SUMMARY
The structural basis for organizational heterogeneity of lipids and proteins underlies fundamental questions about the plasma membrane of eukaryotic cells. A current hypothesis is the participation of liquid ordered (Lo) membrane domains (lipid rafts) in dynamic compartmentalization of membrane function, but it has been difficult to demonstrate the existence of these domains in live cells. Recently, giant plasma membrane vesicles (GPMVs) obtained by chemically induced blebbing of cultured cells were found to phase separate into optically resolvable, coexisting fluid domains containing Lo-like and liquid disordered (Ld)-like phases as identified by fluorescent probes. In the present study, we used these GPMVs to investigate the structural bases for partitioning of selected lipids and proteins between coexisting Lo-like/Ld-like fluid phases in compositionally complex membranes. Our results with lipid probes show that the structure of the polar headgroups, in addition to acyl chain saturation, can significantly affect partitioning. We find that the membrane anchor of proteins and the aggregation state of proteins both significantly influence their distributions between coexisting fluid phases in these biological membranes. Our results demonstrate the value of GPMVs for characterizing the phase preference of proteins and lipid probes in the absence of detergents and other perturbations of membrane structure.
Keywords: lipid rafts, liquid order, liquid disorder, GPMVs, detergent-resistant membranes, RBL mast cells
INTRODUCTION
The cornerstone of the lipid raft hypothesis is the concept derived from model membranes that cell membranes segregate into two distinct fluid phases, liquid ordered (Lo) and liquid disordered (Ld). This is due to tighter packing of cholesterol with phospholipids containing long, saturated acyl chains (Lo) than with phospholipids containing two or more double bonds in their acyl chains (Ld) [1–6]. In cells under normal physiological conditions, these domains are likely sub-microscopic and dynamic. The partitioning of a given membrane component (protein or lipid) into an Lo domain will be governed by its intrinsic preference for the ordered environment, as well as by its interactions with other membrane constituents. Segregation of membrane components due to differential partitioning between coexisting fluid phases provides a basis for various physiological functions attributed to regulated compartmentalization and targeting by lipid rafts. Ample evidence supports the view that these rafts can serve as platforms for differential sorting of molecules that are critical for cellular processes such as stimulated signal transduction [1, 7–9], membrane trafficking [10, 11], cellular adhesion and motility [12], and viral entry into and budding from cells [13]. Raft association or exclusion of a host of proteins, either in native monomeric form or after stimulated clustering by exogenous ligands, can provide environments critical for their optimal function [7–9, 13].
Detailed information about the partitioning preferences of proteins and lipids is necessary to delineate the structural features and the intermolecular interactions that are critical for raft association. Although a number of fluorescence techniques have been used to study the lateral organization of cell membranes [14–19], the partitioning preferences of the fluorophores used to probe these complex biological membranes have not been well characterized. Although detergent-resistant membranes (DRMs) are commonly used as a crude measure of association with lipid rafts in live cells [20], it is well known that detergent extraction introduces a significant perturbation, such that the resulting DRMs cannot be equated to preexisting biological membrane structures [21–23].
Giant unilammelar vesicles (GUVs) have proven to be valuable research preparations [24–27], but these simple model membranes fail to capture the rich complexity of the plasma membrane and the multitude of protein-protein and protein-lipid interactions that take place in that milieu. A further limitation of GUVs is technical difficulties associated with reconstitution, such that partitioning of most membrane proteins between Lo and Ld is not readily evaluated. We recently observed that giant plasma membrane vesicles (GPMVs) obtained by chemically-induced blebbing of cells undergo phase separation into optically detectable coexisting fluid phases [28]. These GPMVs contain a large fraction of lipids and proteins from the plasma membrane and provide a compositionally rich biological membrane system to study the partitioning of lipids and proteins between coexisting fluid phases using fluorescence imaging microscopy. During their formation, GPMVs detach from the cytoskeleton and micron-scale phase separation is readily observed at temperatures lower than ~25°C. Native cell membranes at higher, physiological temperatures are likely to exhibit submicroscopic concentration fluctuations that lead to local differences in composition, related to Lo/Ld phase segregation [28]. These correlated concentration fluctuations may lead to larger scale phase separation and/or membrane reorganization that results from, for example, receptor aggregation by an exogeneous ligand and accompanies various physiological responses of the cell. Thus, the partitioning of membrane components between large coexisting fluid phases in GPMVs provides information about how these same molecules redistribute in the presence of nanoscopic heterogeneities in macroscopically homogeneous, live cell membranes.
In the present study we used GPMVs as a model system to systematically investigate the structural bases for protein and lipid partitioning and, thus, the general physical properties that target membrane components to particular lipid environments. We also evaluated the capacity of detergent extraction to discriminate between molecules preferring Lo- and Ld- like fluid phases. Our results reveal similarities to and some differences for Lo/Ld partitioning from those predicted by DRM fractionation of lysed cells, and they identify several Lo-preferring fluorescent lipid analogues in biological membranes.
MATERIALS AND METHODS
Lipid probes and chemicals
1,2-Dipalmitoyl-sn-glycero-3-phosphoethanolami-ne-N-(7-nitro-2-1,3-benzoxadiazol-4-yl) (NBD-DPPE), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(7-nitro-2-1,3-benzoxadiazol-4-yl) (NBD-DOPE), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(Lissamine Rhodamine B Sulfonyl) (Rh-DOPE), 1,2-dipalmitoyl-sn-glycero-3-phospho-ethanolamine-N-(Lissamine Rhodamine B Sulfonyl) (Rh-DPPE), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(carboxyfluorescein) (FITC-DOPE), N-[12-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]dodecanoyl]-sphingosine-1-phosphocholine (12-NBD-SM), 1-palmitoyl-2-[12-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]dodecanoyl]-sn-glycero-3-phosphocholine (12-NBD-PC), N-[12-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]dodecanoyl]-D-erythro-sphingosine (12-NBD-Ceramide), and 25-{N-[(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-methyl]amino}-27-norcholesterol (25-NBD-Cholesterol) were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). N-(fluorescein-5-thiocarbamoyl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine, triethylammoni-um salt (FITC-DPPE), N-[6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl]-sphingosine-1-phosphocholine (6-NBD-SM), and BODIPY® FL C5-ganglioside GM1 (Bodipy-GM1) were obtained from Invitrogen Molecular Probes (Eugene, OR). Unless otherwise indicated, all other chemicals were from Sigma-Aldrich (St. Louis, MO).
Antibodies
Purified O×7, mAb specific for rat glycosylphosphatidylinositol-linked Thy-1 (BD Pharmingen, San Diego, CA), was labeled with Cy3 (Amersham Pharmacia Biotech, Piscataway, NJ) according to manufacturer’s instructions. Purified AA4, mAb specific for the ganglioside acetylated GD1b (provided by Dr. R. Siraganian, NIH), and mouse monoclonal anti-2,4-dinitrophenyl (DNP) IgE were labeled with AlexaFluor488 (Invitrogen Molecular Probes, Eugene, OR) as previously described for IgE [29]. AlexaFluor488-cholera toxin subunit B (A488-CTB) was obtained from Invitrogen Molecular Probes. AlexaFluor488-AA4 and A488-CTB were added at concentrations of 1–2 μg/ml to GPMV or cell suspensions, and samples were incubated for 15 min at room temperature or on ice prior to imaging.
Fluorescent protein constructs
Vectors containing cDNA for GFP-GPI and YFP-GL-GPI were provided by J. Lippincott-Schwartz (NIH, Bethesda) and T. Baumgart (University of Pennsylvania, Philadelphia), respectively. Plasmids encoding monomeric YFP-GL-GPI (mYFP-GL-GPI) and monomeric YFP-GT46 (mYFP-GT46) were constructed from YFP-GL-GPI and YFP-GT46, respectively, by introducing the A206K mutation [19] using Quickchange site-directed mutagenesis kit (Stratagene, La Jolla, CA). The oligonucleotides 5′-mEYFP (CACTACCTGAGCTACCAGTCCAAACTGAGCAAAGACCCCAACGAG) and 3′-mEYFP (CTCGTTGGGGTCTTTGCTCAGTTTGGACTGGTAGCTCAGGTAGTG) (Integrated DNA Technologies, Coralville, IA) were used as the sense and antisense primers, respectively, and the mutation was verified by sequencing. LAT-EGFP was obtained from L. Samelson (NIH, Bethesda), YFP-GT46 (originally from Kai Simons, Max Planck Institute of Molecular Cell Biology and Genetics, Dresden) was from A. Kenworthy (Vanderbilt University School of Medicine, Nashville, TN), DsRed-PH and VSVG-EGFP were from Andreas Jeromin (U. Texas, Austin), CD63-EGFP was from Paul Roche (NIH), and flotillin-2-EGFP was from Lawrence Rajendran (Max Planck Institute, Dresden). LAT cDNA was excised from LAT-EGFP using the flanking restriction sites EcoRI and Kpn1 and ligated into monomeric AcGFP-N1 vector to obtain LAT-mEGFP.
