Abstract
Brain tumors exhibit marked and aberrant blood vessel formation indicating angiogenic endothelial cells as a potential target for brain tumor treatment. The brain tumor blood vessels are used for nutrient delivery, and possibly for cancer cell migration. The process of angiogenesis is complex and involves multiple players. The current angiogenesis inhibitors used in clinical trials mostly target single angiogenic proteins and so far show limited effects on tumor growth. Besides the conventional angiogenesis inhibitors, RNA-based inhibitors such as small-interfering RNAs (siRNAs) are being analyzed for their capacity to silence the message of proteins involved in neovascularization. More recently, a new family of non-coding RNAs, named angiomirs [microRNAs (miRNAs) involved in angiogenesis] has emerged. These small RNAs have the advantage over siRNAs in that they have the potential of silencing multiple messages at the same time and therefore they might become therapeutically relevant in a “one-hit multiple-target” context against brain tumor angiogenesis. In this review we will discuss the emerging technologies in anti-angiogenesis emphasizing on RNA-based therapeutics.
Key words: glioma, angiogenesis, anti-angiogenesis therapy, siRNA, miRNA, endothelial cells, blood vessels
Brain Tumor Angiogenesis
Malignant gliomas are characterized by a marked increase in blood vessel formation (angiogenesis), which is crucial for tumor growth and colonization in the brain.1 Glioma blood vessels show endothelial cell proliferation which is a key feature of high grade gliomas in the WHO grading system.2,3 Increasing evidence supporting the critical role of angiogenesis in the biological behavior of these tumors, and patients prognosis led to a variety of studies on basic mechanisms of tumor angiogenesis.4 Several factors are involved in this process which results in the recruitment, proliferation and alignment of the endothelial blood vessel cells through a complex interaction between these cells and tumor cells.1 Glioma cells are known to alter the mRNA profile of endothelial cells as shown by microarray analysis in co-cultures of human umbilical vein endothelial cells and glioma cells.5 Further, differences in levels of specific proteins have been observed between normal and tumor endothelial cells, which contribute in part to the distinct angiogenic blood vessel morphology in tumors.6,7
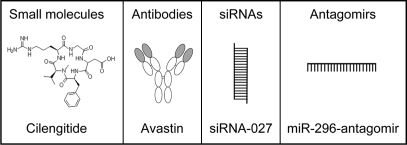
Glioma cells clearly need the vasculature for the delivery of nutrients and oxygen for tumor growth. In addition, many reports demonstrated that high grade glioma cells may also use the vasculature for migration to different parts of the brain.8,9 High grade gliomas show two types of infiltration into the normal brain. The first is a diffuse infiltrative migration of single cells into the brain parenchyma. The second type is a clear perivascular migration along the microvasculature. Brain tumor infiltration has been shown to be one of the main reasons for tumor recurrence.8,9 These infiltrated cells cannot be resected by surgery and are known to be resistant to current chemo- and radiotherapies.10,11 Increasing awareness of the importance of the tumor vasculature for glioma growth and migration has led the effort to focus on developing novel anti-angiogenic drugs which are summarized in Figure 1A.12–16 Two major pathways are undertaken for this type of therapy: (1) cutting the oxygen and nutrients supplies; and (2) limiting possible migration routes for infiltrating glioma cells. Brain tumor angiogenesis is regulated through a complex network of molecules which will be partly discussed here, offering several potential points of intervention.
Figure 1.
Different classes of angiogenesis inhibitors.
Conventional Angiogenesis Inhibitors
Glioma cells secrete many pro-angiogenic factors.13,17 Interestingly, these cells also secrete substances that could inhibit angiogenesis.17,18 Following resection and radiation combined with temozolomide treatment, the median time for glioma recurrence or progression is six months.11,19 The area of the recurrent tumor, defined as progression of the contrast enhanced lesion on a magnetic resonance imaging (MRI) scan, is in the majority of cases localized at the resection cavity of the primary tumor, where infiltrating tumor cells with a low proliferation rate reside. The phenomenon of fast relapse may be explained by the hypothesis that a large primary tumor secretes not only stimulators of its own angiogenesis, but also produces angiogenesis inhibitors that inhibit neovascularization in more distal parts—and thus inhibit further growth—of the infiltrated tumor cells.9,18,20 The use of angiogenesis inhibitors could therefore have a beneficial effect on glioma recurrence.
