Abstract
OBJECTIVE
Although preterm very low birth weight infants have a high prevalence of neuroanatomical abnormalities when evaluated at term-equivalent age, patterns of brain growth in prematurely born infants during school age and adolescence remain largely unknown. Our goal was to test the hypothesis that preterm birth results in long-term dynamic changes in the developing brain.
METHODS
We performed serial volumetric MRI studies at ages 8 and 12 years in 55 preterm infants born weighing 600 to 1250 g and 20 term control children who participated in the follow-up component of a prospective, randomized, placebo-controlled intraventricular hemorrhage prevention study.
RESULTS
Total brain volumes increased 2% to 3% between the ages of 8 and 12 years for both preterm and term children. These changes involved reductions in cerebral gray matter while white matter increased. Between 8 and 12 years of age, preterm subjects experienced a 2% decrease in left cerebral gray matter compared with a 10% reduction in left cerebral gray for term controls. For right cerebral gray matter, preterm children experienced a 3% decrease in volume between years 8 and 12, compared with 9% for term controls (group-by-time). In contrast, preterm subjects had a 10% increase in cerebral white matter volumes bilaterally between ages 8 and 12 years, compared with >26% increases for both hemispheres for term controls. Significant differences in regional volume changes between study groups were found in bilateral temporal gray and in parietal white matter.
CONCLUSIONS
Preterm birth continues to perturb the trajectory of cerebral development during late childhood and early adolescence with preterm children, showing both lower gray matter reduction and less white matter gain over time compared with term control subjects.
Keywords: preterm, brain volume changes, childhood, adolescence, birth weight, gender
Recent data suggest that preterm birth may alter the genetically determined pattern of cerebral development in the developing brain.1–3 The neuroanatomical sequelae of preterm birth have been well described,4–7 but the suggestion that birth at an extremely low gestational age results in dynamic alterations in brain development during childhood and adolescence remains largely unexplored. Numerous cross-sectional MRI studies have documented the structural sequelae of preterm birth in the newborn period, at school age and beyond.8–12 Preterm neonates have reduced cortical surface area measurements13 and lower gray and white matter volumes at term-equivalent age when compared with term controls of the same age,14–16 and studies of school-aged prematurely born children suggest regional vulnerability in the developing preterm brain.17,18 In contrast, MRI studies of prematurely born subjects at young adult ages fail to document differences in total brain or regional volumes when preterm subjects are compared with either control subjects or to their adult-aged siblings.12,19
Although many progressive and regressive events that are critical for cerebral development occur during the third trimester of gestation and in the newborn period, both postmortem examinations and cross-sectional MRI studies of typically developing subjects suggest that dynamic changes in brain composition continue during childhood and adolescence.20–24 Studying normally developing children at ages 5 to 17 years, Reiss et al.25 found that, although there was little change in total cerebral volume, there were prominent changes in cerebral gray and white matter volumes within this time span. The ratio of cortical gray to total cerebral volume was negatively correlated with age, whereas the opposite pattern was observed for white matter in this population. Similarly, Sowell et al.26 described a regional pattern of age-related differences in gray and white matter changes in normally developing children ages 7 to 16 years, although white matter changes were more robust than those for gray.
Because neuroanatomical outcome after preterm birth is complex, with ongoing interactions between the environment and the genome, longitudinal studies seem well suited for evaluating our hypothesis that preterm birth would result in long-term dynamic changes in cerebral development. For this reason, we performed serial volumetric MRI studies at 8 and 12 years of age on very low birth weight (VLBW) prematurely born children and term control subjects as part of our long-term study of preterm children.27
METHODS
Study Subjects
Study subjects were recruited and evaluated at the Yale University School of Medicine, New Haven, Connecticut, and at Brown Medical School, Providence, Rhode Island. MRI scans were performed at Yale University and analyzed at the Stanford Center for Interdisciplinary Brain Sciences Research. All subjects and their parents provided informed written consent/assent for participation, and the institutional review boards of all 3 universities approved the protocols.
The preterm subjects consisted of VLBW children born between September 5, 1989, and August 31, 1992, and enrolled in the Multicenter Indomethacin Intraventricular Hemorrhage Prevention Trial at Yale University and Brown Medical School on the basis of geographic proximity to New Haven (Table 1).28,29 Overall, 287 (84%) of the surviving 340 children were evaluated at 8 and 12 years of age. Two hundred resided within proximity to New Haven; scans were performed on 170 of 200. Usable scans at 1 or both time points were obtained on 133 of 170 children. These numbers are consistent with previous reports for children scanned during this age range.23,30 Children with MRI studies were representative of the entire cohort of available survivors (N = 287) with respect to neonatal variables and cognitive outcome scores.
TABLE 1.
Preterm Study Population
| Subject Population | No. of Children |
|---|---|
| Infants with study permission | 398 |
| Infants enrolled in primary and secondary indomethacin cohort studies | 392 |
| Survivors of the primary and secondary indomethacin cohort studies | 340 |
| Study subjects seen in follow-up and evaluated at 8 and 12 y | 287 |
| Subjects who live within geographic proximity of New Haven, CT | 200 |
| Study subjects with MRI studies | 170 |
| Study subjects with usable scans | 133 |
| Study subjects with usable scans at both 8 and 12 ya | 55 |
Longitudinal cohort.
Term control children were recruited from randomly selected names on a telemarketing list in the same region18 and from local advertisements. The families were identified as having children of similar ages and living in the same zip code areas as the preterm children. One hundred twenty live within proximity of New Haven; MRI studies were performed on 102 and 76 had usable scans at 1 or both times.
Finally, 55 preterm subjects and 20 term control children had usable serial scans at both 8 and 12 years of age. Children with usable scans and scanner type (GE [Waukeha, WI] or Siemens [SN] [Erlangen, Germany]) are shown in Table 2. The most common reason for a subject to be scanned at 1 but not both ages was orthodontia or other contraindication for MRI.31
TABLE 2.
