sampson J. Convection-enhanced delivery of therapeutics for brain disease, and its optimization. Neurosurg Focus. 2006;20:12. doi: 10.3171/foc.2006.20.4.7. [DOI] [PubMed] [Google Scholar]
- 41.Koziara J, Lockman P, Allen D, Mumper R. Paclitaxel nanoparticles for the potential treatment of brain tumors. J Control Release. 2004;99:259–269. doi: 10.1016/j.jconrel.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Menei P, Boisdron-Celle M, Croué A, Guy G, Benoit J. Effect of stereotactic implantation of biodegradable 5-fluorouracil-loaded microspheres in healthy and C6 glioma-bearing rats. Neurosurgery. 1996;39:117–123. doi: 10.1097/00006123-199607000-00023. [DOI] [PubMed] [Google Scholar]
- 43.Yoo H, Lee K, Oh J, Park T. In vitro and in vivo anti-tumor activities of nanoparticles based on doxorubicin-PLGA conjugates. J Control Release. 2000;68:419–431. doi: 10.1016/s0168-3659(00)00280-7. [DOI] [PubMed] [Google Scholar]
- 44.Panyam J, Labhasetwar V. Sustained cytoplasmic delivery of drugs with intracellular receptors using biodegradable nanoparticles. Mol Pharm. 2004;1:77–84. doi: 10.1021/mp034002c. [DOI] [PubMed] [Google Scholar]
- 45.Gulyaev A, Gelperina S, Skidan I, Antropov A, Kivman G JK. Significant transport of doxorubicin into the brain with polysorbate 80-coated nanoparticles. Pharm Res. 1999;16:156. doi: 10.1023/a:1018983904537. [DOI] [PubMed] [Google Scholar]
- 46.Liu L, Guo K, Lu J, Venkatraman S, Luo D, Ng K, et al. Biologically active core/shell nanoparticles self-assembled from cholesterol-terminated PEGeTAT for drug delivery across the bloodebrain barrier. Biomaterials. 2008;29:1509. doi: 10.1016/j.biomaterials.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 47.Sethuraman V, Bae Y. TAT peptide-based micelle system for potential active targeting of anti-cancer agents to acidic solid tumors. J Control Release. 2007;118:216–224. doi: 10.1016/j.jconrel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gummerloch M, Neuwalt E. Drug entry into the brain and its pharmacological manipulationeditor. In: MWB B, editor. Physiology and pharmacology of the bloodebrain barrier Handbook of experimental pharmacology. Berlin: Springer; 1992. p. 525. [Google Scholar]
- 49.Rapoport S. Osmotic Opening of the Blood-Brain Barrier: Principles, Mechanism and Therapeutic Applications. Cell Mol Neurobiol. 2000;20:217–230. doi: 10.1023/A:1007049806660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Folkman J. Angiogenesis inhibitors: a new class of drugs. Cancer Biol Ther. 2003;2:127–133. [PubMed] [Google Scholar]
- 51.Folkman J. Endogenous angiogenesis inhibitors. APMIS. 2004;112:496–507. doi: 10.1111/j.1600-0463.2004.apm11207-0809.x. [DOI] [PubMed] [Google Scholar]
- 52.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 53.Weingart J, Sipos E, Brem H. The role of minocycline in the treatment of intracranial 9L glioma. J Neurosurg. 1995;82:635–640. doi: 10.3171/jns.1995.82.4.0635. [DOI] [PubMed] [Google Scholar]
- 54.Tamargo R, Leong K, Brem H. Growth inhibition of the 9L glioma using polymers to release heparin and cortisone acetate. J Neurooncol. 1990;9:131–138. doi: 10.1007/BF02427833. [DOI] [PubMed] [Google Scholar]
- 55.Benny O, Duvshani-Eshet M, Cargioli T, Bello L, Bikfalvi A, Carroll R, et al. Continuous delivery of endogenous inhibitors from poly(lactic-co-glicolic acid) polymeric microspheres inhibits glioma tumor growth. Clin Cancer Res. 2005;11:768–776. [PubMed] [Google Scholar]
- 56.Benny O, Kim S, Gvili K, Radzishevsky I, Mor A, Verduzco L, et al. In vivo fate and therapeutic efficacy of PF-4/CTF microspheres in an orthotopic human glioblastoma model. FASEB J. 2007 doi: 10.1096/fj.07-8801com. [DOI] [PubMed] [Google Scholar]