A number of endogenous inhibitors of angiogenesis have been discovered. The most commonly studied is angiostatin, a polypeptide of approximately 200 amino acids.21 Angiostatin is produced by the cleavage of plasminogen, a plasma protein that is important for dissolving blood clots. Another commonly used anti-angiogenic inhibitor is endostatin, a polypeptide of 184 amino acids.21 Endostatin is composed of the globular domain found at the C-terminal of Type XVIII collagen (a collagen found in blood vessels), which is cut off from the parent molecule. Understanding the function of collagen and collagen receptors can help in revealing the role of the extracellular matrix in glioma angiogenesis. Glioma cells express integrins, which are collagen receptors, by which they anchor the extracellular matrix to the outside of the cell membrane.22,23 It has been shown that the new blood vessels in tumors express a vascular integrin, designated alphavbeta3, which is not found on blood vessels of normal tissues.23 Endostatin significantly inhibited angiogenesis and tumor growth in several orthotopic human brain tumor models, including the U87 mouse model and the BT4C rat model.24,25 Unfortunately, clinical trials with endostatin showed that, although safe to use, it was not effective in 40 patients when given as a single agent in the treatment of advanced neuroendocrine tumors.26,27 Monoclonal antibodies and small molecule drugs directed against integrins have shown promising results in mice and in clinical trials in humans.22,28,29 One of the promising integrin inhibitors is cilengitide, a potent αvβ3 and αvβ5 integrin inhibitor.22 Preclinical studies and clinical trials evaluating cilengitide showed that the integrin inhibitor is active and synergizes with radiotherapy in preclinical glioblastoma (GBM) models. In clinical trials for recurrent GBM, single-agent cilengitide showed antitumor benefits and minimal toxicity. Among newly diagnosed patients with GBM, cilengitide combined with standard radiotherapy and temozolomide showed promising therapeutic response with no increased toxicity. These results lead to a planned randomized Phase III trial.22 Further testing is required in order to analyze the final effectiveness of these newly developed antibodies and small molecules.
Cancer cells are also known to produce excess quantities of growth factors.1,13,17 These factors have autocrine stimulating capacities to induce tumor growth and endothelial neovascularization. Angiogenic growth factors acting on specific receptors are currently being studied extensively for anti-cancer drug development.16,30 Anti-angiogenesis drugs targeting growth factors like vascular endothelial growth factor (VEGF), or growth factor receptors like VEGFR, epidermal growth factor receptor (EGFR) and platelet-derived growth factor receptor (PDGFR), are being tested in clinical trials to determine their effect in reducing tumor blood vessel formation, or to normalize the aberrant glioma blood vessel system, either alone or in combination with standard or experimental therapeutics. Recently, patients with newly diagnosed WHO grade 3 malignant glioma were treated in a Phase II clinical trial with bevacizumab, a monoclonal antibody to VEGF, in combination with irinotecan.31 Evaluation with physical examination and MRI showed that twenty patients (61%) had at least partial response with limited adverse events. This study concludes that the combination of bevacizumab and irinotecan is an active regimen with acceptable toxicity for patients with recurrent WHO grade 3 malignant gliomas and that anti-VEGF treatment is therapeutically relevant. Although the treatment was shown to be sub-optimal, further studies showing long term effects are warranted.
RNA-Based Angiogenesis Inhibitors
Besides small molecules and antibodies, an additional class of RNA-based inhibitors is arising. Currently, RNA interference (RNAi) is widely used as a mechanism to silence individual genes.32,33 There are marketed small-interfering RNA (siRNA) products being analyzed by ophthalmologists to prevent age-related macular degeneration, caused by aberrant neovascularization in the eye, and resulting in completely blinding of some of the older patients.34,35 Further, several groups are focusing on using siRNAs to knock-down specific genes involved in glioma angiogenesis. A recent study by Gondi et al.36,37 showed that intraperitoneal injection of plasmids encoding short hairpin RNAs (shRNAs) directed against urokinase-type plasminogen activator (uPA) and its receptor (uPAR), as well as proteinase matrix metalloproteinase-9 (MMP-9) resulted in significant reduction of glioma angiogenesis and tumor growth in preclinical mouse models. These results also suggest that the intraperitoneal route of administration can be used for successful delivery of RNA-based drugs. Kargiotis et al.38 showed that the delivery of siRNA against MMP-2 using adenovirus vectors resulted in impaired invasion, as well tumor-induced angiogenesis leading to inhibition of tumor growth. Niola et al.39 showed that siRNAs against VEGF reduced GBM angiogenesis in xenograft mouse model. Clinical trials are also ongoing in which siRNAs are being delivered into target cells in order to prevent neovascularization. For example, siRNAs designed to target and to abrogate the expression of VEGF or its receptor are currently being tested. Clinical trials were initiated to analyze intraocular injection of siRNAs in patients with choroidal neovascularization (CNV), a late stage of age-related macular degeneration. Preclinical CNV mouse models were used to show that injections with naked VEGF siRNA (Bevasiranib) or VEGFR1 siRNA (AGN211745; siRNA-027) suppressed laser-injury-induced CNV.40–43