Cell culture and transfection
RBL-2H3 cells were maintained in monolayer cultures for 3 to 5 days after passage as described [30] and washed twice with phosphate buffered saline (PBS) before being subjected to the blebbing procedure as described below. RBL cells were transiently transfected with the EGFP/DsRed constructs using GenePORTER (Gene Therapy Systems, San Diego), as previously described [29]. In some cases, RBL-2H3 monolayer cultures were incubated at 37°C with 0.5 μg/ml of Alexa488-IgE for 8 hours or with 1–2 μg/ml of Cy3-OX7 for 1 hour before GPMVs were induced.
Giant Plasma Membrane Vesicles
GPMVs were generated as described previously [28, 31]. In brief, monolayers of adherent, confluent RBL-2H3 cells in tissue culture flasks were washed twice with GPMV buffer (2 mM CaCl2, 10 mM Hepes, 150 mM NaCl, pH 7.4), and incubated with freshly prepared GPMV active reagent consisting of 25 mM formaldehyde and 2 mM dithiothreitol in GPMV buffer, for 1 hour at 37°C with gentle shaking (60 cycles per minute). GPMVs were then gently decanted into a conical tube. For microscopy experiments the tube was allowed to stand undisturbed in ice for 30–45 minutes to allow the larger GPMVs to sediment, and then 20% of the total volume was collected from the bottom of the tube. For this preparation, >75% of the GPMVs showed Lo-like/Ld-like phase separation at 15°C as indicated by selective probes [28]. The sucrose gradient experiments were performed using the entire preparation of GPMVs.
Labeling with lipid probes
To label GPMVs with fluorescent lipid analogues, GPMVs suspended in GPMV buffer was incubated with these probes in methanol for 5–10 minutes at 4°C. The final probe concentration was 2–8 μM, and methanol concentration was between 0.5 and 2 % (v/v). Identical partitioning results were obtained with an alternative protocol: adherent cells were washed twice with buffered salt solution (BSS: 135 mM NaCl, 5.0 mM KCl, 1.8 mM CaCl2, 1.0 mM MgCl2, 5.6 mM glucose, 20 mM HEPES, 1mg/mL BSA) and incubated with labeling solution at room temperature for 10 minutes. Labeling solution was prepared by mixing lipid probes in methanol with BSS. The final probe concentration varied between 1.0–5.0 μM, and methanol concentration was between 0.2% and 1% (v/v). Cells were then washed twice with GPMV buffer to remove the methanol and unincorporated probe, then incubated with GPMV reagents as described above to generate labeled GPMVs. It is possible that incorporated lipid probes could alter the phase separation observed, but lipid probe-free GPMVs labeled with fluorescent antibodies or generated from cells expressing EGFP/EYFP-labled endogeneous proteins also exhibit robust phase separation under identical conditions, indicating that the observed phase behavior is not dependent on the incorporated lipid probes.
Sucrose Gradient Analysis
Detergent lysed GPMVs were fractionated on sucrose step gradients using the procedure described previously [32] with some modifications. GPMVs were initially lysed at 4 × 106 cell equivalents/ml with a final concentration of 0.04% Triton X-100 (Pierce). The lysates were then diluted with an equal volume of 80% (wt/vol) sucrose to adjust to a final concentration of 40% sucrose. Sucrose step gradients were formed by the successive addition of 40% (containing the GPMV lysate), 30% and 10% sucrose. After centrifugation at 250,000 × g for 12–18 hours, gradients were divided into nine fractions. Fluorescence of isolated fractions was measured in an SLM 8100 fluorescence spectrophotometer (SLM instruments), and background signal due to buffer alone was subtracted.
Imaging
GPMVs were imaged using a Leica TCS SP2 spectral confocal system (Leica Microsystems Inc, Bannockburn, IL). GPMVs were added in the middle of a grease cage on a glass slide and sealed with a cover slip. The glass slide was placed on a temperature-controlled aluminum block, and the GPMVs were imaged with an upright objective (63x, 1.2NA, water, coverslip corrected). The sample temperature was measured near the sample chamber by means of a thermal couple (Bat-10, Physitemp). For each lipid probe or protein, ~5–15 phase separated GPMVs were used to characterize the phase preference, based on visual assessment of differences in fluorescence intensity of each probe in the two phases. Representative images are shown in Figures 1–7, and overall results are summarized in Tables 1 and 2. In principle, partition coefficients of lipid and protein fluorophores between coexisting Lo-like and Ld-like phases could be determined from fluorescence imaging, but uncertainties in the quantum yields and difficulties in accurately quantifying fluorescence intensities from the curved surfaces of the GPMVs make this very difficult.
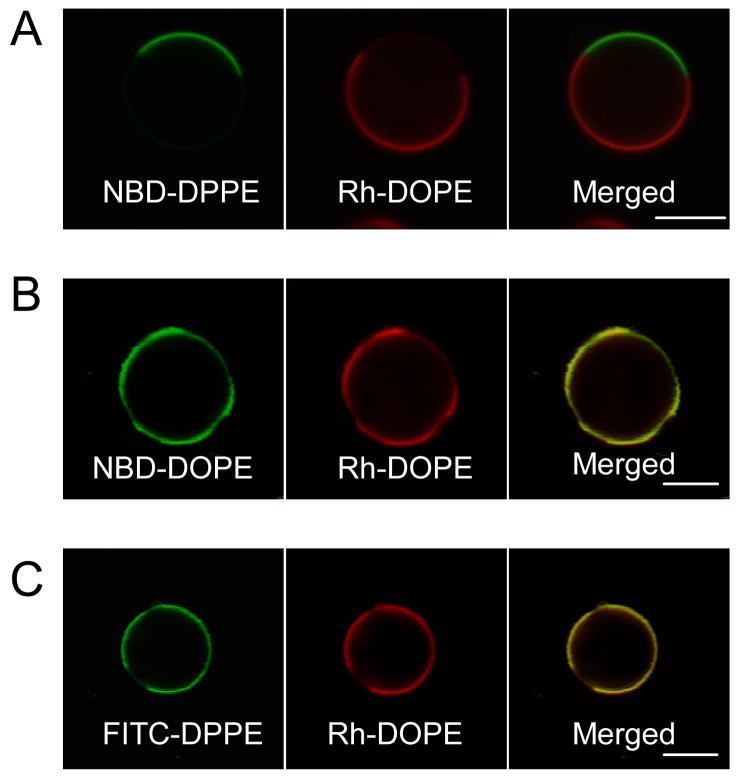
Figure 1.
Partitioning of headgroup-labeled phosphoglycerolipids in phase separated GPMVs. GPMVs were labeled subsequent to isolation from unlabeled cells. Equatorial (A, C) or tangential (B) sections of GPMVs colabeled with fluorescent lipid analogues NBD-DPPE, NBD-DOPE or FITC-DPPE, together with Rh-DOPE were imaged at 20°C. (A) Headgroup-labeled NBD-DPPE and Rh-DOPE exhibit complementary partitioning in GPMVs, while, (B) headgroup-labeled NBD-DOPE partitions similarly to Rh-DOPE. (C) FITC-DPPE, unlike NBD-DPPE, preferentially partitions into the same phase as Rh-DOPE. (Scale bars, 5 μm.)
Figure 7.
Fluorescence images of tangential (A) or equatorial sections (B–C) through GPMVs comparing the partitioning of transmembrane proteins with the Ld-preferring lipid probe Rh-DOPE in the presence of coexisting fluid phases in GPMVs. GPMVs were isolated from Rh-DOPE-labeled RBL cells, transiently expressing either VSVG-EGFP, LAT-EGFP, or LAT-mEGFP. (A) VSVG-EGFP partitions strongly into the Ld-like phase colabeled with Rh-DOPE, whereas (B) LAT-EGFP shows equal partitioning between coexisting fluid phases, and (C) LAT-mEGFP preferentially partitions into the Ld-like phase colabeled with Rh-DOPE. (Scale bars, 5 μm.).
Table 1.
Summary of Distributions of Labeled Lipids and Lipid Probes in GPMVs.
| Lipid probe | Partitioning* | Figure | |
|---|---|---|---|
| Headgroup labeled glycerophospholipids | NBD-DPPE | Lo# | 1 A |
| NBD-DOPE | Ld | 1 B | |
| FITC-DPPE | Ld | 1 C | |
| Rh-DPPE | Ld | - | |
| Rh-DOPE | Ld | 1–4 (and ref. 28) | |
| Acyl chain labeled glycerophospholipids and sphingolipids | 12-NBD-SM | Lo/Ld¶ | - |
| 12-NBD-Ceramide | Lo/Ld¶ | 2 A | |
| 12-NBD-PC | Ld | 2 C | |
| 6-NBD-SM | Lo# | 2 B | |
| Gangliosides | CTB-GM1 | Lo | 3 A |
| AA4-GD1b | Lo | 3 B | |
| Bodipy-GM1 | Lo§ | 3 C | |
| Fluorophore labeled cholesterol | FITC-Cholesterol | Ld | 4 A |
| 25-NBD-Cholesterol | Ld | 4 B |
Lo and Ld indicate preferential partitioning into Lo-like and Ld-like phases, respectively. For each lipid probe, ~10–15 GPMVs were examined and the difference in fluorescence intensity of these lipid probes between the two phases, identified using a second marker of known phase preference, was used to determine the partitioning preference. Ld-like phase-preferring probes exhibited stronger Ld-like preference in all the GPMVs examined, though the extent of Ld-like preference varied across the different GPMVs.
