Abstract
Using the genetic estimates of paternity available for 22 species of socially monogamous mammals, we investigated the impact of the social structure and of the type of pair bonding on the interspecific variations of extra-pair paternity rates. To this purpose, we classified species in three categories of social structure—solitary, pair or family-living species—and in two categories of pair bonding—intermittent or continuous. We show that interspecific variations of extra-pair paternity rates are better explained by the social structure than by the type of pair bonding. Species with intermittent and continuous pair bonding present similar rates of extra-pair paternity, while solitary and family-living species present higher extra-pair paternity rates than pair-living species. This can be explained by both higher male–male competition and higher female mate choice opportunities in solitary and family-living species than in pair-living species.
Keywords: paternity loss, mating system, social structure, social organization, monogamy
1. Introduction
In socially monogamous species, individuals are expected to engage in extra-pair copulations (Trivers 1972). Indeed, through extra-pair copulations, males may enhance their reproductive success without increasing their parental investment (Trivers 1972) while females may modify their mate choice and gain direct and/or indirect benefits (Jennions & Petrie 2000; Akcay & Roughgarden 2007). While extra-pair paternities are widespread among socially monogamous bird species (Griffith et al. 2002), evidence has also started to accumulate in socially monogamous mammals. Although high variability of extra-pair paternity rates has been reported among socially monogamous species of mammals (table 1), the causes of this variability remain largely unknown.
Table 1.
Proportion of extra-pair young (EPY) in 22 socially monogamous mammals.
| common name | Latin name | social structure | pair bonding | n total | n EPY | proportion EPY | references (see electronic supplementary material, appendix 1) |
|---|---|---|---|---|---|---|---|
| Ethiopian wolf | Canis simensis | family | continuous | 47 | 13 | 0.28 | Randall et al. (2007) |
| North American beavera | Castor Canadensis | family | intermittent | 23 | 20 | 0.87 | Crawford et al. (2008) |
| North American beavera | Castor Canadensis | family | intermittent | 16 | 10 | 0.63 | Crawford et al. (2008) |
| fat-tailed dwarf lemur | Cheirogaleus medius | family | intermittent | 16 | 7 | 0.44 | Fietz et al. (2000) |
| greater white-toothed shrew | Crocidura russula | pair | intermittent | 63 | 0 | 0.00 | Bouteiller & Perrin (2000) |
| common mole rata | Cryptomys hottentotus hottentotus | family | continuous | 24 | 21 | 0.88 | Bishop et al. (2004) |
| common mole rata | C. hottentotus hottentotus | family | continuous | 49 | 22 | 0.45 | Bishop et al. (2004) |
| feral cat | Felis catus | solitary | intermittent | 40 | 0 | 0.00 | Say et al. (2002) |
| alaotran gentle lemur | Hapalemur griseus alaotrensis | family | continuous | 59 | 5 | 0.08 | Nievergelt et al. (2002) |
| dwarf mongoose | Helogale parvula | family | continuous | 45 | 14 | 0.31 | Keane et al. (1994) |
| Bornean gibbon | Hylobates muelleri | family | continuous | 6 | 0 | 0.00 | Oka & Takenaka (2001) |
| Malagasy giant jumping rat | Hypogeomys antimena | pair | intermittent | 48 | 2 | 0.04 | Sommer (2003) |
| African wild dog | Lycaon pictus | family | continuous | 29 | 3 | 0.10 | Girman et al. (1997) |
| Kirk's dik-dika | Madoqua kirkii | pair | continuous | 8 | 0 | 0.00 | Brotherton et al. (1997) |
| Kirk's dik-dika | M. kirkii | pair | continuous | 4 | 0 | 0.00 | Brotherton et al. (1997) |
| Alpine marmot | Marmota marmota | family | intermittent | 369 | 36 | 0.10 | Cohas et al. (2008) |
| California mouse | Peromyscus californicus | pair | continuous | 82 | 0 | 0.00 | Ribble (1991) |
| rock-wallaby | Petrogale assimilis | pair | continuous | 63 | 21 | 0.33 | Spencer et al. (1998) |
| fork-marked lemur | Phaner furcifer | solitary | intermittent | 7 | 4 | 0.57 | Schulke et al. (2004) |
| meerkata | Suricata suricatta | family | continuous | 121 | 15 | 0.12 | Griffin et al. (2003) |
| meerkata | S. suricatta | family | continuous | 389 | 99 | 0.25 | Young et al. (2007) |
| bobuck (mountain brushtail possum) | Trichosurus cunninghami | pair | intermittent | 31 | 11 | 0.35 | Martin et al. (2007) |
| large tree shrewa | Tupaia tana | solitary | intermittent | 15 | 9 | 0.60 | Munshi-South (2007) |
| large tree shrewa | T. tana | solitary | intermittent | 7 | 2 | 0.29 | Munshi-South (2007) |
| island fox | Urocyon littoralis | solitary | intermittent | 16 | 4 | 0.25 | Roemer et al. (2001) |
| swift fox | Vulpes velox | solitary | intermittent | 19 | 10 | 0.53 | Kitchen et al. (2006) |
| red fox | Vulpes vulpes | family | intermittent | 50 | 46 | 0.92 | Baker et al. (2004) |
Data available from two populations.