Intriguingly, it was recently found that siRNAs in general can cause toll-like receptor 3 (TLR3) activation, irrespective of their sequence.44 TLR3 is a member of the toll-like receptor family of pattern recognition receptors of the innate immune system and recognizes double-stranded RNA, resulting in activation of the transcription factor nuclear factor-kappaB (NF.B) thereby inducing interferons as an immune defense mechanism.45 Kleinman et al.44 showed that endothelial TLR3 activation by siRNAs had a direct effect on neovascularization. This work reveals an unexpected aspect of RNAi for angiogenesis which demonstrates that any 21-nucleotide or longer double-strand RNA could affect angiogenesis and that the two siRNAs tested in clinical trials could owe their anti-angiogenic effect not to target knock-down only, but also because of TLR3 activation.44,46 Since many different proteins are involved in angiogenesis, it is unlikely that single-target strategies are sufficient to treat complex processes such as brain tumor angiogenesis inhibition. Although the activation of TLR3 by siRNAs can have anti-angiogenic effect, it probably will need complementation from other anti-angiogenic molecules in order to inhibit brain tumor angiogenesis. One possibility may be to use a cocktail of multiple siRNAs to silence multiple genes, besides activating TLR3. A recent study in a mouse model for collagen-induced arthritis showed that siRNA directed against different targets can act synergistically. Khoury et al.47 showed that weekly injections of siRNAs against either interleukin 1 (IL-1), IL-6 or IL-18 reduced the collagen-induced arthritis, however, the most striking therapeutic effect was observed when a combination of all three siRNAs were delivered. The disadvantage of such a complex multi-component silencing cocktail is that it might result in numerous off-target effects (the unexpected silencing of ‘good’ genes) and therefore seems currently not yet practically feasible.48
The discovery of microRNAs (miRNAs) and their role in the complex control of gene expression may offer new strategies to change gene expression profiles. miRNAs are part of a highly conserved group of small non-coding RNAs that can block mRNA translation.49–51 One important principle of miRNA function is that the complementarity between their nucleotides and their targets is not complete and therefore a single miRNA can regulate multiple protein species by interacting with up to hundreds of different target mRNAs.52,53 miRNAs are usually about 22 nucleotides long, which is relatively short for a genetic transcript. The human genome is estimated to contain thousands of them. miRNAs can efficiently base pair with their protein-coding mRNA targets in order to block their expression. This biological mechanism is described as a “one-hit multiple target” mechanism, which enables a single miRNA to control whole sets of protein-coding genes.52,53 All the accumulating evidence indicates that regulation of miRNA expression is very important for proper development and differentiation of many cell types and tissues and that deregulated miRNA expression is a common feature of angiogenesis.52–61
Angiogenesis modulation in a “one-hit multiple target” fashion may be a more attractive strategy then single-target anti-angiogenesis therapy. The use of our own miRNAs to regulate many genes at the same time may have several advantages, mainly based on the use of miRNAs to regulate genes in a more natural phenomenon shaped by evolution. Several methods have been developed to alter single miRNA expression levels, thereby changing the expression pattern of multiple genes. The action of overexpressed intra-cellular miRNAs can be inhibited by the delivery of anti-sense single-strand RNA-based oligonucleotides. Inhibition of specific endogenous miRNAs has been achieved by the administration of synthetic anti-sense oligonucleotides that are complementary to the mature endogenous miRNAs.62–68 Improved miRNA inhibitors are now available containing various chemical modifications. These modifications were shown to have different effects on specificity and efficacy.69 In addition, efficient locked nucleic acid (LNA) anti-sense oligonucleotides have been designed and tested for their silencing capacity in vitro and vivo.70–73 More recently, antagomirs, miRNA inhibitors conjugated to cholesterol groups, have been developed. Antagomirs have been described to efficiently inhibit miRNA activity in various organs as well as in tumor vasculature, when injected into mice, and may have therapeutic potential.74–76
Various laboratories are currently working on improving the targeting of miRNA modulators into the cells of interest. Since tumors take up small molecules more efficiently than normal cells, they form a logical target for the delivery of miRNA mimics and miRNA inhibitors. Nevertheless, the delivery of inhibitors of these small RNAs to cancer cells in order to block tumor growth remains challenging, more likely due to the heterogeneity of cancer cells. On the other hand, tumor blood vessels offer an attractive target for the delivery of miRNA inhibitors or precursor/mimics in order to reduce tumor growth since these cells are not transformed and consequently less heterogeneous.