Usable Scans for Children in the Longitudinal Cohort
| Scanner | Group | Total | |
|---|---|---|---|
| Preterm | Term | ||
| GE ages 8 and 12 y | 39 | 6 | 45 |
| GE age 8 and Siemens age 12 y | 16 | 14 | 30 |
| Total | 55 | 20 | 75 |
Neonatal Assessment and Neurodevelopmental Outcome
The assessments of neonatal health status, outcome, data collection, and the grading systems for intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), and ventriculomegaly were previously described. 28,29
At both ages, children were assessed by using the Wechsler Intelligence Scale for Children–III32 and the Peabody Picture Vocabulary Test–Revised33 by research staff blinded to the child’s study group and medical status.
MRI Protocols, Image Processing, and Measurement
Image Acquisition
Imaging was performed on a GE-Signa 1.5-T scanner or, after this magnet was no longer available, a Siemens-Sonata 1.5-T scanner. Numbers for subjects undergoing serial scans on the GE and those scanned on the GE at 8 and the Siemens at 12 are shown in Table 2.
For the GE scanner, sagittal images were acquired with a three-dimensional volumetric radio frequency spoiled gradient echo pulse sequence using the following parameters: repetition time, 24 milliseconds; echo time, 5 milliseconds; flip angle, 45°; number of excitations (NEX) 1; matrix size, 256 × 192; field of view, 30 cm; slice thickness, 1.2 mm; 124 contiguous slices.
For the Siemens-Sonata scanner, sagittal images were acquired with a three-dimensional volumetric radio frequency spoiled gradient echo pulse sequence by using parameters designed to be most compatible with acquired GE image data sets: repetition time, 24 milliseconds; echo time, 2.9 milliseconds; flip angle, 45°; NEX, 2; matrix size, 256 × 192; field of view, 30 cm; slice thickness, 1.2 mm; 124 contiguous slices.
Volumetric Imaging Analysis
Scans were transferred digitally from Yale University to Stanford University, and all image processing was completed at Stanford University consistent with Health Insurance Portability and Accountability Act guidelines for subject confidentiality. Raters blinded to diagnosis and results of previous scan visually inspected the images to exclude those that could not be processed owing to excessive motion and/or flow artifact. There were no significant differences in IQ scores between subjects with usable serial scan data and those without serial data.
Volumetric image processing was conducted by using BrainImage (cibsr.stanford.edu/tools) and FSL (www.fmrib.ox.ac.uk/fsl) software. Data processing included removal of nonbrain tissues from the images, bias field correction, gray, white and cerebrospinal fluid segmentation, normalization of image position, and parcellation of the cerebral cortex based on a stereotaxic atlas template. 34,35 This procedure as described and validated in previous reports, results in reliable measurements for gray, white, deep gray, and whole brain and regional volumes.36 Intrarater reliabilities for volumes described in this study were all ≥.95 as determined by the intra-class correlation coefficient.
Statistical Methods
Demographic and cognitive data were analyzed by using standard χ2 statistics for categorical data, and nonparametric Wilcoxon rank-sum tests for continuous-valued data.
Generalized linear models were used to evaluate group differences in gray and white matter tissue volumes across time. Total brain volume and age at time of scan at time 1 (ie, 8 years old), the difference in ages between scan time 1 and scan time 2 (ie, 12 years old), time interval, scanner (GE versus Siemens), group (ie, preterm versus term control), and gender were used as covariates. Brain volume summary statistics are reported as model-based least square means ± SEM.
In anticipation of the GE scanner no longer being available and the necessity of moving future scans to the Siemens magnet at Yale University, we obtained MRI data from 7 healthy adults and 2 preterm subjects from both magnets by using the protocols described earlier. Even with highly compatible pulse sequences on 1.5T magnets, it is recognized that images obtained from different scanner platforms can result in variations in scaling. As suggested by Schnack et al,37 to better understand the nature of this variation in our study, we subjected MRI data from these 9 subjects to standard image processing procedures as described earlier. Although tissue volumes derived from the 2 scanners were highly correlated (cerebral gray matter: R = 0.93; cerebral white matter: R = 0.89; cerebral spinal fluid: R = 0.90), results from the GE scanner generally showed higher gray matter (mean ± SD: 60.9 ± 33.7 mL) and lower white matter (−67.0 ± 27.4 mL) volumes relative to the Siemens data. We first chose a priori to evaluate the effect of different scanners in the measurements observed. By including “scanner” as a covariate in our generalized linear models, we were able to use the true longitudinal nature of these data to address key brain development issues presented here.
A secondary analysis was also performed by using regression models for individual brain volumes (ie, total brain volume, left cerebral gray, left cerebral white, right cerebral gray, right cerebral white, and cerebellum). To accomplish this, region-specific regression formulae (ie, y = mx + b) were derived from the data for the 9 subjects imaged on both scanners, then used to mathematically “convert” preterm study subject data collected on the Siemens scanner to “GE” comparable data. For example, the formula used to convert left cerebral white volumes obtained on the Siemens scanner to GE comparable volumes was: GE = 15.5 + (0.83*L White SN), where y is the “converted GE” volume, x is the original Siemens volume, 0.83 is the slope, and 15.5 is the intercept.
Interaction terms for group-by-time interval, group-by- gender, and group-by-gender-by-time interval were assessed. Interactions are intended to address the issue of whether the between-group effects vary according to another factor; for example, the group-by-time examines whether preterm versus term group comparisons vary over time. Using a priori hypotheses, the relationship between neuroanatomical variation and neonatal variables was assessed in the preterm group by using general linear models. Neonatal variables included birth weight, gestational age, small for gestational age status, randomization to indomethacin, bronchopulmonary dysplasia (BPD), IVH, cystic PVL, low pressure ventriculomegaly, and years of maternal education. These analyses controlled for gender and scanner. Neuroanatomical variables included those that differentiated the preterm group from the term control group at 8 years old; neonatal variables were chosen on the basis of those previously shown to be associated with outcome in preterm subjects.
All statistical analyses were performed by using SAS 9.1.3 (SAS Institute, Inc, Cary, NC), and all P values are 2-sided.
RESULTS
Study Subjects
Neonatal data for the study subjects are shown in Table 3. Fifty-eight percent of the preterm subjects were males, as were 45% of controls, and there were no significant differences in maternal education for the groups.
TABLE 3.