Jennions & Petrie (2000) pointed out that two critical determinants of extra-pair paternity should be the availability of extra-pair males and the capacity of social males to control accessibility of females to these extra-pair males. The direct control of competitors by males determines the availability of extra-pair males and this control may depend on the social setting (Cohas et al. 2006). We then expected social structure to affect both the ability of males to control competitors and the mate choice opportunities for females. We predicted that extra-pair paternity should be higher in species living in family groups or solitarily, two situations where males should be less able to control competitors available to the females than among those living in pairs. We also expected that extra-pair paternity in socially monogamous mammals should depend on the strength of pair bonding between social mates, which affects the accessibility of females to these extra-pair males. We predicted that extra-pair paternity should be higher in species where pair members are only intermittently associated (Clutton-Brock & Isvaran 2006). We tested these two predictions in 22 socially monogamous mammals.
2. Material and methods
We searched the ISI Web of Knowledge for the keywords ‘paternity(ies)’ or ‘parentage analysis(es)’. We retained studies only from free-ranging populations where parentage was assessed by molecular analyses. We extracted the proportions of extra-pair young (EPY) for 22 socially monogamous species from 27 populations (table 1), social monogamy being characterized, at a behavioural level, by an exclusive association between a breeding male and a breeding female during the breeding season in addition to the fact that mating is observed exclusively between these two individuals. When similar information was available from the same species and population in several studies, we kept only the information with the greatest sample size. Based on the social unit observed throughout the year (and during the breeding season), we distinguished three categories of social structure: solitary; pairs; and family groups. A species is categorized as solitary when members of the two sexes live alone, as pairs when the stable social unit is a male and a female, and as living in families when the social unit characteristic of the species is the association of a dominant pair with subordinates (subordinates can be related to both, to one or to none of the dominant individuals) and their offspring. Based on the strength of the social pair bonding during the breeding season, we distinguished species having continuous bonds (the male remains spatially close to or actively guards its female partner during the fertile period) from species having intermittent bonds (the female is not continuously guarded, or both partners forage separately although their territories largely overlap) (Clutton-Brock & Isvaran 2006).
Although the species in our sample are widely distributed across multiple clades, some species are closely related, suggesting that phylogenetic dependence may exist. Consequently, we used phylogenetic generalized least square to estimate the measure of phylogenetic correlation proposed by Pagel (1999). Using the procedure given by Freckleton et al. (2002) and the script of Duncan et al. (2007), we found no phylogenetic signal (λ=0), indicating phylogenetic independence. We then modelled the effect of social structure, strength of pair bonding, and their interaction on the proportion of EPY without taking phylogeny into account. We accounted for pseudo-replication (i.e. two populations with EPY estimates in five species) by using generalized linear mixed models (glmer function) with species as a random factor. We used a logit link with number of EPY as the response variable and total number of offspring as the binomial denominator. We assessed the significance of fixed terms using the z-statistics of parameter estimates. Statistical analyses were performed using R 2.7.0 software (R Development Core Team 2008), ape and nlme libraries for phylogenetic analyses and lme4 library (Bates et al. 2008) for linear modelling. All tests are two-tailed, the level of significance is set to 0.05, parameter estimates are given with 95 per cent confidence intervals and effect sizes are given as generalized R2 (Nagelkerke 1991).