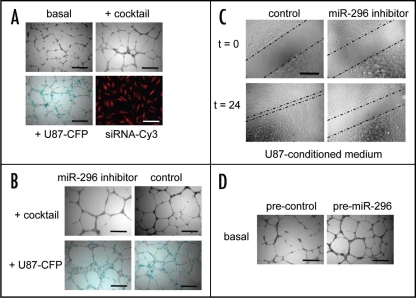
Recently, a new group of miRNAs related to angiogenesis was identified and named angiomirs.76,77 These miRNAs were shown to play a significant role in neovascularization. Specifically, miR-296 controls the overexpression of growth factor receptors on tumor endothelial cells. Our group showed that inhibition of miR-296 resulted in reduced tumor angiogenesis in vitro and in vivo.76 We showed that glioma cells and angiogenic growth factors (including VEGF), induce miR-296 in primary human brain microvascular endothelial cells (HBMVECs) in culture, as well as in primary tumor endothelial cells isolated from human brain tumors. Exposing endothelial cells to glioma cells or a cocktail composed of angiogenic growth factors resulted in increased tubule formation in an in vitro matrigel angiogenesis assay (Fig. 2A). miR-296 knockdown resulted in inhibition of both glioma-induced tubule formation as well as endothelial cell migration (Fig. 2B and C). On the other hand, overexpression of miR-296 using pre-miR-296 molecules resulted in an increased tubule formation in a similar manner to endothelial cells exposed to glioma cells or growth factors (Fig. 2D). Further, inhibition of miR-296 inhibited tumor vascularization in mice.76 Angiomirs, such as miR-296, can regulate a broad range of ‘natural’ mRNA targets and therefore they might prove more efficient for fine-tuning cellular switch programs than siRNAs.78 Wang et al.,61 Fish et al.56 and Kuhnert et al.58 showed that miR-126 plays an important role in developmental angiogenesis and vascular integrity. Knock-out experiments in zebrafish and mice demonstrated aberrant vasculature attributed to the absence of miR-126 molecules. Poliseno et al.59 showed that miR-221 and miR-222 block endothelial cell migration, proliferation and angiogenesis in vitro by targeting the stem cell factor receptor c-Kit and indirectly regulating expression of endothelial nitric oxide synthase. Further, Kuehbacher et al.57 showed that inhibition of microRNAs let-7f and miR-27b also inhibited angiogenesis.
Figure 2.
miR-296-mediated induction and inhibition of angiogenesis. (A) HBMVEC cells were cultured on Matrigel-coated plates in basal medium (EBM; Cambrex) only, basal medium supplemented with a cocktail of angiogenic factors (EGM), or with U87-human glioma cells expressing the cyan fluorescent protein (CFP) in order to induce miR-296 and tubule formation. Transfection efficiency of endothelial cells in monolayer culture was determined (>99%) by using siRNA-Cy3 molecules. (B) HBMVECs were transfected with miR-296 inhibitor or non-related control molecules and analyzed for tubule formation. At 48 hr after inhibition of U87 or cocktail-induced miR-296, less chaotic tubule networks were observed. (C) HBMVECs were transfected with anti-miR-296 inhibitor or non-related control molecules and analyzed for migration capacity. Inhibition of miR-296 resulted in a significant decrease in migration. (D) Overexpression of miR-296 at 24 hr after transfection of premiR-296 molecules resulted in increased angiogenesis in vitro. Size bars, 300 µm (Adapted from Würdinger et al. Cancer Cell 2008).76
The advantage of manipulating angiomir levels by single strand RNA derivatives could be that it may avoid the activation of TLR3 as was described to occur when using double-strand siRNAs thereby maintaining their specific inhibitory function and possibly avoiding possible TLR3-mediated interferon responses.44,45 Since many potential miRNA targets are known to be protein-coding mRNAs implicated in the disease state, the use of natural or synthetic miRNA regulators to correct defects in mRNA expression profiles may become clinically important. The “one-hit multiple target” property of angiomir modulators may be exploited to “normalize” or “correct” deregulated angiogenic expression profiles and to develop novel drugs to control complex processes such as brain tumor angiogenesis.
Conclusions
Anti-angiogenesis therapy for the treatment of brain cancer has so far produced disappointing results. Combinations of conventional angiogenesis inhibitors along with standard chemo-and radiotherapy are currently being tested. However, it is apparent that optimization and re-evaluation is needed since these angiogenesis inhibitors are not blocking neovascularization in patients sufficiently in order to produce a therapeutic response. Recent reports suggest that normalization of the blood vessel system can be beneficial in order to increase the therapeutic window for chemotherapeutic drug delivery.13 The development of novel angiogenesis inhibitors as well as the identification of angiogenesis targets are of importance and need to be validated further in clinical settings. The use of siRNAs and miRNA inhibitors to silence angiogenic factors should be re-considered especially in the context of TLR3 activation.44,46 The therapeutic efficacy of miRNAs remains to be determined and requires the development of efficient targeting methods to specific cells. Currently, studies are focusing on elucidating tumor-induced changes in miRNA expression in endothelial blood vessel cells. Our group found that 5% of the human miRNAs are deregulated upon exposure of endothelial cells to glioma cells.76 These results support the hypothesis that glioma cells stimulate endothelial blood vessel formation at least in part by altering the expression levels of specific endothelial miRNAs. The discovery of these small non-coding RNAs has increased our knowledge regarding the complex control of gene expression involved in regulating tumor growth and is an attractive strategy for anti-angiogenesis therapy, especially in view of a “one-hit multiple target” approach.