NBD-DPPE and 6-NBD-SM strongly partitioned into the Lo-like phase in 80–90 % of the GPMVs and partitioned more evenly between Lo-like and Ld-like phases in the rest.
12-NBD-SM and 12-NBD-ceramide evenly partitioned between Lo-like and Ld-like phase in ~80% of the GPMVs, and showed stronger Lo-like partitioning in the rest.
Bodipy-GM1 showed stronger Lo-partitioning in ~60% of the GPMVs, and even distribution between Lo and Ld phases in the rest.
Table 2.
Summary of Distributions of Labeled Proteins in GPMVs.
| Protein | Partitioning* | Figure | |
|---|---|---|---|
| GPI-anchored proteins | YFP-GL-GPI | Lo | 5 A |
| mYFP-GL-GPI | Lo | 5 B | |
| GFP-GPI | Lo | - | |
| Thy1 | Lo | (ref. 28) | |
| Inner leaflet-associated proteins | PH-DsRed | Ld | 6 A |
| Flotillin-2-EGFP | Ld | 6 B | |
| Lyn-EGFP | Ld | (ref. 28) | |
| Transmembrane proteins | VSVG-EGFP | Ld | 7 A |
| YFP-GT46 | Ld | - | |
| mYFP-GT46 | Ld | - | |
| CD63-EGFP | Ld | - | |
| LAT-EGFP | Lo/Ld# | 7 B | |
| LAT-mEGFP | Ld | 7 C | |
| IgE-FcεRI | Ld | (ref. 28) |
Lo and Ld indicate preferential partitioning into Lo-like and Ld-like phases respectively. Phase preference was determined by comparing the fluorescence intensity of the EGFP/YFP/DsRed-tagged proteins in the two phases. For each protein construct, 5–15 GPMVs were examined. Lo-like phase-preferring constructs partitioned preferably into Lo-like phase, and Ld-like phase-preferring constructs showed stronger Ld-like partitioning in all the GPMVs.
LAT-EGFP partitioned evenly between Lo-like and Ld-like phases in ~80% of the GPMVs, and showed slightly stronger partitioning into the Ld-like phase in the rest.
RESULTS
A large percentage of GPMVs generated from RBL cells undergo visible phase separation into coexisting fluid domains below 25°C, as revealed by probes that partition differentially between these two phases [28; Fig. 1–7 described below]. Cholera toxin B subunit bound to the ganglioside GM1 (CTB-GM1) is highly enriched in the DRM fraction of cells and is considered an archetypical raft marker [10]. We previously demonstrated that A488-labeled CTB-GM1 (A488-CTB-GM1) and Cy3-OX7-labeled Thy1, a GPI-anchored protein (Cy3-OX7-Thy1) partition strongly into the Lo-like phase in GPMVs, whereas Rh-DOPE preferentially labels the Ld-like phase [28]. We took advantage of these reference Ld and Lo markers to examine the relative partitioning of selected lipid and protein probes that represent distinctive structural features. Our results are summarized in Tables 1 and 2 and described below.
Partitioning of lipids (Table 1)
Headgroup labeled glycerophospholipids
To evaluate the effect of acyl chain structure on partitioning, we compared partitioning of NBD-DPPE and NBD-DOPE which have identical polar headgroups. NBD-DPPE has saturated C16-acyl chains whereas NBD-DOPE has unsaturated C18:1Δ9-acyl chains with a single double bond in each acyl chain. As shown in Fig. 1 (A, B), NBD-DPPE preferentially partitions into the Lo-like phase and shows complementary partitioning to Rh-DOPE, whereas NBD-DOPE partitions similarly to Rh-DOPE. Interestingly, unlike NBD-DPPE, FITC-DPPE, with two saturated C16 acyl chains, preferentially partition into the Ld-like phase (Figure 1 C). Similar results are obtained with Rh-DPPE (data not shown).
Acyl chain labeled glycerophospholipids and sphingolipids
DRMs and the Lo phase in model membranes are enriched in sphingolipids, glycosphingolipids and cholesterol. Long chain ceramides can also promote formation of gel (Lβ) and Lo domains [33] and can displace cholesterol from ordered domains in model membranes [34]. Ceramides generated in the plasma membrane by the hydrolysis of sphingomyelin by acid sphinomyelinease in T-cells, B-cells, and other cell types in response to stimulation have been proposed to form ordered domains critical for compartmentalized signal propagation and induction of apoptosis [35]. Thus, to gain insights into how sphingolipids partition into rafts and/or promote new raft domains during membrane remodeling and biogenesis, we examined NBD-SM, NBD-ceramide and NBD-PC in GPMVs. As shown in Fig 2 (A, B), 12-NBD-SM partitions equally between coexisting Lo-like and Ld-like phases, whereas 12-NBD-PC, with matched acyl chains, shows a higher preference for the Ld-like phase. 12-NBD-ceramide exhibits partitioning between the two fluid phases similar to that for 12-NBD-SM (Fig. 2C). Somewhat surprisingly, 6-NBD-SM partitions preferentially into the Lo-like phase in GPMVs (Fig. 2 D).
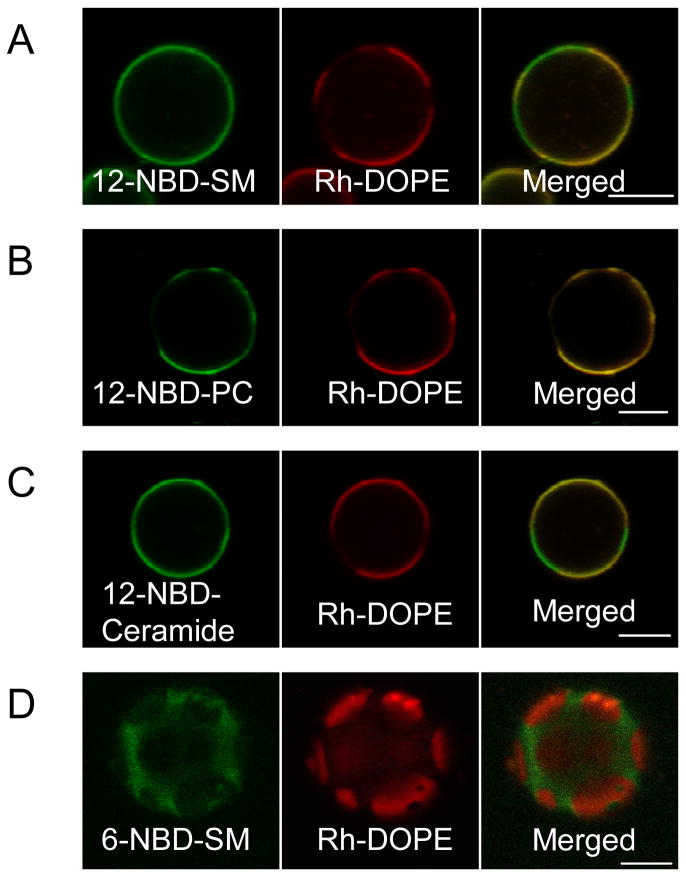
Figure 2.
Partitioning of acyl chain-labeled lipid analogues in coexisting fluid phases in GPMVs. Equatorial (A–C) or tangential (D) sections of GPMVs, colabeled after formation, with Rh-DOPE and 12-NBD-SM, 12-NBD-PC, 12-NBD-Ceramide, or 6-NBD-SM and imaged at 20°C. (A–B) Acyl-chain labeled 12-NBD-SM and 12-NBD-PC exhibit differential partitioning in GPMVs: NBD-SM partitions equally between Lo-like and Ld-like phases (A), whereas, NBD-PC shows similar partitioning as Rh-DOPE (Ld-phase) (B). (C) Acyl chain labeled 12-NBD-Ceramide exhibits equal partitioning between Lo-like and Ld-like phases. (D) Acyl chain labeled 6-NBD-SM prefers the Lo-like phase and exhibits partitioning complementary to Rh-DOPE. (Scale bars, 5 μm.)
Gangliosides
In some experiments, GPMVs were generated from cells pre-labeled with lipid probes and antibodies. In other experiments, we found the partitioning of lipid probes and antibody-bound gangliosides to be identical when GPMVs were labeled after isolation from cells (see Materials and Methods), indicating that the small amount of methanol present during GPMV labeling with lipid probes did not alter the membrane phase behaviour. We compared the endogenous gangliosides, GM1 and acetylated GD1b, using A488-CTB and a specific monoclonal antibody, AA4, respectively. Both A488-CTB-GM1 and Alexa 488-AA4 bound to acetylated GD1b (A488-AA4-GD1b) show a strong preference for the ordered Lo-like phase in GPMVs (Fig. 3A, B). Exogenously added bodipy-C5-GM1, however, partitions preferentially into the Lo-like phase in ~60% of the GPMVs (Fig. 3 C) and exhibits approximately similar partitioning between the two fluid phases in the rest, with a slight preference for Lo-like over Ld-like phases.