Neonatal Data for the Longitudinal Study Children
| Variable | Preterm | Term | P |
|---|---|---|---|
| No. | 55 | 20 | |
| No. (%) of males | 32 (58) | 9 (45) | .31 |
| Birth weight, mean ± SD, g | 972 ± 157 | 3328 ± 565 | <.001 |
| Gestational age, mean ± SD, wk | 28.4 ± 1.7 | 39.4 ± 3.0 | <.001 |
| No. (%) from twin/triplet gestation | 6 (11) | 0 | .18 |
| No. (%) small for gestational age | 4 (7) | 3/18 (17) | .24 |
| Nonwhite, n (%) | 30 (56) | 4 (20) | .008 |
| Chorioamnionitis, n (%) | 8 (16.7) | NA | |
| Randomization to indomethacin, n (%) | 30 (54.6) | NA | |
| IVH, n (%) | 14 (25) | NA | |
| Grade 1 IVH, n (%) | 5 (9) | NA | |
| Grade 2 IVH, n (%) | 9 (16) | NA | |
| PVL, n (%) | 2 (3.6) | NA | |
| Ventriculomegaly, n (%) | 1 (1.8) | NA | |
| BPD, n (%) | 26 (47) | NA | |
| Maternal education, mean ± SD, y | 13.7 ± 2.3 | 14.9 ± 2.9 | .11 |
| Maternal education less than high school, n (%) | 4/53 (7.6) | 1 (5.0) | .70 |
NA indicates not applicable.
Although the preterm subjects were on average 6 months older than the term children at the first scan (9.2 ± 0.7 vs 8.6 ± 0.7 years; P = .002), there was no significant difference between subjects’ ages at the second scan (12.3 ± 0.4 vs 12.1 ± 0.6 years) or in the time interval between scans (P = .17 and .10, respectively). Forty-seven (85%) preterm children and 19 (95%) term controls were right-handed (P = .26). At 12 years old, preterm subjects had lower verbal (93.6 ± 16.2 vs 102.6 ± 16.1), performance (87.8 ± 14.9 vs 103.8 ± 20.1), and full-scale IQ (89.9 ± 15.1 vs 103.4 ± 18.1) scores (P = .034, .002, .006), but no significant difference was noted in the Peabody Picture Vocabulary Test–Revised scores (P = .30).
Neuroanatomical Volumes
As shown in Table 4, the total brain volume (TBV) data suggested a 2% to 3% increase in volumes for both groups (group-by-time: F = 4.35; df = 1,71; P = .041). Females had lower TBVs than males (F = 12.83; df = 1,68; P < .001), but there were no gender-by-time or gender-by-group effects for TBV in our population (F = 0.00; df = 1,71; P = .97; and F = 2,67; df = 1,68; P = .11, respectively).
TABLE 4.
Volumetric Data for the Study Subjects
| Region | Least Square Mean±SE, cc3 | P | |||||
|---|---|---|---|---|---|---|---|
| Preterm | Term | ||||||
| 8 y | 12 y | 8 y | 12 y | Group | Time | Group × Time | |
| Total brain volume | 1319.56 ± 14.53 | 1348.91 ± 14.53 | 1391.42 ± 23.54 | 1439.17 ± 23.54 | .007 | <.001 | .041 |
| Cerebral gray | |||||||
| Left | 321.41 ± 2.40 | 314.27 ± 2.40 | 332.69 ± 3.99 | 298.33 ± 3.99 | .52 | <.001 | <.001 |
| Right | 321.69 ± 2.42 | 311.88 ± 2.42 | 326.55 ± 4.03 | 297.99 ± 4.03 | .25 | <.001 | .003 |
| Cerebral white | |||||||
| Left | 202.24 ± 2.57 | 222.18 ± 2.57 | 192.14 ± 4.28 | 243.26 ± 4.28 | .20 | <.001 | <.001 |
| Right | 201.49 ± 2.54 | 221.40 ± 2.55 | 193.71 ± 4.23 | 244.04 ± 4.23 | .07 | <.001 | <.001 |
| Total cerebellar volume | 126.31 ± 1.74 | 132.03 ± 1.74 | 125.87 ± 2.92 | 138.46 ± 2.92 | .40 | <.001 | <.001 |
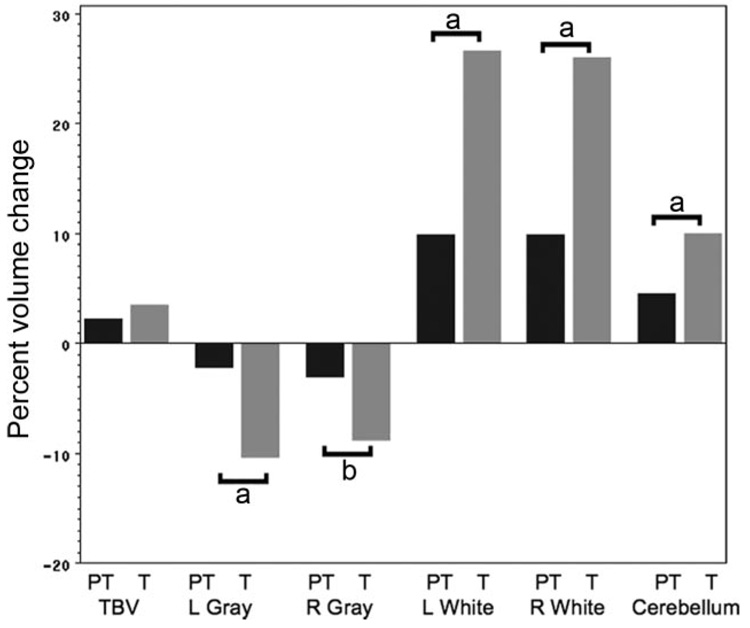
The changes in TBV over time for the preterm and term groups involve both cerebral gray and white matter. As shown in Fig 1, cerebral gray volumes decreased between ages 8 and 12 years. Left cerebral gray decreased 2% for the preterm children, compared with 10% for term control subjects (F = 17.92; df = 1,71; P < .001). In addition, although female preterm subjects had significantly less left cerebral gray matter than preterm males (F = 4.00; df = 1,67; P = .050), there were no group-by-gender-by-time effects for the study groups. Similarly, right cerebral gray volumes decreased 3% for preterm subjects, compared with 9% for term controls (F = 9.72; df = 1,71; P = .003). Unlike values for left cerebral gray, we found no group-by-gender effects for right cerebral gray.