Acknowledgements
We would like to acknowledge the Steve Kaplan Fellowship from the American Brain Tumor Association and we would like to thank Dr. David P. Noske for critical reading of the manuscript, Mrs. Suzanne McDavitt and Mrs. Lee-Ann Tjon for skilled editorial assistance.
Abbreviations
- GBM
glioblastoma
- CNV
choroidal neovascularization
- MRI
magnetic resonance imaging
- siRNAs
small-interfering RNAs
- miRNAs
microRNAs
- uPA
urokinase-type plasminogen activator
- uPAR
urokinase-type plasminogen activator receptor
- MMP-9
matrix metalloproteinase-9
- LNA
locked nucleic acid
- HBMVECs
human brain microvascular endothelial cells
- VEGF
vascular endothelial growth factor
- EGFR
epidermal growth factor receptor
- PDGFR
platelet-derived growth factor receptor
- shRNAs
short hairpin RNAs
- TLR3
toll-like receptor 3
- NFκB
nuclear factor κB
- IL
interleukin
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/7910
References
- 1.Fischer I, Gagner JP, Law M, Newcomb EW, Zagzag D. Angiogenesis in gliomas: biology and molecular pathophysiology. Brain Pathol. 2005;15:297–310. doi: 10.1111/j.1750-3639.2005.tb00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brem S, Cotran R, Folkman J. Tumor angiogenesis: a quantitative method for histologic grading. J Natl Cancer Inst. 1972;48:347–356. [PubMed] [Google Scholar]
- 3.Folkerth RD. Descriptive analysis and quantification of angiogenesis in human brain tumors. J Neurooncol. 2000;50:165–172. doi: 10.1023/a:1006499824379. [DOI] [PubMed] [Google Scholar]
- 4.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 5.Khodarev NN, Yu J, Labay E, Darga T, Brown CK, Mauceri HJ, et al. Tumour-endothelium interactions in co-culture: coordinated changes of gene expression profiles and phenotypic properties of endothelial cells. J Cell Sci. 2003;116:1013–1022. doi: 10.1242/jcs.00281. [DOI] [PubMed] [Google Scholar]
- 6.Karcher S, Steiner HH, Ahmadi R, Zoubaa S, Vasvari G, Bauer H, et al. Different angiogenic phenotypes in primary and secondary glioblastomas. Int J Cancer. 2006;118:2182–2189. doi: 10.1002/ijc.21648. [DOI] [PubMed] [Google Scholar]
- 7.Miebach S, Grau S, Hummel V, Rieckmann P, Tonn JC, Goldbrunner RH. Isolation and culture of microvascular endothelial cells from gliomas of different WHO grades. J Neurooncol. 2006;76:39–48. doi: 10.1007/s11060-005-3674-6. [DOI] [PubMed] [Google Scholar]
- 8.Anderson JC, McFarland BC, Gladson CL. New molecular targets in angiogenic vessels of glioblastoma tumours. Expert Rev Mol Med. 2008;10:23. doi: 10.1017/S1462399408000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demuth T, Berens ME. Molecular mechanisms of glioma cell migration and invasion. J Neurooncol. 2004;70:217–228. doi: 10.1007/s11060-004-2751-6. [DOI] [PubMed] [Google Scholar]
- 10.Bansal K, Liang ML, Rutka JT. Molecular biology of human gliomas. Technol Cancer Res Treat. 2006;5:185–194. doi: 10.1177/153303460600500302. [DOI] [PubMed] [Google Scholar]
- 11.Mrugala MM, Chamberlain MC. Mechanisms of disease: temozolomide and glioblastoma—look to the future. Nat Clin Pract Oncol. 2008;5:476–486. doi: 10.1038/ncponc1155. [DOI] [PubMed] [Google Scholar]
- 12.Chakravarti A, Palanichamy K. Overcoming therapeutic resistance in malignant gliomas: current practices and future directions. Cancer Treat Res. 2008;139:173–189. [PubMed] [Google Scholar]
- 13.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 14.Rosen L. Antiangiogenic strategies and agents in clinical trials. Oncologist. 2000;5:20–27. doi: 10.1634/theoncologist.5-suppl_1-20. [DOI] [PubMed] [Google Scholar]
- 15.Sathornsumetee S, Rich JN. New treatment strategies for malignant gliomas. Expert Rev Anticancer Ther. 2006;6:1087–1104. doi: 10.1586/14737140.6.7.1087. [DOI] [PubMed] [Google Scholar]
- 16.Wong ET, Brem S. Antiangiogenesis treatment for glioblastoma multiforme: challenges and opportunities. J Natl Compr Canc Netw. 2008;6:515–522. doi: 10.6004/jnccn.2008.0039. [DOI] [PubMed] [Google Scholar]
- 17.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 18.Folkman J. Endogenous angiogenesis inhibitors. Apmis. 2004;112:496–507. doi: 10.1111/j.1600-0463.2004.apm11207-0809.x. [DOI] [PubMed] [Google Scholar]