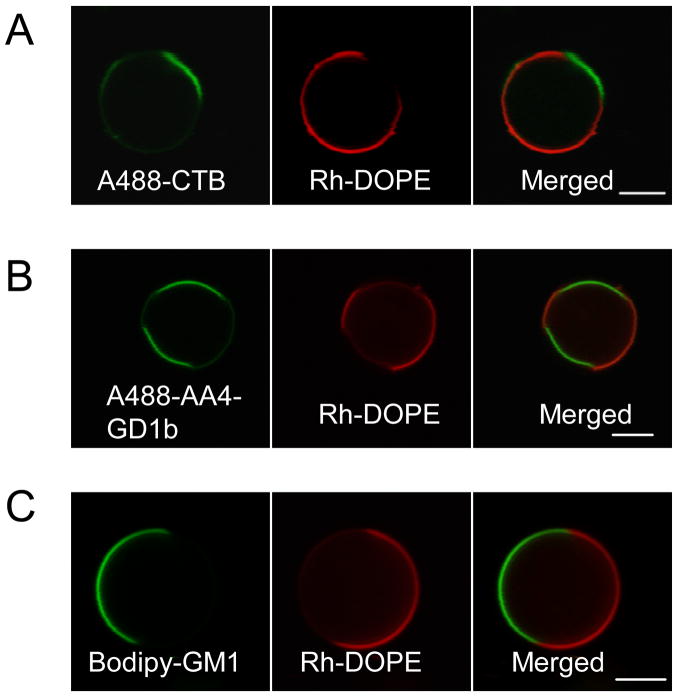
Figure 3.
Partitioning of glycosphingolipids between coexisting fluid phases in GPMVs. GPMVs were generated from cells pre-labeled with Rh-DOPE and either A488-CTB (A), A488-AA4 (B) or bodipy-C5-GM1 (C). A488-CTB bound to GM1 (A), A488-AA4 bound to acetylated GD1b (B), and acyl chain-labeled Bodipy-C5-GM1 (C) prefer the Lo-like phase and shows partitioning complementary to Rh-DOPE in GPMVs. (Scale bars, 5 μm.)
Fluorophore labeled cholesterol
Cholesterol is an essential component of the Lo phase. GPMVs have a phospholipid:cholesterol ratio of 2:1, and about 30% of the phospholipids are detergent insoluble [36]. Comparisons between GPMVs and simpler model systems strongly suggest that cholesterol should be concentrated in the Lo-like phase of the GPMVs, although this has not been formally demonstrated. Furthermore, DRMs isolated from mammalian cells are characteristically cholesterol- and sphingolipid-rich, with highly ordered, Lo-like phase properties [36, 37]. We find that both headgroup-labeled FITC-Cholesterol and alkyl chain labeled 25-NBD-Cholesterol partition strongly into Ld-like phase (Fig. 4). The presence of FITC is expected to disrupt the packing of cholesterol with neighboring lipids at the membrane–water interface (“umbrella model”) [38]. Similarly, the NBD fluorophore attached to the alkyl chain of cholesterol appears not to be accommodated by the restrictive environment of cholesterol in the ordered Lo-like phase. Thus, neither of the fluorophore-labeled cholesterol probes behave like unmodified cholesterol in phase-separated GPMVs. This suggests that the partitioning of cholesterol into the ordered phase is very sensitive to the structure of the cholesterol derivative, including both the small headgroup and the rigid and flat hydrophobic portion.
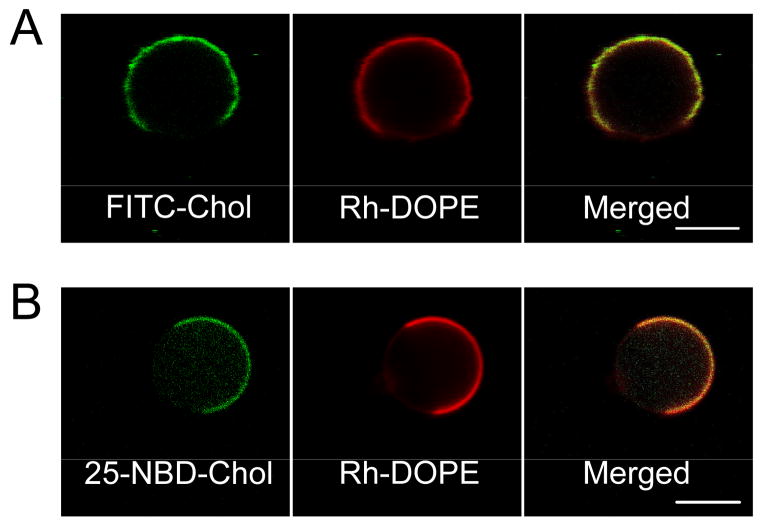
Figure 4.
Fluorescence images depicting equatorial confocal sections of GPMVs labeled with Rh-DOPE and the cholesterol analogues FITC-Cholesterol or 25-NBD-Cholesterol after isolation from cells. Both (A) headgroup labeled FITC-Cholesterol, and (B) alkyl chain labeled 25-NBD-Cholesterol, exhibit similar partitioning into Ld-like phase similar to Rh-DOPE in phase separated GPMVs. (Scale bars, 5 μm.)
Partitioning of proteins (Table 2)
GPI-linked proteins
We previously reported that the GPI-AP, Thy-1, strongly partitions into the Lo-like phase, with a partition coefficient K (Lo-like/Ld-like) greater than 1 [28]. To extend our characterization, we examined the partitioning of endogenously labeled GPI anchored proteins in GPMVs. YFP-GL-GPI has YFP attached to consensus N-glycosylation site fused to the GPI-attachment sequence of lymphocyte-function-associated antigen 3 [39], and it exhibits strong partitioning into the Lo-like phase in GPMVs (Fig. 5 A). Because YFPs have a tendency to dimerize at high concentrations [19], we constructed mYFP-GL-GPI, a monomeric version of YFP-GL-GPI. As shown in Fig. 5 B, mYFP-GL-GPI also strongly partitions into the Lo-like phase in GPMVs, indicating that YFP dimerization is not causing this result.
Figure 5.
Partitioning of GPI-anchored proteins between coexisting fluid phases in GPMVs. GPMVs were generated from cells transiently expressing YFP-GPI or mYFP-GL-GPI and subsequently labeled with Rh-DOPE. Confocal images of equatorial (A) or tangential sections (B) through GPMVs comparing the partitioning preference of GPI-anchored proteins with the Ld-marker Rh-DOPE. (A) YFP-GL-GPI is strongly enriched in the Lo-like phase in GPMVs while Rh-DOPE labels the Ld-like phase, and (B) mYFP-GL-GPI partitions preferentially into the Lo-like phase, complementary to the Rh-DOPE labeled Ld-like phase. (Scale bars, 5 μm.)
Inner leaflet-associated proteins
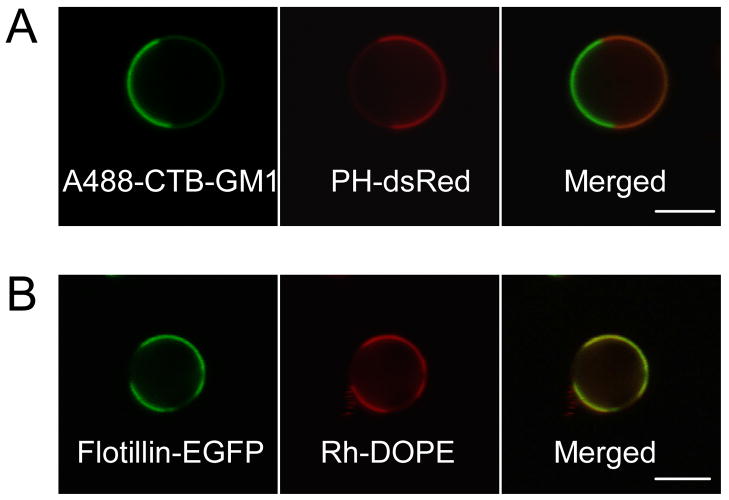
The DsRed-PH construct localizes to the inner leaflet of the plasma membrane via the PH domain of PLCδ that binds with high selectivity to phosphtidylinositol 4,5 biphosphate (PIP2) [40]. PIP2 is known to modulate membrane-cytoskeletal adhesion and cytoskeletal reorganization, and it plays an important role in regulating vesicle trafficking and signaling events [41, 42]. Although PIP2 was initially reported to be enriched in DRMs [43], recent electron microscopy and FRET experiments showed that PLCδ PH-EGFP bound to PIP2 is homogeneously distributed in the inner leaflet of the plasma membrane, but treatment of cells with very dilute concentration of Triton-X 100 can lead to clustering of PIP2 [44]. In GPMVs, DsRed-PH is enriched in the phase that is complementary to CTB-GM1, indicating that PIP2 bound to this cytoplasmic protein preferentially partitions into the Ld-like phase (Fig. 6A).
Figure 6.