FIGURE 1.
Percent volume changes between 8 and 12 years for the preterm (PT) and term control (T) subjects for TBV, left cerebral gray, right cerebral gray, left cerebral white, right cerebral white, and cerebellar volumes. Volume changes are given in Table 5; percent changes are given for data comparison. a P< .001; b P < .01.
In contrast, cerebral white matter volumes increased over time for the study groups. Left cerebral white matter increased 10% for the preterm group, compared with a 27% increase for term controls (F = 24.91; df = 1,71; P < .001). In addition, preterm males had significantly less left cerebral white than preterm females (F = 4.61; df = 1,67; P = .035), but similar to data for left cerebral gray, we found no group-by-gender-by-time effects for the study groups. Right cerebral white matter also increased 10% for the preterm subjects, whereas term controls experienced a 26% increase in volume for right cerebral white matter during the same time interval (F = 22.34; df = 1,71; P < .001). No group-by-gender or group-by-gender-by-time effects were noted for right cerebral white.
Likewise, total cerebellar volumes increased 5% for the preterm subjects, compared with 10% increase in total volume for the term controls (F = 12.95; df = 1,71; P < .001). Finally, there were no significant group-by-gender or group-by-gender-by-time effects for total cerebellum data.
As a secondary analysis, neuroanatomical volumes were analyzed after using the regression formulae described. These data, shown in Table 6 (which is published as supporting information at www.pediatrics.org/content/full/123/2/503), confirm the differential volumetric changes for the preterm and term control subjects described earlier.
Finally, to test the hypotheses that IVH, PVL, or low-pressure ventriculomegaly may have inordinately influenced brain volume changes in our preterm study group and that those term subjects requiring special school services may have abnormal cerebral development, we omitted 15 preterm subjects with abnormal cranial ultrasounds and 2 control children requiring special school services and completed the previous analyses. As shown in Table 7 (which is published as supporting information at www.pediatrics.org/content/full/123/2/503), even after removing these children, all study results remained significant.
Analysis of Regional Volumes
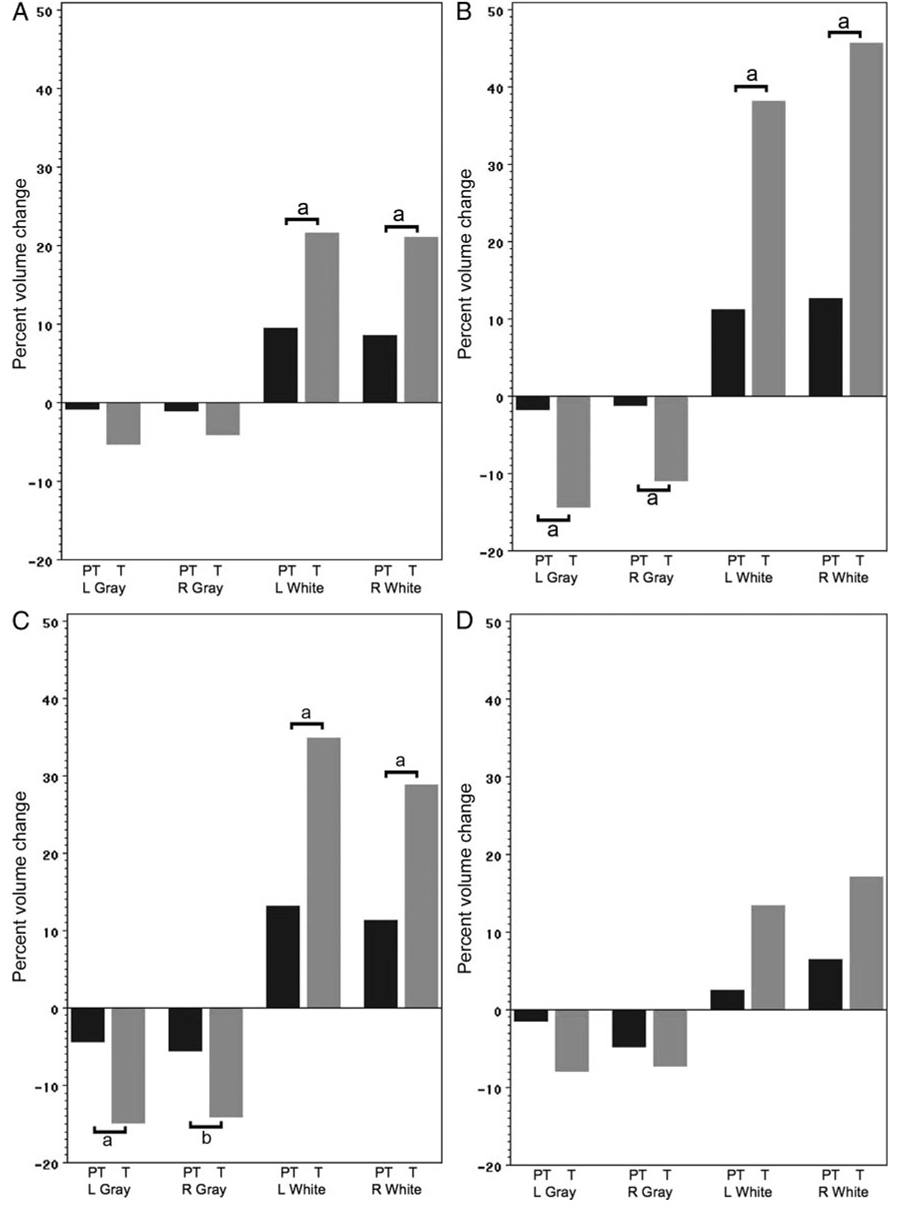
When examining regional longitudinal volume changes for the preterm and term control subjects, we noted widespread significant group-by-time effects for volume changes (Table 5). As shown in Fig 2 and Table 8 (which is published as supporting information at www.pediatrics.org/content/full/123/2/503), differential decreases in regional gray matter volumes were most significant in the bilateral temporal and parietal regions, as well as in the left deep gray region. In all regions, regressive events were significantly less in the preterm subjects than in the term control group.