- 19.Reardon DA, Rich JN, Friedman HS, Bigner DD. Recent advances in the treatment of malignant astrocytoma. J Clin Oncol. 2006;24:1253–1265. doi: 10.1200/JCO.2005.04.5302. [DOI] [PubMed] [Google Scholar]
- 20.Claes A, Idema AJ, Wesseling P. Diffuse glioma growth: a guerilla war. Acta Neuropathol. 2007;114:443–458. doi: 10.1007/s00401-007-0293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 22.Reardon DA, Nabors LB, Stupp R, Mikkelsen T. Cilengitide: an integrin-targeting arginineglycine-aspartic acid peptide with promising activity for glioblastoma multiforme. Expert Opin Investig Drugs. 2008;17:1225–1235. doi: 10.1517/13543784.17.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uhm JH, Gladson CL, Rao JS. The role of integrins in the malignant phenotype of gliomas. Front Biosci. 1999;4:188–199. doi: 10.2741/uhm. [DOI] [PubMed] [Google Scholar]
- 24.Read TA, Sorensen DR, Mahesparan R, Enger PO, Timpl R, Olsen BR, et al. Local endostatin treatment of gliomas administered by microencapsulated producer cells. Nat Biotechnol. 2001;19:29–34. doi: 10.1038/83471. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt NO, Ziu M, Carrabba G, Giussani C, Bello L, Sun Y, et al. Antiangiogenic therapy by local intracerebral microinfusion improves treatment efficiency and survival in an orthotopic human glioblastoma model. Clin Cancer Res. 2004;10:1255–1262. doi: 10.1158/1078-0432.ccr-03-0052. [DOI] [PubMed] [Google Scholar]
- 26.Kulke MH, Bergsland EK, Ryan DP, Enzinger PC, Lynch TJ, Zhu AX, et al. Phase II study of recombinant human endostatin in patients with advanced neuroendocrine tumors. J Clin Oncol. 2006;24:3555–35561. doi: 10.1200/JCO.2006.05.6762. [DOI] [PubMed] [Google Scholar]
- 27.Twombly R. First clinical trials of endostatin yield lukewarm results. J Natl Cancer Inst. 2002;94:1520–1521. doi: 10.1093/jnci/94.20.1520. [DOI] [PubMed] [Google Scholar]
- 28.Bello L, Lucini V, Giussani C, Carrabba G, Pluderi M, Scaglione F, et al. IS20I, a specific alphavbeta3 integrin inhibitor, reduces glioma growth in vivo. Neurosurgery. 2003;52:177–185. doi: 10.1097/00006123-200301000-00023. [DOI] [PubMed] [Google Scholar]
- 29.Chatterjee S, Matsumura A, Schradermeier J, Gillespie GY. Human malignant glioma therapy using anti-alpha(v)beta3 integrin agents. J Neurooncol. 2000;46:135–144. doi: 10.1023/a:1006444300504. [DOI] [PubMed] [Google Scholar]
- 30.Ren H, Yang BF, Rainov NG. Receptor tyrosine kinases as therapeutic targets in malignant glioma. Rev Recent Clin Trials. 2007;2:87–101. doi: 10.2174/157488707780599384. [DOI] [PubMed] [Google Scholar]
- 31.Desjardins A, Reardon DA, Herndon JE, 2nd, Marcello J, Quinn JA, Rich JN, et al. Bevacizumab Plus Irinotecan in Recurrent WHO Grade 3 Malignant Gliomas. Clin Cancer Res. 2008;14:7068–7073. doi: 10.1158/1078-0432.CCR-08-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boutros M, Ahringer J. The art and design of genetic screens: RNA interference. Nat Rev Genet. 2008;9:554–566. doi: 10.1038/nrg2364. [DOI] [PubMed] [Google Scholar]
- 33.Hannon GJ, Rossi JJ. Unlocking the potential of the human genome with RNA interference. Nature. 2004;431:371–378. doi: 10.1038/nature02870. [DOI] [PubMed] [Google Scholar]
- 34.Chappelow AV, Kaiser PK. Neovascular age-related macular degeneration: potential therapies. Drugs. 2008;68:1029–1036. doi: 10.2165/00003495-200868080-00002. [DOI] [PubMed] [Google Scholar]
- 35.de Fougerolles AR. Delivery vehicles for small interfering RNA in vivo. Hum Gene Ther. 2008;19:125–132. doi: 10.1089/hum.2008.928. [DOI] [PubMed] [Google Scholar]