Partitioning of fluorescently labeled inner leaflet-associated proteins in phase-separated GPMVs. GPMVs were labeled either with A488-CTB or Rh-DOPE after isolation from cells transiently expressing PH-dsRed or flotillin-2-EGFP. (A) PH-dsRed shows partitioning complementary to Lo marker A488-CTB, and, (B) flotillin-2-EGFP cosegregates with the Ld marker Rh-DOPE. (Scale bars, 5 μm.)
Flotillins associate with the cytoplasmic leaflet of the membrane, co-isolate with DRMs, and have been implicated in raft-related signaling and trafficking [45, 46]. Flotillin-2 has been shown to associate with GPI-linked proteins in the plasma membrane of T-cells and neurons and to cocluster with T-cell receptors when T-cells are activated by crosslinking GPI-linked proteins or GM1 [47]. As shown in Fig. 6B, flotillin-2-EGFP preferentially partitions into the Ld-like phase in GPMVs. These results are consistent with previous results for lipid anchored, inner leaflet proteins including Lyn kinase [28], and they indicate an interesting discrepancy between DRM association in lysed cells and Lo-like partitioning in GPMVs. The basis for this difference is considered in the Discussion.
Integral membrane proteins
Several integral membrane proteins, including transmembrane domains of influenza hemagglutinin, neuraminidase, CD40, LAT, and HIV-envelope are found to associate with DRMs and are thought to partition into ordered domains in plasma membranes [4, 48–50]. Protein structure, protein-protein interactions, or lipid-protein interactions may account for these findings [45, 49, 51].
Vesicular stomatitis virus G protein (VSVG) was shown to be detergent soluble when extracted with cold nonionic detergent like Triton X-100 and to traffic to the basolateral surface of polarized epithelial cells [48, 52]. In other studies, VSVG was found to copatch with raft markers when crosslinked with antibodies [39], and this protein was found to pseudotype with other enveloped viruses, a process believed to involve localization to lipid rafts [48]. We find that VSVG-EGFP strongly partitions into the Ld-like phase marked with Rh-DOPE in GPMVs (Fig 7 A). Other transmembrane proteins we tested, CD63-EGFP, LYFP-GT46 and monomeric LYFP-GT46 (mLYFP-GT46), also partition strongly into the Ld-like phase (data not shown).
LAT plays an important role as an adaptor protein during signal transduction in RBL mast cells and T cells [7, 8, 50]. We find that LAT-EGFP shows similar partitioning between Lo-like and Ld-like phases (Fig. 7 B), consistent with partitioning following detergent lysis of cells [52]. We tested a LAT-monomeric EGFP construct (LAT-mEGFP) which partitioned preferably into the Ld-like phase in GPMVs (Fig. 7 C). These contrasting results suggest aggregation plays a role in the partitioning behavior of this protein.
Sucrose Gradient Analysis of detergent extracted GPMVs
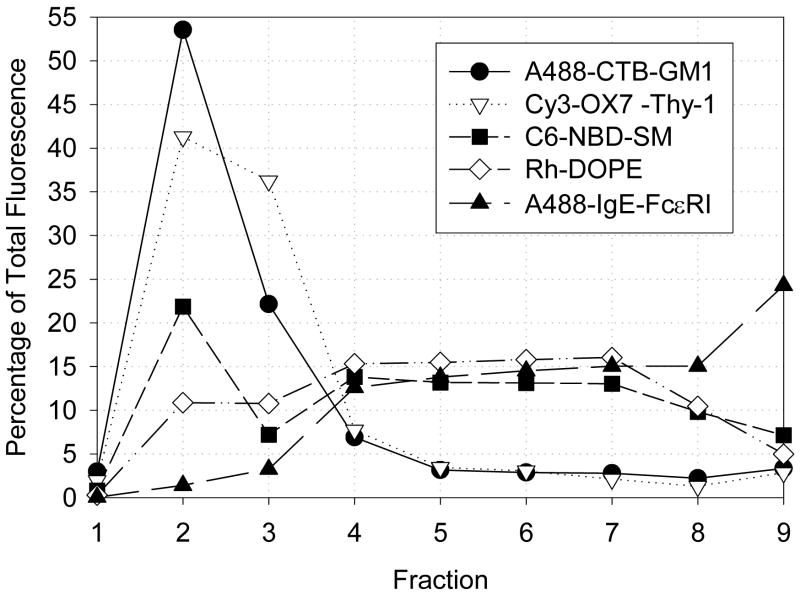
Because association of a particular membrane component (protein or lipid) with lipid rafts is commonly inferred from association with DRMs derived from whole cells following sucrose gradient fractionation [1, 3, 4, 20], we investigated this relationship for GPMVs. As described above, we find that A488-CTB-GM1 [28] and 6-NBD-SM (Fig. 2 D) strongly partition into the Lo-like phase, whereas Rh-DOPE preferentially partitions into the Ld-like phase in phase-separated GPMVs. Cy3-OX7-Thy1 exhibits strong preference for the Lo-like phase, whereas A488-IgE bound to FcεRI (A488-IgE-FcεRI) preferentially partitions into the Ld-like phase in GPMVs [28]. Sucrose gradient analysis was performed on GPMVs after treatment with cold Triton X-100 (0.04%) under conditions in which DRMs are recovered in fractions 1–3. As shown in Fig. 8, Lo-like phase-preferring A488-CTB-GM1 and Cy3-Ox7-Thy1 strongly associate with DRMs under these given conditions, such that 75–80 % of these components are present in fractions 1–3. In contrast, Rh-DOPE and monomeric A488-IgE-FcεRI primarily fractionate with the solubilized membrane components in fractions 4–8, with only ~20–25% of Rh-DOPE and ~5–10% of A488-IgE-FcεRI present in DRM fractions, as expected from previous studies of IgE-FcεRI on cells [7, 32]. 6-NBD-SM, which preferentially partitions into the Lo-like phase in GPMVs, shows an intermediate sensitivity to detergent extraction, with ~40–45% of the lipid probe being associated with DRM fractions. Thus, visible partitioning of these particular components into the Lo-like phase of GPMVs correlates qualitatively with their co-fractionation with DRMs prepared from GPMV samples.
Figure 8.
Distributions of antibody-labeled proteins and lipid probes associated with GPMVs, following lysis of GPMVs with 0.04% TritonX-100 and sucrose gradient fractionation. In this representative experiment, percentages of total fluorescence are shown for A488-CTB-GM1 (filled circles), Cy3-OX7-Thy1 (open triangles), 6-NBD-SM (filled squares), Rh-DOPE (open diamonds), and A488-IgE-FcεRI (filled triangles) in different fractions. Fractions 2–3 (10–20 % sucrose) include DRMs, whereas fractions 4–8 (~40% sucrose) contain solubilized proteins and lipids.
DISCUSSION
Partitioning of fluorescent lipid analogues
Our comparative investigation with phase separated GPMVs shows that the chemical nature of the phospholipid headgroups, as well as the degree of acyl chain saturation, can influence Lo partitioning. NBD-DPPE is the only fluorescent phosphatidylglycerol lipid examined in this study that preferentially partitions into the Lo-like phase; all others exhibit preferential enrichment in the Ld-like phase (Fig. 1, Table 1). Packing of cholesterol with the all-trans extended conformation of long, saturated acyl chains of sphingolipids and saturated phosphatidylglycerolipids is energetically more favorable than packing with unsaturated acyl chains, and this contributes significantly to close packing in the ordered Lo phase [53, 54]. Correspondingly, NBD-DOPE is not accommodated in the Lo-like phase. The partitioning of these lipid probes is consistent with results obtained in model membranes [20, 24, 55, 56], and provides direct evidence that the acyl chain structure is a key determinant in the partitioning of lipids between coexisting fluid phases in compositionally complex biological membranes. This result furthermore supports our previous FRET studies on live cells which showed that carbocyanine lipid probes with different alkyl chains laterally segregate in the plasma membrane [57].
The stronger preference of 12-NBD-SM and 12-NBD-ceramide for Lo-like phase compared to that of 12-NBD-PC (Fig. 2, Table 1) suggests that the sphingosine backbone confers a preference for the Lo-like phase, consistent with model membrane studies [58], and this may be responsible for the high sphingosine content of DRMs isolated from cells [59]. According to the “umbrella model”, proposed by Feigenson [38], the tight packing of cholesterol with sphingolipids might be energetically favorable because the large headgroups of neighboring sphingolipids can serve to compensate for the small polar headgroup (-OH) of cholesterol and thereby shield the hydrophobic sterol rings from the aqueous phase. Furthermore, the amide group of SM has the capacity to donate hydrogen bonds [4], such that SM, unlike PC, can potentially create a more extensive network of hydrogen bonds.