TABLE 5.
Regional Volumetric Data for the Study Subjects
| Region | Preterm, Least Square Mean ± SE, cc3 |
Term, Least Square Mean ± SE, cc3 |
P |
||||
|---|---|---|---|---|---|---|---|
| 8 y | 12 y | 8 y | 12 y | Group | Time | Group × Time | |
| Gray matter | |||||||
| Frontal | |||||||
| Left | 116.80 ± 1.06 | 115.82 ± 1.06 | 116.86 ± 1.77 | 110.62 ± 1.77 | .16 | .004 | .032 |
| Right | 117.69 ± 1.69 | 116.39 ± 1.15 | 115.99 ± 1.91 | 111.27 ± 1.91 | .11 | .008 | .122 |
| Temporal | |||||||
| Left | 66.57 ± 0.91 | 65.40 ± 0.91 | 71.59 ± 1.52 | 61.32 ± 1.52 | .77 | <.001 | <.001 |
| Right | 64.71 ± 0.77 | 63.90 ± 0.77 | 67.53 ± 1.28 | 60.19 ± 1.28 | .73 | <.001 | <.001 |
| Parietal | |||||||
| Left | 80.69 ± 0.90 | 77.22 ± 0.90 | 81.18 ± 1.50 | 69.14 ± 1.50 | .009 | <.001 | <.001 |
| Right | 81.50 ± 0.92 | 76.99 ± 0.92 | 80.23 ± 1.53 | 68.94 ± 1.53 | .003 | <.001 | .004 |
| Occipital | |||||||
| Left | 37.07 ± 0.60 | 36.54 ± 0.60 | 39.85 ± 1.00 | 36.69 ± 1.00 | .19 | <.001 | .015 |
| Right | 38.44 ± 0.65 | 36.61 ± 0.65 | 40.87 ± 1.09 | 37.90 ± 1.09 | .13 | <.001 | .351 |
| Deep gray | |||||||
| Left | 20.27 ± 0.25 | 19.29 ± 0.54 | 23.20 ± 0.42 | 20.55 ± 0.42 | <.001 | <.001 | .009 |
| Right | 19.34 ± 0.23 | 17.99 ± 0.23 | 21.92 ± 0.38 | 19.69 ± 0.38 | <.001 | <.001 | .073 |
| White matter | |||||||
| Frontal | |||||||
| Left | 70.35 ± 0.97 | 76.99 ± 0.97 | 67.46 ± 1.62 | 82.03 ± 1.62 | .55 | <.001 | <.001 |
| Right | 72.90 ± 1.10 | 79.13 ± 1.10 | 69.19 ± 1.83 | 83.70 ± 1.83 | .83 | <.001 | <.001 |
| Temporal | |||||||
| Left | 32.26 ± 0.84 | 35.86 ± 0.84 | 31.86 ± 1.40 | 44.01 ± 1.40 | .008 | <.001 | <.001 |
| Right | 31.12 ± 0.89 | 35.03 ± 0.89 | 30.70 ± 1.48 | 44.71 ± 1.48 | .003 | <.001 | <.001 |
| Parietal | |||||||
| Left | 55.57 ± 0.92 | 62.89 ± 0.92 | 51.15 ± 1.53 | 68.99 ± 1.53 | .58 | <.001 | <.001 |
| Right | 55.24 ± 0.84 | 61.47 ± 0.84 | 52.23 ± 1.39 | 67.23 ± 1.39 | .31 | <.001 | <.001 |
| Occipital | |||||||
| Left | 25.46 ± 0.57 | 26.08 ± 0.57 | 23.76 ± 0.96 | 26.93 ± 0.96 | .69 | .003 | .013 |
| Right | 22.94 ± 0.65 | 24.41 ± 0.65 | 22.32 ± 1.08 | 26.14 ± 1.08 | .66 | <.001 | .032 |
| Deep white | |||||||
| Left | 18.60 ± 0.28 | 20.36 ± 0.28 | 17.91 ± 0.46 | 21.29 ± 0.46 | .80 | <.001 | .015 |
| Right | 19.29 ± 0.25 | 21.35 ± 0.25 | 19.27 ± 0.42 | 22.26 ± 0.42 | .33 | <.001 | .073 |
FIGURE 2.
Percent volume changes between 8 and 12 years for the preterm (PT) and term control (T) subjects for frontal gray and white (A), temporal gray and white (B), parietal gray and white (C), and occipital gray and white (D) volumes. Volume changes are given in Table 6; percent changes are given for data comparison. a P<.001; b P<0.01.
Regional white matter volumes also changed differently across time in our subject groups. Also as shown in Fig 2 and Table 8, differential increases in regional white matter volumes were most significant in the bilateral frontal, temporal, and parietal regions.
Relationship Among Neuroanatomical and Perinatal Variables
Evaluating the preterm cohort only, we tested the hypotheses that birth weight, gestational age, gender, small for gestational age status, neonatal randomization to early indomethacin, BPD, IVH, PVL, and/or low-pressure ventriculomegaly and maternal education would significantly contribute to the brain volumes reported in Table 4.
These analyses demonstrated that birth weight and gender were significantly associated with cerebral white matter volumes (at both time points). Preterm infants with lower birth weights had less cerebral white matter than those with higher weights, and preterm girls had significantly less cerebral white matter than preterm boys. For left cerebral white matter, these effects were as follows: birth weight (F = 4.30; df = 1,46; P = .044); gender (F = 5.93; df = 1,46; P = .019). We also noted an interaction between birth weight and gender for left cerebral white (F = 6.28; df = 1,46; P = .016); increasing birth weight was significantly correlated with increased white matter volume at both 8 and 12 years for female subjects but not for male preterm children. For right cerebral white matter, the significant effects were as follows: birth weight (F = 5.06; df = 1,46; P = .029), gender (F = 5.77; df = 1,46; P = .020), and the gender-by-birth weight interaction (F = 6.58; df = 1,46; P = .014). For right cerebral white matter, this interaction was similar to that for left cerebral white matter; with increasing birth weight, preterm females had greater white matter at both 8 and 12 years. This relationship was not true for preterm male subjects.