- 36.Gondi CS, Lakka SS, Dinh DH, Olivero WC, Gujrati M, Rao JS. Downregulation of uPA, uPAR and MMP-9 using small, interfering, hairpin RNA (siRNA) inhibits glioma cell invasion, angiogenesis and tumor growth. Neuron Glia Biol. 2004;1:165–176. doi: 10.1017/s1740925x04000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gondi CS, Lakka SS, Dinh DH, Olivero WC, Gujrati M, Rao JS. Intraperitoneal injection of a hairpin RNA-expressing plasmid targeting urokinase-type plasminogen activator (uPA) receptor and uPA retards angiogenesis and inhibits intracranial tumor growth in nude mice. Clin Cancer Res. 2007;13:4051–4060. doi: 10.1158/1078-0432.CCR-06-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kargiotis O, Chetty C, Gondi CS, Tsung AJ, Dinh DH, Gujrati M, et al. Adenovirus-mediated transfer of siRNA against MMP-2 mRNA results in impaired invasion and tumor-induced angiogenesis, induces apoptosis in vitro and inhibits tumor growth in vivo in glioblastoma. Oncogene. 2008;27:4830–4840. doi: 10.1038/onc.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Niola F, Evangelisti C, Campagnolo L, Massalini S, Bue MC, Mangiola A, et al. A plasmid-encoded VEGF siRNA reduces glioblastoma angiogenesis and its combination with interleukin-4 blocks tumor growth in a xenograft mouse model. Cancer Biol Ther. 2006;5:174–179. doi: 10.4161/cbt.5.2.2317. [DOI] [PubMed] [Google Scholar]
- 40.Krzystolik MG, Afshari MA, Adamis AP, Gaudreault J, Gragoudas ES, Michaud NA, et al. Prevention of experimental choroidal neovascularization with intravitreal anti-vascular endothelial growth factor antibody fragment. Arch Ophthalmol. 2002;120:338–346. doi: 10.1001/archopht.120.3.338. [DOI] [PubMed] [Google Scholar]
- 41.Reich SJ, Fosnot J, Kuroki A, Tang W, Yang X, Maguire AM, et al. Small interfering RNA (siRNA) targeting VEGF effectively inhibits ocular neovascularization in a mouse model. Mol Vis. 2003;9:210–216. [PubMed] [Google Scholar]
- 42.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 43.Shen J, Samul R, Silva RL, Akiyama H, Liu H, Saishin Y, et al. Suppression of ocular neovascularization with siRNA targeting VEGF receptor 1. Gene Ther. 2006;13:225–234. doi: 10.1038/sj.gt.3302641. [DOI] [PubMed] [Google Scholar]
- 44.Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NFkappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 46.Rossi J, Zamore P, Kay MA. Wandering eye for RNAi. Nat Med. 2008;14:611. doi: 10.1038/nm0608-611. [DOI] [PubMed] [Google Scholar]
- 47.Khoury M, Escriou V, Courties G, Galy A, Yao R, Largeau C, et al. Efficient suppression of murine arthritis by combined anticytokine small interfering RNA lipoplexes. Arthritis Rheum. 2008;58:2356–2367. doi: 10.1002/art.23660. [DOI] [PubMed] [Google Scholar]
- 48.Jackson AL, Bartz SR, Schelter J, Kobayashi SV, Burchard J, Mao M, et al. Expression profiling reveals off-target gene regulation by RNAi. Nat Biotechnol. 2003;21:635–637. doi: 10.1038/nbt831. [DOI] [PubMed] [Google Scholar]
- 49.Doench JG, Petersen CP, Sharp PA. siRNAs can function as miRNAs. Genes Dev. 2003;17:438–442. doi: 10.1101/gad.1064703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petersen CP, Bordeleau ME, Pelletier J, Sharp PA. Short RNAs repress translation after initiation in mammalian cells. Mol Cell. 2006;21:533–542. doi: 10.1016/j.molcel.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 51.Zeng Y, Yi R, Cullen BR. MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. Proc Natl Acad Sci USA. 2003;100:9779–9784. doi: 10.1073/pnas.1630797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 53.Wurdinger T, Costa FF. Molecular therapy in the microRNA era. Pharmacogenomics J. 2007;7:297–304. doi: 10.1038/sj.tpj.6500429. [DOI] [PubMed] [Google Scholar]
- 54.Chen C, Chai H, Wang X, Jiang J, Jamaluddin MS, Liao D, et al. Soluble CD40 ligand induces endothelial dysfunction in human and porcine coronary artery endothelial cells. Blood. 2008;112:3205–3216. doi: 10.1182/blood-2008-03-143479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, Wentzel E, et al. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet. 2006;38:1060–1065. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, et al. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007;101:59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- 58.Kuhnert F, Mancuso MR, Hampton J, Stankunas K, Asano T, Chen CZ, et al. Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Development. 2008 doi: 10.1242/dev.029736. [DOI] [PubMed] [Google Scholar]
- 59.Poliseno L, Tuccoli A, Mariani L, Evangelista M, Citti L, Woods K, et al. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108:3068–3071. doi: 10.1182/blood-2006-01-012369. [DOI] [PubMed] [Google Scholar]