The nature of the polar headgroups and their interaction with the local membrane environment also influences the partitioning behavior (Fig. 1, Table 1). Our results are consistent with those of Dietrich et. al., who showed that NBD-DPPE significantly partitions into the Lo phase, whereas Rh-DPPE and FITC-DPPE are excluded from this phase in supported POPC/cholesterol/sphingomyelin (2:1:1) lipid monolayers [24]. The bulky FITC and Rh fluorophores can apparently disrupt favorable interactions in other parts of the labeled lipids. Solvent molecules around the polar headgroups in the membrane-water interface of the Lo-like phase are probably substantially more ordered than those of the Ld-like phase [60] and the smaller NBD fluorophore would introduce a smaller perturbation to this ordered environment. Additionally, it has been suggested that the magnitude of the dipole moment of the polar headgroup and its orientation relative to the dipolar potential of the lipid phase can significantly affect the partitioning of amphiphiles between Ld and Lo phases [60]. Thus, differential interactions of the fluorophore dipole moments might also contribute to the observed differences in partitioning.
Our observation that 6-NBD-SM partitions preferentially into Lo-like phase, in contrast to the even distribution of 12-NBD-SM between the two phases, probably reflects the differences in the location of the polar NBD moiety in the membrane. The small C6-acyl chain can flip upwards and the NBD-fluorophore in 6-NBD-SM might locate preferentially near the membrane–water interface [61]. In this case, 6-NBD-SM in GPMVs might have the configuration of a lysophospholipid with an effectively big polar headgroup comprising the choline of SM and the polar NBD fluorophore. The membrane-water interfacial environment and interactions of 6-NBD-SM is then expected to be significantly different from those of 12-NBD-SM, resulting in differential partitioning. The effectively bigger headgroup of 6-NBD-SM would also be expected to shield cholesterol molecules more efficiently [38]. Our results suggest that headgroup-labeled lysophospholipids may show similar partitioning in GPMVs. Lysophospholipids can serve as ligands for certain G-protein coupled signaling processes and cell-cell interactions, and the membrane distribution of these lysophospholipids may be relevant to the spatial organization of these processes [62]. It has been recently shown that exogeneously added alkyl-lysophospholipids accumulate in rafts and can trigger apopotosis following endocytosis [63].
Gangliosides strongly partition into the Lo-like phase in GPMVs
Because CTB is pentameric and presumably crosslinks GM1, it is possible that oligomerization of GM1 by CTB has some effect on the pronounced Lo-like preference we observe for Alexa488 CTB-GM1 in GPMVs [28]. Consistent with this view, Bacia et. al recently reported that the ganglioside GM1 preferentially partitions into Ld in model membranes, but migrates to Lo on binding CTB [26]. However, GM1 often fractionates with DRMs in the absence of CTB [64] indicating a preference for an Lo environment on cells even in the absence of CTB crosslinking. Furthermore, the mAb AA4 can only form dimers of acetylated GD1b gangliosides, and yet strong Lo-like partitioning is observed (Fig. 3).
Headgroup-labeled N-Bodipy-GM1 preferentially partitions into the Lo phase in model membranes [65], and we find that acyl chain-labeled bodipy-C5-GM1 partitions either more strongly into the Lo-like phase in GPMVs (~60% of the GPMVs) or equally between Lo-like and Ld-like phases. These results indicate that the core GM1 structure has an intrinsically strong preference for the Lo-like phase. Specific interactions mediated by the sugar residues of this ganglioside in its headgroup region may strengthen its Lo-like partitioning, and additional interactions may be provided by the sphingosine backbone. Consistent with our results, the presence of a bulky fluorophore in the backbone would be expected to perturb the packing of lipid acyl chains and thus decrease the partitioning of bodipy-C5-GM1 into Lo-like domains.
GPI-anchored proteins show strong preference for the Lo-like phase in GPMVs
Lipid-anchored membrane proteins with saturated acyl chains, including GPI-anchored proteins (GPI-APs) and dual palmitoylation or tandem myristoylation and palmitoylation-anchored proteins generally associate with DRMs [20, 66], indicating that acyl chain order of lipidated proteins appears to be a key determinant for partitioning into lipid rafts. In reconstituted lipid vesicles, GPI-linked human placental alkaline phosphatase (PLAP) was found in DRMs [20]. In contrast, Thy-1 and PLAP were found to partition preferentially (60–80%) into the Ld-phase in model membrane monolayers and GUVs, respectively [67, 27], although Lo-partitioning was increased by polyclonal antibody-mediated crosslinking of these proteins. We used Cy3-labeled mAb (Cy3-OX7) specific for Thy-1 to examine its partitioning in GPMVs, and we found a high degree of Lo-like partitioning [28]. In comparison, mYFP-GL-GPI was directly visualized without the need for a labeling antibody, and strong Lo-like phase partitioning was observed in the absence of crosslinking.
This strong partitioning of GPI-APs into the Lo-like phase in GPMVs suggests that partitioning of proteins between coexisting fluid phases in biological membranes can be different from model membranes composed of 3 or 4 components. Lo phases in synthetic lipid mixtures are likely to be more compact than in plasma membrane vesicles of complex composition and consequently more restrictive towards accommodating proteins and disordered lipids [37]. Specific protein-protein and protein-lipid interactions can also influence partitioning in biological membranes. Thus the partitioning of proteins in GPMVs is likely to reflect more accurately their properties in the plasma membrane of live cells.
Inner leaflet-anchored proteins partition into the Ld-like phase
Both inner leaflet-associated proteins examined in this study, PH-dsRed and flotillin-2-EGFP, partition preferentially into the Ld-like phase in GPMVs, and this is consistent with our previous results with Lyn-EGFP, PM-EGFP and GG-EGFP [28]. These results contrast with findings that these lipid anchored proteins associate substantially with DRMs derived from whole cells [68; P.S., unpublished results]. Although the GFP-module might influence the partitioning of these inner leaflet anchored proteins, the substantial Lo-like partitioning of YFP-GL-GPI (Fig. 5) and LAT-EGFP in GPMVs (Fig. 8 B) indicate that the GFP-moiety does not prevent Lo-like phase association. Recovery of these inner leaflet-associated proteins in DRMs from whole cells, and colocalization of another, Fyn, with outer leaflet raft markers in co-crosslinking experiments [39] suggest that they exhibit some preference for Lo domains in live cell membranes.
This apparent discrepancy in partitioning observed in cells compared to GPMVs for the inner leaflet-associated proteins points to the possibility that loss of cytoskeletal attachment or leaflet asymmetry in GPMVs [28] may have a significant effect. In this regard, the extent of plasma membrane lipid remodeling during the formation of GPMVs is unknown, and the organization of some membrane components may be affected. It is likely that the inner leaflet-associated proteins and some transmembrane proteins might be particularly sensitive, leading to different distributions between coexisting phases in GPMVs as compared to regulated plasma membranes of intact cells.
Partitioning of transmembrane proteins is sensitive to oligomerization
Most transmembrane integral proteins are not expected to be readily accommodated in the ordered lipid environment of lipid rafts, based on experimental [3, 4] and theoretical considerations [69]. The transmembrane proteins examined in this study (Table 2), with the exception of LAT-EGFP, strongly partition into the Ld-like phase in GPMVs. Recently, a peptide corresponding to the transmembrane domain of LAT was found to partition strongly to the Ld phase in model membrane GUVs [70]. We found that LAT-mEGFP, unlike LAT-EGFP, preferentially partitions into the Ld-like phase in GPMVs. GFPs are known to dimerize at high concentrations [19], and the difference in partitioning for the two LAT constructs can be rationalized on the basis of their different aggregation states. Crosslinking and clustering of proteins is a critical step in various membrane-associated processes, including signal transduction in immune cells, where clustering of transmembrane receptors often leads to large scale membrane reorganization and recruitment of downstream signaling components. The mechanisms involved can include changes in partitioning and membrane localization of clustered molecules, and stabilization of membrane domains [7–9]. For example, in RBL-mast cells, considerable evidence supports the view that crosslinking of IgE receptors leads to their stable association with ordered domains where they have increased probability of encountering functionally active Lyn kinase sequestered from transmembrane phosphatases [71].
Palmitoylation of two cysteine residues near the cytoplasm–membrane interface was shown to cause DRM association of LAT and to enable its function as a signaling adaptor [49]. An increase in the number of palmitoylated cysteine residues per molecular cluster upon oligomerization of LAT caused by EGFP in the cells prior to GPMV formation might be responsible for the enhanced Lo-like preference of LAT-EGFP. Similarly, protein-protein interactions mediated by the intracellular domain of wild type LAT during the course of cell activation might change its oligomerization state and lead to its association with the Lo phase. Future experiments will determine whether specific transmembrane amino acid residues contribute to Lo-partitioning of LAT and other transmembrane proteins in cells and GPMVs.
Sucrose Gradient Analysis of detergent extracted GPMVs correlates with partitioning in GPMVs
The correlation between partitioning of membrane components into Lo domains in live cells and the recovery of specific molecules in DRMs is a matter of controversy. The micron-scale fluid-fluid phase separation in GPMVs allows unambiguous assignment of partitioning preference for proteins and lipids in a detergent-free, complex biological membrane. Subsequent detergent extraction of phase separated GPMVs allowed us to relate directly the partitioning of proteins and lipids between coexisting Lo-like/Ld-like fluid phases and their presence in DRMs. The correlation observed confirms that detergent resistance provides useful information about the tendencies of membrane constituents to associate with Lo domains. The correlation is not quantitative, as indicated by the strong Lo-like preference of C6-NBD-SM visualized with confocal microscopy compared to the more moderate DRM association.