- 57.Giussani C, Carrabba G, Pluderi M, Lucini V, Pannacci M, Caronzolo D, et al. Local Intracerebral delivery of endogenous inhibitors by osmotic minipumps effectively suppresses glioma growth in vivo. Cancer Res. 2003;63:2499–2505. [PubMed] [Google Scholar]
- 58.Schmidt N, Ziu M, Carrabba G, Giussani C, Bello L, Sun Y, et al. Antiangiogenic therapy by local intracerebral microinfusion improves treatment efficiency and servival in an orthotopic human glioblastoma model. Clin Cancer Res. 2004;10:1255–1262. doi: 10.1158/1078-0432.ccr-03-0052. [DOI] [PubMed] [Google Scholar]
- 59.Joki T, Machluf M, Atala A, Zhu J, Seyfried N, Dunn I, et al. Continuous release of endostatin from microencapsulated engineered cells for tumor therapy. Natu Biotechnology. 2001;19:35–39. doi: 10.1038/83481. [DOI] [PubMed] [Google Scholar]
- 60.Read T, Sorensen D, Mahesparan R, Enger P, Timpl R, Olsen B, et al. Local endostatin treatment of gliomas administered by microencapsulated producer cells. Nat Biotechnol. 2001;19:29–34. doi: 10.1038/83471. [DOI] [PubMed] [Google Scholar]
- 61.Cirone P, Bourgeois J, Chang P. Antiangiogenic cancer therapy with microencapsulated cells. Hum Gene Ther. 2003;14:1065–1077. doi: 10.1089/104303403322124783. [DOI] [PubMed] [Google Scholar]
- 62.De Boüard S, Guillamo J, Christov C, Lefévre N, Brugières P, Gola E, et al. Antiangiogenic therapy against experimental glioblastoma using genetically engineered cells producing interferon-□, angiostatin or endostatin. Hum Gene Ther. 2003;14:883–895. doi: 10.1089/104303403765701178. [DOI] [PubMed] [Google Scholar]
- 63.Sengupta S, Eavarone D, Capila I, Zhao G, Watson N, Kiziltepe T, et al. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature. 2005;436:568–572. doi: 10.1038/nature03794. [DOI] [PubMed] [Google Scholar]
- 64.Sonabend A, Velicu S, Ulasov I, Han Y, Tyler B, Brem H, et al. A safety and efficacy study of local delivery of interleukin-12 transgene by PPC polymer in a model of experimental glioma. Anticancer Drugs. 2008;19:133–142. doi: 10.1097/CAD.0b013e3282f24017. [DOI] [PubMed] [Google Scholar]
- 65.Reynolds A, Moein Moghimi S, Hodivala-Dilke K. Nanoparticle-mediated gene delivery to tumour neovasculature. Trends Mol Med. 2003;9:2–4. doi: 10.1016/s1471-4914(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 66.Chen X, Park R, Shahinian A, Tohme M, Khankaldyyan V, Bozorgzadeh M, et al. 18F-labeled RGD peptide: initial evaluation for imaging brain tumor angiogenesis. Nucl Med Biol. 2004;31:179–189. doi: 10.1016/j.nucmedbio.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 67.Park J, Kwon S, Nam J, Park R, Chung H, Seo S, et al. Self-assembled nanoparticles based on glycol chitosan bearing 5beta-cholanic acid for RGD peptide delivery. J Control Release. 2004;95:579–588. doi: 10.1016/j.jconrel.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 68.Ingber D, Fugita T, Kishimoto S, Sudo K, Kanamaru T, Brem H, et al. Synthetic analogue of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature. 1990;348:555–557. doi: 10.1038/348555a0. [DOI] [PubMed] [Google Scholar]
- 69.Folkman J, Kalluri R. Tumor angiogenesis. In: Kufe D, Pollock RE, Weischselbaum RR, Bast RC, Gansler TS, Holland JF, Frei E, editors. Cancer Medicine. Hamilton, Ontario: BC Decker Inc; 2003. pp. 161–194. [Google Scholar]
- 70.Shusterman S, Grupp SA, Barr R, Carpentieri D, Zhao H, Maris JM. The angiogenesis inhibitor tnp-470 effectively inhibits human neuroblastoma xenograft growth, especially in the setting of subclinical disease. Clin Cancer Res. 2001;7:977–984. [PubMed] [Google Scholar]
- 71.Takamiya Y, Brem H, Ojeifo J, Mineta T, Martuza R. AGM-1470 inhibits the growth of human glioblastoma cells in vitro and in vivo. Neurosurgery. 1994;34:869–875. doi: 10.1227/00006123-199405000-00013. [DOI] [PubMed] [Google Scholar]