- 60.Suarez Y, Fernandez-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100:1164–1173. doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- 61.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baker BF, Lot SS, Condon TP, Cheng-Flournoy S, Lesnik EA, Sasmor HM, et al. 2′-O-(2-Methoxy)ethyl-modified anti-intercellular adhesion molecule 1 (ICAM-1) oligonucleotides selectively increase the ICAM-1 mRNA level and inhibit formation of the ICAM-1 translation initiation complex in human umbilical vein endothelial cells. J Biol Chem. 1997;272:11994–12000. doi: 10.1074/jbc.272.18.11994. [DOI] [PubMed] [Google Scholar]
- 63.Cheng AM, Byrom MW, Shelton J, Ford LP. Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res. 2005;33:1290–1297. doi: 10.1093/nar/gki200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davis S, Lollo B, Freier S, Esau C. Improved targeting of miRNA with antisense oligonucleotides. Nucleic Acids Res. 2006;34:2294–2304. doi: 10.1093/nar/gkl183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Esau C, Kang X, Peralta E, Hanson E, Marcusson EG, Ravichandran LV, et al. MicroRNA-143 regulates adipocyte differentiation. J Biol Chem. 2004;279:52361–52365. doi: 10.1074/jbc.C400438200. [DOI] [PubMed] [Google Scholar]
- 66.Hutvagner G, Simard MJ, Mello CC, Zamore PD. Sequence-specific inhibition of small RNA function. PLoS Biol. 2004;2:98. doi: 10.1371/journal.pbio.0020098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee YS, Kim HK, Chung S, Kim KS, Dutta A. Depletion of human micro-RNA miR-125b reveals that it is critical for the proliferation of differentiated cells but not for the downregulation of putative targets during differentiation. J Biol Chem. 2005;280:16635–16641. doi: 10.1074/jbc.M412247200. [DOI] [PubMed] [Google Scholar]
- 68.Meister G, Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431:343–349. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- 69.Bhat B, Esau C, Davis S, Propp S, Kinberger G, Gaus H, et al. 2′-O-Methoxyethyl/2′-Fluoro Modified Oligonucleotides Result in More Potent Inhibition of micro RNA-122 in Vivo: A Target implicatedin HCV Replication. Nucleic Acids Symp Ser (Oxf) 2008;69 [Google Scholar]
- 70.Elmen J, Lindow M, Schutz S, Lawrence M, Petri A, Obad S, et al. LNA-mediated microRNA silencing in non-human primates. Nature. 2008;452:896–899. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
- 71.Elmen J, Lindow M, Silahtaroglu A, Bak M, Christensen M, Lind-Thomsen A, et al. Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to upregulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Res. 2008;36:1153–1162. doi: 10.1093/nar/gkm1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Orom UA, Kauppinen S, Lund AH. LNA-modified oligonucleotides mediate specific inhibition of microRNA function. Gene. 2006;372:137–141. doi: 10.1016/j.gene.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 73.Vester B, Wengel J. LNA (locked nucleic acid): high-affinity targeting of complementary RNA and DNA. Biochemistry. 2004;43:13233–13241. doi: 10.1021/bi0485732. [DOI] [PubMed] [Google Scholar]
- 74.Bonci D, Coppola V, Musumeci M, Addario A, Giuffrida R, Memeo L, et al. The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat Med. 2008;14:1271–1277. doi: 10.1038/nm.1880. [DOI] [PubMed] [Google Scholar]
- 75.Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 76.Wurdinger T, Tannous BA, Saydam O, Skog J, Grau S, Soutschek J, et al. miR-296 regulates growth factor receptor overexpression in angiogenic endothelial cells. Cancer Cell. 2008;14:382–393. doi: 10.1016/j.ccr.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang XH, Qian RZ, Zhang W, Chen SF, Jin HM, Hu RM. MicroRNA-320 Expression in myocardial microvascular endothelial cells and its relationship with insulin-like growth factor-1 in type 2 diabetic rats. Clin Exp Pharmacol Physiol. 2008 doi: 10.1111/j.1440-1681.2008.05057.x. [DOI] [PubMed] [Google Scholar]
- 78.Miranda KC, Huynh T, Tay Y, Ang YS, Tam WL, Thomson AM, et al. A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell. 2006;126:1203–1217. doi: 10.1016/j.cell.2006.07.031. [DOI] [PubMed] [Google Scholar]