Conclusions
GPMVs enable new insights into how lipids and proteins laterally segregate into regions of different order in the complex milieu of the plasma membrane. We found that the phase preference of a lipid molecule is determined by a complicated interplay between the headgroup and its acyl chains and their interactions with their immediate environment. In this manner, subtle changes in molecular interactions may shift the phase preference of a probe. Our characterization of the phase-preference of fluorescent lipid analogues in GPMVs provides valuable information for selecting probes for studying lateral heterogeneities in live cell membranes, as well as for developing and fine tuning new fluorescent probes. GPMVs also provide a convenient model system to examine a wide range of plasma membrane proteins. Our results show that a number of factors, including the nature of the membrane anchor, the oligomerization state of proteins, and location of the protein at inner versus the outer leaflet, can significantly influence partitioning between different environments in biological membranes.
Acknowledgments
We thank Drs. G. Feigenson and T. Baumgart for helpful discussions. This work was supported by the Nanobiotechnology Center (National Science Foundation ECS-9876771) and by National Institutes of Health grant AI22449.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simons K, Ikonen E. Functional rafts in cell membrane. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 2.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 3.Edidin M. The state of lipid rafts: from model membranes to cells. Annu Rev Biophys Biomol Struct. 2003;32:257–283. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- 4.Simons K, Vaz WL. Model systems, lipid rafts, and cell membranes. Annu Rev Biophys Biomol Struct. 2004;33:351–378. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- 5.Silvius JR. Role of cholesterol in lipid raft formation: lessons from lipid model systems. Biochim Biophys Acta. 2001;1610:174–183. doi: 10.1016/s0005-2736(03)00016-6. [DOI] [PubMed] [Google Scholar]
- 6.Brown DA, London E. Structure and function of sphingolipid and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 7.Holowka D, Gosse JA, Hammond AT, Han X, Sengupta P, Smith NL, Wagenknecth-Wiesner A, Wu M, Young RM, Baird B. Lipid segregation and IgE receptor signaling: a decade of progress. Biochim Biophys Acta. 2005;1746:252–259. doi: 10.1016/j.bbamcr.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Kabouridis PS. Lipid rafts in T cell receptor signaling. Mol Membr Biol. 2006;23:49–57. doi: 10.1080/09687860500453673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pierce SK. Lipid rafts and B-cell activation. Nat Rev Immunol. 2002;2:96–105. doi: 10.1038/nri726. [DOI] [PubMed] [Google Scholar]
- 10.Parton RG, Richards AA. Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic. 2003;4:724–738. doi: 10.1034/j.1600-0854.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 11.Salaun C, James DJ, Chamberlain LH. Lipid rafts and the regulation of exocytosis. Traffic. 2004;5:255–264. doi: 10.1111/j.1600-0854.2004.0162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manes S, Viola A. Lipid rafts in lymphocyte activation and migration. Mol Membr Biol. 2006;23:59–69. doi: 10.1080/09687860500430069. [DOI] [PubMed] [Google Scholar]
- 13.del Real Manes SG, Martinez-A C. Pathogens: raft hijackers. Nat Rev Immunol. 2003;3:557–568. doi: 10.1038/nri1129. [DOI] [PubMed] [Google Scholar]
- 14.Pralle A, Keller P, Florin EL, Simons K, Horber JK. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J Cell Biol. 2000;148:997–1008. doi: 10.1083/jcb.148.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas JL, Holowka D, Baird B, Webb WW. Large-scale co-aggregation of fluorescent lipid probes with cell surface proteins. J Cell Biol. 1994;125:795–802. doi: 10.1083/jcb.125.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hao M, Mukherjee S, Maxfield FR. Cholesterol depletion induces large scale domain segregation in living cell membranes. Proc Natl Acad Sci U S A. 2001;98:13072–13077. doi: 10.1073/pnas.231377398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kusumi A, Nakada C, Ritchie K, Murase K, Suzuki K, Murakoshi H, Kasai RS, Kondo J, Fujiwara T. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu Rev Biophys Biomol Struct. 2005;34:351–378. doi: 10.1146/annurev.biophys.34.040204.144637. [DOI] [PubMed] [Google Scholar]
- 18.Sharma P, Verma R, Sarasji RC, Ira, Gousset K, Krishnamoorthy G, Rao M, Mayor S. Nanoscale Organization of Multiple GPI-Anchored Proteins in Living Cell Membranes. Cell. 2004;116:577–589. doi: 10.1016/s0092-8674(04)00167-9. [DOI] [PubMed] [Google Scholar]
- 19.Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–916. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- 20.Schroeder R, London E, Brown D. Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc Natl Acad Sci U S A. 1994;91:12130–12134. doi: 10.1073/pnas.91.25.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lichtenberg D, Goni FM, Heerklotz H. Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem Sci. 2005;30:430–436. doi: 10.1016/j.tibs.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Heerklotz H. Triton promotes domain formation in lipid raft mixtures. Biophys J. 2002;83:2693–2701. doi: 10.1016/S0006-3495(02)75278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heerklotz H, Szadkowska H, Anderson T, Seelig J. The sensitivity of lipid domains to small perturbations demonstrated by the effect of Triton. J Mol Biol. 2003;329:793–799. doi: 10.1016/s0022-2836(03)00504-7. [DOI] [PubMed] [Google Scholar]
- 24.Dietrich C, Bagatolli LA, Volovyk ZN, Thompson NL, Levi M, Jacobson K, Gratton E. Lipid rafts reconstituted in model membranes. Biophys J. 2001;80:1417–1428. doi: 10.1016/S0006-3495(01)76114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammond AT, Heberle FA, Baumgart T, Holowka D, Baird B, Feigenson GW. Crosslinking a lipid raft component triggers liquid ordered-liquid disordered phase separation in model plasma membranes. Proc Natl acad Sci U S A. 2004;102:6320–6325. doi: 10.1073/pnas.0405654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bacia K, Schwille P, Kurzchalia T. Sterol structure determines the separation of phases and the curvature of the liquid-ordered phase in model membranes. Proc Natl acad Sci U S A. 2005;102:3272–3277. doi: 10.1073/pnas.0408215102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahya N, Brown DA, Schwille P. Raft partitioning and dynamic behavior of human placental alkaline phosphatase in giant unilamellar vesicles. Biochemistry. 2005;44:7479–7489. doi: 10.1021/bi047429d. [DOI] [PubMed] [Google Scholar]
- 28.Baumgart T, Hammond A, Sengupta P, Hess S, Holowka D, Baird B, Webb WW. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc Natl Acad Sci U S A. 2007;104:3165–3170. doi: 10.1073/pnas.0611357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larson DR, Gosse JA, Holowka DA, Baird BA, Webb WW. Temporally resolved interactions between antigen-stimulated IgE receptors and Lyn kinase on living cells. J Cell Biol. 2005;171:527–536. doi: 10.1083/jcb.200503110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierini L, Holowka D, Baird B. Fc epsilon RI-mediated association of 6-micron beads with RBL-2H3 mast cells results in exclusion of signaling proteins from the forming phagosome and abrogation of normal downstream signaling. J Cell Biol. 1996;134:1427–1439. doi: 10.1083/jcb.134.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott RE. Plasma membrane vesiculation: a new technique for isolation of plasma membranes. Science. 1976;194:743–545. doi: 10.1126/science.982044. [DOI] [PubMed] [Google Scholar]
- 32.Field KA, Holowka D, Baird B. Fc epsilon RI-mediated recruitment of p53/56lyn to detergent-resistant membrane domains accompanies cellular signaling. Proc Natl Acad Sci U S A. 1995;92:9201–9205. doi: 10.1073/pnas.92.20.9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massey JB. Interaction of ceramides with phosphatidylcholine, sphingomyelin and sphingomyelin/cholesterol bilayers. Biochim Biophys Acta. 2001;1510:167–18. doi: 10.1016/s0005-2736(00)00344-8. [DOI] [PubMed] [Google Scholar]
- 34.Megha, London E. Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): implications for lipid raft structure and function. J Biol Chem. 2004;279:9997–10004. doi: 10.1074/jbc.M309992200. [DOI] [PubMed] [Google Scholar]
- 35.Cremesti E, Goni EFAM, Kolesnick R. Role of sphingomyelinase and ceramide in modulating rafts: do biophysical properties determine biologic outcome? FEBS Lett. 2001;531:47–53. doi: 10.1016/s0014-5793(02)03489-0. [DOI] [PubMed] [Google Scholar]