DISCUSSION
Cerebral development is a dynamic process, and brain growth continues for several decades after birth.22,38,39 This study reports longitudinal total brain and regional cerebral volume changes during late childhood and early adolescence in a well-characterized population of VLBW preterm subjects and term control children. Girls had smaller total brain volumes than boys, and between 8 and 12 years of age both preterm and term study groups exhibited a modest 2% to 3% increase in total brain volume. For both groups, significant volumetric changes putatively associated with central nervous system regressive and progressive changes were found.
Overall, percent white matter increases were greater than corresponding decreases in cerebral gray matter volumes for both study groups, but both the regressive gray matter changes and increases in cerebral white matter volumes were significantly less robust in preterm subjects when compared with term controls. For both groups, reduction in gray matter was most significant in the parietal region, whereas regional increases in white matter occurred most significantly in both temporal and parietal regions. Finally, although multiple perinatal variables including BPD, low-grade IVH, PVL, and randomization to indomethacin had no influence on cerebral volumes, white matter volumes at both 8 and 12 years were significantly correlated with birth weight for preterm female subjects, but not for preterm males.
Advances in neuroimaging have permitted study of the changes in cerebral structure that occur between childhood and adolescence. Multiple cross-sectional and cross-sectional/longitudinal investigations of typically developing children between 4 and 20 years of age have demonstrated linear increases in white matter of 12% to 25% during that time interval.22,24,38–40 Whereas data for term controls are consistent with these results, white matter increased only 10% for the preterm children between ages 8 and 12 years.
In addition, several authors have reported nonlinear changes in cortical gray with overall decreases of 23% to 32% between ages 4 and 30 years.38,39 Cortical gray matter increases during late childhood and then, in the postadolescent period, decreases before adulthood. Furthermore, depending on the ages of the children studied, gray matter changes have been shown to be regionally specific, with changes in frontal and parietal lobe gray matter peaking at ~12 years of age and those in the temporal lobe reaching greatest values at 16 years old.26,38 Consistent with these data, between ages 8 and 12 years both the term control and preterm subjects experienced the greatest decreases in gray matter in the parietal lobes. They differed, however, in magnitude of change. The term control children experienced a 14% to 15% decrease in parietal gray, whereas the parietal gray for the preterm subjects decreased just 4% to 6%.
The true effects of preterm birth on the developing brain remain unresolved. Previous studies have suggested alterations in brain volumes at term equivalent in VLBW survivors of preterm birth when compared with term controls,14 but evolving changes in newborn special care practices and the inclusion of widely differing populations of study infants have made comparisons of different populations difficult over time. Indeed, a recent report suggests that cerebral volume is not reduced in preterm infants who were compared with term controls at term-equivalent age,41 whereas 2 others used serial MRI to suggest both persistent white matter abnormalities at term equivalent and disruption of the scaling relation determining brain growth in the prematurely born.1,2 In addition, both the need for oxygen supplementation at postnatal age 28 days and IVH have both been identified as risk factors for abnormal brain growth at term-equivalent age.3,41,42
Evaluation of volumetric imaging studies of prematurely born subjects during late childhood and early adolescence demonstrates the long-term impact of preterm birth on brain development at a static point in time. At 8 years old, preterm subjects were shown to have significantly lower volumes for TBV and cerebral gray and white matter regions.11,43 In contrast, at age 14 to 15 years, Nosarti et al.5 noted no difference in cerebral white matter when comparing 72 preterms and 48 term controls, and Allin et al.12 reported no significant whole brain, gray matter, or white matter differences between 32 VLBW adults and 18 sibling controls.
The introduction of both voxel-based morphometry and diffusion tensor imaging has permitted more detailed assessment of regional white matter volumes and fractional anisotropy, a measure of fiber tract organization. Diffusion tensor imaging studies suggest changes in neural connectivity throughout the brain of preterm children when compared with term controls at 12 years old,44 and 2 recent studies of prematurely born children at adolescence demonstrate significant abnormalities in numerous white matter regions throughout the cerebrum in the preterm groups.6,45 Furthermore, significant correlations were reported between white matter alterations and both birth weight and gestational age such that the lower the birth weight and gestational age, the lower the white matter integrity.
Our study has benefited from a well-characterized, longitudinal cohort design. The preterm subjects have been studied since the sixth postnatal hour with serial cranial ultrasonography and prenatal, perinatal, and neonatal data collection. Both the preterm and term control subjects have undergone serial neurodevelopmental assessments, and all have been invited to participate in our MRI studies at 8 and 12 years of age. Similarly, the weaknesses of our study are well described. Because of the years in which they were born and the difficulties transporting VLBW preterm infants to MRI facilities at the participating sites, the preterm subjects did not undergo MRI studies in the newborn period and thus we have no data concerning the presence of diffuse excessive high signal intensity or other subtle white matter injuries, which may alter cortico-genesis in the developing preterm brain.46–48 In addition, the sample size for term controls in our longitudinal study is relatively modest. Finally, our institution changed MRI scanners for research studies when our study subjects were turning 12 years of age. Although we optimized scan acquisition and processing procedures to allow for the greatest degree of compatibility possible, images from the 2 scanner platforms showed variation in tissue volumes. Previous authors have investigated scanner associated variability in volumetric data while also using statistical or image postprocessing methods to avoid compromising the validity of the results, and our correlation coefficients compare well with those reported by Schnack et al.37 In the present study, we chose to statistically adjust for scanner effects in our analyses a priori. This was accomplished through the use of generalized linear models incorporating the following factors: total brain volume and exact chronologic age at first scan, years between scans, scanner, group, gender, and time. As a secondary analysis strategy, we also created “conversion formulae” by which regional volumes on the Siemens scanner were “converted” to “GE values” for generalized linear models that excluded scanner but otherwise included all of the previously described variables. These analytic methods resulted in similar significant findings, but most certainly would have benefited from additional preterm and term control subjects who were scanned on both scanners on the same calendar day.