- 72.Takamiya Y, Friedlander RM, Brem H, Malick A, Martuza RL. Inhibition of angiogenesis and growth of human nerve-sheath tumors by AGM-1470. J Neurosurg. 1993;78:470–476. doi: 10.3171/jns.1993.78.3.0470. [DOI] [PubMed] [Google Scholar]
- 73.Wilson J, Penar P. The effect of AGM-1470 in an improved intracranial 9L gliosarcoma rat model. Neurol Res. 1994;16:121–124. doi: 10.1080/01616412.1994.11740208. [DOI] [PubMed] [Google Scholar]
- 74.Kruger E, Figg WD. TNP-470: an angiogenesis inhibitor in clinical development for cancer. Expert Opin Investig Drugs. 2000;9:1383–1396. doi: 10.1517/13543784.9.6.1383. [DOI] [PubMed] [Google Scholar]
- 75.Kudelka A, Verschraegen C, Loyer E. Complete remission of metastatic cervical cancer with the angiogenesis inhibitor TNP-470. N Engl J Med. 1998;338:991–992. doi: 10.1056/NEJM199804023381412. [DOI] [PubMed] [Google Scholar]
- 76.Kudelka A, Levy T, Verschraegen C, Edwards C, Piamsomboon S, Termrungruanglert W, et al. A phase I study of TNP-470 administered to patients with advanced squamous cell cancer of the cervix. Clin Cancer Res. 1997;3:1501–1505. [PubMed] [Google Scholar]
- 77.Bhargava P, Marshall J, Rizvi N, Dahut W, Yoe J, Figuera M, et al. A phase I and pharmacokinetic study of TNP-470 administered weekly to patients with advanced cancer. Clin Cancer Res. 1999;5:1989–1995. [PubMed] [Google Scholar]
- 78.Tran H, Blumenschein GJ, Lu C, Meyers C, Papadimitrakopoulou V, Fossella F, et al. Clinical and pharmacokinetic study of TNP-470, an angiogenesis inhibitor, in combination with paclitaxel and carboplatin in patients with solid tumors. Cancer Chemother Pharmacol. 2004;54:308–314. doi: 10.1007/s00280-004-0816-z. [DOI] [PubMed] [Google Scholar]
- 79.Herbst R, Madden T, Tran H, Blumenschein GJ, Meyers C, Seabrooke L, et al. Safety and pharmacokinetic effects of TNP-470, an angiogenesis inhibitor, combined with paclitaxel in patients with solid tumors: evidence for activity in non-small-cell lung cancer. J Clin Oncol. 2002;20:4440–4447. doi: 10.1200/JCO.2002.04.006. [DOI] [PubMed] [Google Scholar]
- 80.Stadler W, Kuzel T, Shapiro C, Sosman J, Clark J, Vogelzang NJ. Multi-institutional study of the angiogenesis inhibitor TNP-470 in metastatic renal carcinoma. J Clin Oncol. 1999;17:2541–2545. doi: 10.1200/JCO.1999.17.8.2541. [DOI] [PubMed] [Google Scholar]
- 81.Logothetis C, Wu K, Finn L, Daliani D, Figg W, Ghaddar H, et al. Phase I trial of the angiogenesis inhibitor TNP-470 for progressive androgen-independent prostate cancer. Clin Cancer Res. 2001;7:1198–1203. [PubMed] [Google Scholar]
- 82.Satchi-Fainaro R, Puder M, Davies J, Tran H, Sampson D, Greene A, et al. Targeting angiogenesis with a conjugate of HPMA copolymer and TNP-470. Nat Med. 2004;10:255–261. doi: 10.1038/nm1002. [DOI] [PubMed] [Google Scholar]
- 83.Benny O, Fainaru O, Adini A, Cassiola F, Bazinet L, Adini I, et al. An orally delivered small-molecule formulation with antiangiogenic and anticancer activity. Nat Biotechnol. 2008;26:799–807. doi: 10.1038/nbt1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wong E, Brem S. Antiangiogenesis treatment for glioblastoma multiforme: challenges and opportunities. J Natl Compr Canc Netw. 2008;6:515–522. doi: 10.6004/jnccn.2008.0039. [DOI] [PubMed] [Google Scholar]
- 85.Kieran M. Anti-angiogenic chemotherapy in central nervous system tumors. Cancer Treat Res. 2004;117:337–349. doi: 10.1007/978-1-4419-8871-3_19. [DOI] [PubMed] [Google Scholar]
- 86.Dietrich J, Norden A, Wen P. Emerging antiangiogenic treatments for gliomas—efficacy and safety issues. Curr Opin Neurol. 2008;21:736–744. doi: 10.1097/WCO.0b013e3283131370. [DOI] [PubMed] [Google Scholar]