- 36.Gidwani A, Holowka D, Baird B. Fluorescence Anisotropy Measurements of Lipid Order in Plasma Membranes and Lipid Rafts from RBL-2H3 Mast Cells. Biochemistry J. 2001;40:12422–12429. doi: 10.1021/bi010496c. [DOI] [PubMed] [Google Scholar]
- 37.Ge M, Field KA, Aneja R, Holowka D, Baird B, Freed JH. Electron spin resonance characterization of liquid ordered phase of detergent-resistant membranes from RBL-2H3 cells. Biophys J. 1999;77:925–933. doi: 10.1016/S0006-3495(99)76943-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang J, Feigenson GW. A microscopic interaction model of maximum solubility of cholesterol in lipid bilayers. Biophys J. 1999;76:2142–2157. doi: 10.1016/S0006-3495(99)77369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Czech MP. PIP2 and PIP3: Complex roles at the cell surface. Cell. 2000;100:603–606. doi: 10.1016/s0092-8674(00)80696-0. [DOI] [PubMed] [Google Scholar]
- 41.Caroni P. Actin cytoskeleton regulation through modulation of PI(4,5)P(2) rafts. EMBO J. 2001;20:4332–4336. doi: 10.1093/emboj/20.16.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janmey PA, Lindberg U. Cytoskeletal regulation: rich in lipids. Nat Rev Mol Cell Biol. 2004;5:658–666. doi: 10.1038/nrm1434. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y, Casey L, Pike LJ. Compartmentalization of phosphatidylinositol 4,5-bisphosphate in low-density membrane domains in the absence of caveolin. Biochem Biophys Res Commun. 1998;245:684–690. doi: 10.1006/bbrc.1998.8329. [DOI] [PubMed] [Google Scholar]
- 44.van Rheenen J, Achame EM, Janssen H, Calafat J, Jalink K. PIP2 signaling in lipid domains: a critical re-evaluation. EMBO J. 2005;24:1664–1673. doi: 10.1038/sj.emboj.7600655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langhorst MA, Reuter A, Stuermer CA. Scaffolding microdomains and beyond: the function of reggie/flotillin proteins. Cell Mol Life Sci. 2005;62:2228–2240. doi: 10.1007/s00018-005-5166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dykstra M, Cherukuri A, Sohn HW, Tzeng SJ, Pierce SK. Location is everything: lipid rafts and immune cell signaling. Annu Rev Immunol. 2003;21:457–481. doi: 10.1146/annurev.immunol.21.120601.141021. [DOI] [PubMed] [Google Scholar]
- 47.Stuermer CA, Lang DM, Kirsch F, Wiechers M, Deininger SO, Plattner H. Glycosylphosphatidyl inositol-anchored proteins and fyn kinase assemble in noncaveolar plasma membrane microdomains defined by reggie-1 and -2. Mol Biol Cell. 2001;12:3031–3045. doi: 10.1091/mbc.12.10.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brigg JA, Wilk T, Fuller SD. Do lipid rafts mediate virus assembly and pseudotyping? J Gen Virol. 2003;84(Pt 4):757–768. doi: 10.1099/vir.0.18779-0. [DOI] [PubMed] [Google Scholar]
- 49.McIntosh TJ, Simon SA. Roles of bilayer material properties in function and distribution of membrane proteins. Annu Rev Biophys Biomol Struct. 2006;35:177–198. doi: 10.1146/annurev.biophys.35.040405.102022. [DOI] [PubMed] [Google Scholar]
- 50.Zhang W, Trible RP, Samelson LE. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–246. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- 51.Walmsle AR, Zheng F, Hooper NM. The N-terminal region of the prion protein ectodomain contains a lipid raft targeting determinant. J Biol Chem. 2003;278:37241–37248. doi: 10.1074/jbc.M302036200. [DOI] [PubMed] [Google Scholar]
- 52.Kenworthy AK, Nichols BJ, Remmert CL, Hendrix GM, Kumar M, Zimmerberg J, Lippincott-Schwartz J. Dynamics of putative raft-associated proteins at the cell surface. J Cell Biol. 2004;165:735–746. doi: 10.1083/jcb.200312170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elliott R, Szleifer I, Schick M. Phase diagram of a ternary mixture of cholesterol and saturated and unsaturated lipids calculated from a microscopic model. Phys Rev Lett. 2006;96:098101. doi: 10.1103/PhysRevLett.96.098101. [DOI] [PubMed] [Google Scholar]
- 54.Pandit SAE, Jacobsson E, Scott HL. Simulation of the early stages of nano-domain formation in mixed bilayers of sphingomyelin, cholesterol and dioleylphosphatidylcholine. Biophys J. 2004;87:3312–3322. doi: 10.1529/biophysj.104.046078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silvius JR. Partitioning of membrane molecules between raft and non-raft domains: insights from model-membrane studies. Biochim Biophys Acta. 2005;1746:193–202. doi: 10.1016/j.bbamcr.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 56.Koivusalo M, Alvesalo J, Virtanen JA, Somerharju P. Partitioning of pyrene-labeled phospho- and sphingolipids between ordered and disordered bilayer domains. Biophys J. 2004;86:923–935. doi: 10.1016/S0006-3495(04)74168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sengupta P, Holowka D, Baird B. Fluorescence Resonance Energy Transfer Between Lipid Probes Detects Nanoscopic Heterogeneity in the Plasma Membrane of Live Cells. Biophys J. 2007;92:3564–3574. doi: 10.1529/biophysj.106.094730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang TY, Silivius JR. Sphingolipid partitioning into ordered domains in cholesterol-free and cholesterol-containing lipid bilayers. Biophys J. 2003;84:367–378. doi: 10.1016/S0006-3495(03)74857-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fridriksson EK, Shipkova PA, Sheets ED, Holowka D, Baird B, McLafferty FW. Quantitative analysis of phospholipids in functionally important membrane domains from RBL-2H3 mast cells using tandem high-resolution mass spectrometry. Biochemistry. 1999;38:8056–8063. doi: 10.1021/bi9828324. [DOI] [PubMed] [Google Scholar]
- 60.Estronca LMBB, Moreno MJ, Aberu MSC, Melo E, Vaz WLC. Solubility of amphiphiles in membranes: influence of phase properties and amphiphile headgroup. Biochem Biophys Res Commun. 2002;296:596–603. doi: 10.1016/s0006-291x(02)00905-1. [DOI] [PubMed] [Google Scholar]
- 61.Chattopadhyay A, London E. Parallax method for direct measurement of membrane penetration depth utilizing fluorescence quenching by spin-labeled phospholipids. Biochemistry. 1987;26:39–45. doi: 10.1021/bi00375a006. [DOI] [PubMed] [Google Scholar]
- 62.Meyer zu Heringdorf D, Jacobs KH. Lysophospholipid receptors: signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim Biophys Acta. 2007;1768:923–940. doi: 10.1016/j.bbamem.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 63.van der Luit AH, Budde M, Ruurs P, Verheij M, van Blitterswijk WJ. Alkyl-lysophospholipid accumulates in lipid rafts and induces apoptosis via raft-dependent endocytosis and inhibition of phosphatidylcholine synthesis. J Biol Chem. 2002;277:39541–39547. doi: 10.1074/jbc.M203176200. [DOI] [PubMed] [Google Scholar]
- 64.Montixi C, Langlet C, Bernard AM, Thimonier J, Dubois C, Wurbel MA, Chauvin JP, Pierres M, He HT. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO J. 1998;17:5334–5348. doi: 10.1093/emboj/17.18.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Samsonov AV, Mihalyov I, Cohen FS. Characterization of cholesterol-sphingomyelin domains and their dynamics in bilayer membranes. Biophys J. 2001;81:1486–1500. doi: 10.1016/S0006-3495(01)75803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Melkonian KA, Ostermeyer AG, Chen JZ, Roth MG, Brown DA. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J Biol Chem. 1997;274:3910–39. doi: 10.1074/jbc.274.6.3910. [DOI] [PubMed] [Google Scholar]
- 67.Dietrich C, Volovyk ZN, Levi M, Thompson NL, Jacobson K. Partitioning of Thy-1, GM1, and cross-linked phospholipid analogs into lipid rafts reconstituted in supported model membrane monolayers. Proc Natl Acad Sci U S A. 2001;98:10642–10647. doi: 10.1073/pnas.191168698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kovarova M, Tolar P, Arudchandran R, Draberova L, Rivera J, Draber P. Structure-function analysis of Lyn kinase association with lipid rafts and initiation of early signaling events after Fcepsilon receptor I aggregation. Mol Biol Cell. 2001;21:8318–8328. doi: 10.1128/MCB.21.24.8318-8328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lundbaek JA, Anderson OS, Werge T, Nielsen C. Cholesterol-induced protein sorting: an analysis of energetic feasibility. Biophys J. 2003;84:2080–2089. doi: 10.1016/S0006-3495(03)75015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shogomori H, Hammond AT, Ostermeyer-Fay AG, Barr DJ, Feigenson GW, London E, Brown DA. Palmitoylation and intracellular domain interactions both contribute to raft targeting of linker for activation of T cells. J Biol Chem. 2005;280:18931–18942. doi: 10.1074/jbc.M500247200. [DOI] [PubMed] [Google Scholar]
- 71.Young RM, Zheng X, Holowka D, Baird B. Reconstitution of regulated phosphorylation of FcepsilonRI by a lipid raft-excluded protein-tyrosine phosphatase. J Biol Chem. 2005;280:1230–1235. doi: 10.1074/jbc.M408339200. [DOI] [PubMed] [Google Scholar]