CONCLUSIONS
Knowledge of longitudinal cerebral changes and the risk factors mediating them is critical for understanding the impact of preterm birth on the developing brain. The serial volumetric MRI studies we report provide evidence for the dynamic influence of preterm birth on brain development during late childhood and early adolescence. Future studies of infants who are ≤26 weeks’ gestation from term-equivalent age through childhood and adolescence will provide important information concerning the interaction of the environment and preterm birth on developing brain.
What’s Known on This Subject
Previous studies have reported volume MRI data at single time points in preterm subjects and term controls.
What This Study Adds
To our knowledge, this is the first report of serial volumetric MRI studies at late childhood and early adolescence in a large cohort of prematurely born children and term controls. Preterm birth has a long-lasting and dynamic influence on brain development.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health grants NS 27116, MO1-RR06022, and MO1- RR00125.
We thank Drs Walter Allan and Deborah Hirtz, Ms Marjorene Ainley, Ms Jill Maller-Kesselman, Ms Victoria Watson, Ms Christa Watson, Ms Cheryl Lacadie, Ms Hedy Sarofin, and Ms Terry Hickey.
Abbreviations
- VLBW
very low birth weight
- IVH
intraventricular hemorrhage
- PVL
periventricular leukomalacia
- BPD
bronchopulmonary dysplasia
- TBV
total brain volume
Footnotes
Reprints Information about ordering reprints can be found online: http://www.pediatrics.org/misc/reprints.shtml
References
- 1.Kapellou O, Counsell S, Kennea NL, et al. Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Medicine. 2006;3(8):e265. doi: 10.1371/journal.pmed.0030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mewes AUJ, Huppi PS, Als H, et al. Regional brain development in serial magnetic resonance imaging of low-risk preterm infants. Pediatrics. 2006;118(1):23–33. doi: 10.1542/peds.2005-2675. [DOI] [PubMed] [Google Scholar]
- 3.Thompson DK, Warfield SK, Carlin JB, et al. Perinatal risk factors altering regional brain structure in the preterm infant. Brain. 2007;130(pt 3):667–677. doi: 10.1093/brain/awl277. [DOI] [PubMed] [Google Scholar]
- 4.Nosarti C, Rushe TM, Woodruff PWR, Stewart AL, Rifkin L, Murray RM. Corpus callosum size and very preterm birth: relationship to neuropsychological outcome. Brain. 2004;127(pt 9):2080–2089. doi: 10.1093/brain/awh230. [DOI] [PubMed] [Google Scholar]
- 5.Nosarti C, M.H.S A-A, Frangou S, Stewart A, Rifkin L, Murray RM. Adolescents who were born very preterm have decreased brain volumes. Brain. 2002;125(pt 7):1616–1623. doi: 10.1093/brain/awf157. [DOI] [PubMed] [Google Scholar]
- 6.Giménez M, Junque C, Narberhaus A, Bargallo N, Botet F, Mercader JM. White matter volume and concentration reductions in adolescents with history of very preterm birth: a voxel-based morphometry study. Neuroimage. 2006;32(4):1485–1498. doi: 10.1016/j.neuroimage.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Giménez M, Junque C, Narberhaus A, Botet F, Bargallo N, Mercader JM. Correlations of thalamic reductions with verbal fluency impairment in those born prematurely. Neuroreport. 2006;17(5):463–466. doi: 10.1097/01.wnr.0000209008.93846.24. [DOI] [PubMed] [Google Scholar]
- 8.Huppi PS, Schuknecht B, Boech C, Bossi Eea Structural and neurobehavioral delay in postnatal brain development of preterm infants. Pediatr Res. 1996;39:895–901. doi: 10.1203/00006450-199605000-00026. [DOI] [PubMed] [Google Scholar]
- 9.Inder TE, Warfield S, Wang H. Impaired cerebral cortical development in the premature infant without periventricular white matter injury. Pediatr Res. 2002;51:439A. [Google Scholar]
- 10.Isaacs EB, Edmonds CJ, Chong WK, Lucas A, Morley R, Gadian DG. Brain morphometry and IQ measurement in preterm children. Brain. 2004;127(pt 12):2595–2607. doi: 10.1093/brain/awh300. [DOI] [PubMed] [Google Scholar]
- 11.Lodygensky GA, Rademaker KJ, Zimine S, et al. Structural and functional brain development after hydrocortisone treatment for neonatal chronic lung disease. Pediatrics. 2005;116(1):1–7. doi: 10.1542/peds.2004-1275. [DOI] [PubMed] [Google Scholar]
- 12.Allin M, Henderson M, Suckling J, et al. Effects of very low birthweight on brain structure in adulthood. Dev Med Child Neurol. 2004;46(1):46–53. doi: 10.1017/s0012162204000088. [DOI] [PubMed] [Google Scholar]
- 13.Ajayi-Obe M, Saeed N, Cowan FM, Rutherford MA, Edwards AD. Reduced development of cerebral cortex in extremely preterm infants. Lancet. 2000;356(9236):1162–1163. doi: 10.1016/s0140-6736(00)02761-6. [DOI] [PubMed] [Google Scholar]
- 14.Counsell SJ, Boardman JP. Differential brain growth in the infant born preterm: Current knowledge and future developments from brain imaging. Semin Fetal Neonatal Med. 2005;10:403–410. doi: 10.1016/j.siny.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Peterson BS, Anderson AW, Ehrenkranz RA, et al. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics. 2003;111(5 pt 1):939–948. doi: 10.1542/peds.111.5.939. [DOI] [PubMed] [Google Scholar]
- 16.Srinivasan L, Dutta R, Counsell S, et al. Quantification of deep gray matter in preterm infants at term-equivalent age using manual volumetry of 3-tesla magnetic resonance images. Pediatrics. 2007;119(4):759–765. doi: 10.1542/peds.2006-2508. [DOI] [PubMed] [Google Scholar]
- 17.Giménez M, Junque C, Vendrell P, et al. Abnormal orbitofrontal development due to prematurity. Neurology. 2006;67(10):1818–1822. doi: 10.1212/01.wnl.0000244485.51898.93. [DOI] [PubMed] [Google Scholar]
- 18.Peterson BS, Vohr B, Cannistraci CJ, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284(15):1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- 19.Fearon P, O’Connell P, Frangou S, et al. Brain volumes in adult survivors of very low birth weight: a sibling-controlled study. Pediatrics. 2004;114(2):367–371. doi: 10.1542/peds.114.2.367. [DOI] [PubMed] [Google Scholar]
- 20.Kostovic I, Jovanov-Milosevic N. The development of cerebral connections during the first 20-45 weeks’ gestation. Semin Fetal Neonatal Med. 2006;11:415–422. doi: 10.1016/j.siny.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional Development of the Brain in Early Life. Oxford: United Kingdom: Blackwell Scientific; 1967. pp. 3–70. [Google Scholar]
- 22.Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends Neurosci. 2006;29(3):148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lenroot RK, Gogtay N, Greenstein DK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36(4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children: a volumetric imaging study. Brain. 1996;119(pt 5):1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- 26.Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol. 2002;44(1):4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- 27.Vohr BR, Allan WC, Westerveld M, et al. School age outcomes of very low birth weight infants in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics. 2003;111(4 pt 1) doi: 10.1542/peds.111.4.e340. Available at www.pediatrics.org/cgi/content/full/111/4/e340. [DOI] [PubMed] [Google Scholar]
- 28.Ment LR, Oh W, Ehrenkranz RA, Philip AGS. Low dose indo-methacin and prevention of intraventicular hemorrhage: a multicenter randomized trial. Pediatrics. 1994;93(4):543–450. [PubMed] [Google Scholar]
- 29.Ment LR, Oh W, Ehrenkranz RA. Low dose indomethacin and extension of intraventricular hemorrhage: a multicenter randomized trial. J Pediatr. 1994;124(6):951–955. doi: 10.1016/s0022-3476(05)83191-9. [DOI] [PubMed] [Google Scholar]
- 30.Reiss AL, Kesler SR, Vohr BR, et al. Sex differences in cerebral volumes of 8-year-olds born preterm. J Pediatr. 2004;145(2):242–249. doi: 10.1016/j.jpeds.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 31.Okunseri C, Pajewski NM, McGinley EL, Hoffman RG. Racial/ethnic disparities in self-reported pediatric orthodontic visits in the United States. J Public Health Dent. 2007;67(4):217–223. doi: 10.1111/j.1752-7325.2007.00032.x. [DOI] [PubMed] [Google Scholar]
- 32.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence-Revised. New York, NY: Psychological Corporation Harcourt Brach Jovanovich; 1989. [Google Scholar]
- 33.Dunn LM, Dunn LM. Peabody Picture Vocabulary Test-Revised. Circle Pines, NM: American Guidance Service; 1981. [Google Scholar]
- 34.Reiss AL, Hennessey JG, Rubin M, Breach L, Abrams MT, Warsofsky IS. Reliability and validity of an algorithm for fuzzy tissue segmentation of MRI. J Comput Assist Tomogr. 1998;22:471–479. doi: 10.1097/00004728-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 35.Talairach J, Tournous P. Co-planar Stereotaxic Atlas of the Human Brain. New York, NY: Thieme Medical Publishers; 1988. [Google Scholar]
- 36.Kates WR, Warsofsky IS, Patwardhan A, Abrams MT, Liu A, Naidu S. Automated Talairach atlas-based parcellation and measurement of cerebral lobes in children. Psychiatry Res. 1999;91(1):11–30. doi: 10.1016/s0925-4927(99)00011-6. [DOI] [PubMed] [Google Scholar]
- 37.Schnack HG, van Haren NEM, Pol HEH, et al. Reliability of brain volumes from multicenter MRI acquisition: a calibration study. Hum Brain Mapp. 2004;22(4):312–320. doi: 10.1002/hbm.20040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giedd JN, Blumental J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 39.Durston S, Hulshoff Pol HE, Casey BJ, Giedd JN, Buitelaar JK, van Engeland H. Anatomical MRI of the developing human brain: what have we learned? J Am Acad Child Adolesc Psychiatry. 2001;40(9):1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6(3):309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 41.Boardman JP, Counsell SJ, Rueckert D, et al. Early growth in brain volume is preserved in the majority of preterm infants. Ann Neurol. 2007;62(2):185–192. doi: 10.1002/ana.21171. [DOI] [PubMed] [Google Scholar]
- 42.Vasileiadis GT, Gelman N, Han VKM, et al. Uncomplicated intraventricular hemorrhage is followed by reduced cortical volume at near-term age. Pediatrics. 2004;114(3) doi: 10.1542/peds.2004-0500. Available at www.pediatrics.org/cgi/content/full/114/3/e367. [DOI] [PubMed] [Google Scholar]
- 43.Kesler SR, Ment LR, Vohr B, et al. Volumetric analysis of regional cerebral development in preterm children. Pediatr Neurol. 2004;31(5):318–325. doi: 10.1016/j.pediatrneurol.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Constable RT, Ment LR, Vohr BR, et al. Prematurely born children demonstrate white matter microstructural differences at age 12 years relative to term controls: an investigation of group and gender effects. Pediatrics. 2008;121(2):306–316. doi: 10.1542/peds.2007-0414. [DOI] [PubMed] [Google Scholar]
- 45.Nosarti C, Giouroukou E, Healy E, et al. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain. 2008;131(pt 1):205–217. doi: 10.1093/brain/awm282. [DOI] [PubMed] [Google Scholar]
- 46.Dyet LE, Kennea NL, Counsell SJ, et al. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics. 2006;118(2):536–548. doi: 10.1542/peds.2005-1866. [DOI] [PubMed] [Google Scholar]
- 47.Inder TE, Huppi PS, Warfield S, et al. Periventricular white matter injury in the premature neonate is followed by reduced cerebral cortical gray matter volume at term. Ann Neurol. 1999;46(5):755–760. doi: 10.1002/1531-8249(199911)46:5<755::aid-ana11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 48.Aylward GP. Neurodevelopmental outcomes of infants born prematurely. Devel Behav Pediatr. 2005;26:427–440. doi: 10.1097/00004703-200512000-00008. [DOI] [PubMed] [Google Scholar]