3. Results
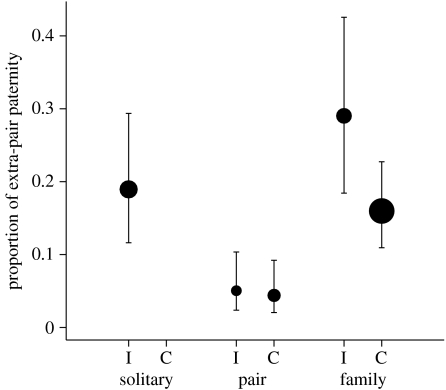
No interaction (tested only in pair and family-living species since solitary were always intermittently associated) occurred between the effects of the strength of pair bonding and social structure (Χ2=1.15, d.f.=2, p=0.56). The strength of pair bonding did not influence the amount of EPY (Χ2=0.98, d.f.=1, p=0.32; %EPY equal to 10.73% (4.65; 22.83) and 16.62% (8.27; 30.59), respectively, in species where pair members were continuously and intermittently associated; figure 1). The strength of pair bonding explained nearly no variations of the proportion of EPY (generalized R2=2.60%). On the other hand, the social structure influenced the amount of EPY (Χ2=6.44, d.f.=2, p=0.04). Species living solitarily or in families had similar rates of EPY (solitary: 18.97% (7.08; 41.85), family: 20.19% (10.95; 34.24), z=−0.11, p=0.91), whereas species living in pairs had lower rates of EPY (4.54% (1.52; 12.76)) than species living either solitarily (z=1.96, p=0.05) or in families (z=2.46, p=0.01; figure 1). The social structure explained a substantial amount of the observed variations of the proportion of EPY (generalized R2=21.05%).
Figure 1.
Mean proportion (±1 s.e.) of extra-pair paternity in species with contrasting social structure (solitary, pair, family) and contrasting strength of pair bonding between pair members (I, intermittently associated; C, continuously associated) in 22 socially monogamous mammal species (table 1). The size of the dots is proportional to the sample size.
In pair-living species, very similar rates of EPY (z=0.40, p=0.69; figure 1) occurred in species with continuously (4.41% (2.05; 9.23)) and intermittently (5.03% (2.37; 10.36)) associated pair members. Although still not significant, the difference was larger in species living in families (continuously associated: 15.98% (10.94; 22.74); intermittently associated: 29.03% (18.42; 42.6), z=1.33, p=0.18; figure 1).
4. Discussion
An important determinant of extra-pair paternity is the ability of males to monopolize breeding females (Gowaty 1996; Westneat & Stewart 2003). Males may ensure paternity by controlling their female partner through efficient mate guarding and/or by directly controlling potential competitors (Johnsen et al. 1998; Hoi-Leitner et al. 1999). Although Clutton-Brock & Isvaran (2006) concluded that the strength of pair bonding satisfactorily accounted for variation in the amount of extra-dominant paternities observed in mammals as a whole, our results demonstrate that this does not hold for socially monogamous mammals. The difference in %EPY between species with continuously and intermittently associated pair members (5.89%) was weak. This means that neither the occurrence of mate guarding nor a continuous association between mates is sufficient to ensure paternity in socially monogamous mammals. In the socially polygynous species with continuous bonding considered by Clutton-Brock & Isvaran, males were probably able to monopolize paternity because harem sizes were always small (less than five females). In those socially polygynous species with continuous bonding, the %EPY markedly increased when additional males were present in the social unit (e.g. Semnopithecus entellus in Clutton-Brock & Isvaran 2006). This suggests again that the social setting rather than the strength of pair bonding may primarily affect %EPY in non-monogamous mammal species.
High extra-pair paternity rates are better predicted by the social structure. Living in families with subordinate males or solitarily may not only prevent males from efficiently monopolizing paternity, since those males may have difficulties in controlling both their female and male competitors, but also increases females mate choice opportunities. In accordance with Cockburn's (2003) review on birds, we also found that the %EPY is especially high, on average, in species living in families. In the case of a family social structure, males may face particularly high intrasexual competition since males, both within and outside the family, may be potential competitors. Within the family group, if reproduction among males results from a tug of war, dominant males may be unable to completely control their subordinates (limited control hypothesis, Reeve 1998), or if the dominant males are able to control the reproduction of subordinates, they may concede reproduction to some offspring to keep them in the family (optimal skew hypothesis, Reeve 1998). Moreover, achieving control of subordinate males within the family can be at the cost of being unable to achieve any control of males outside of the family group (e.g. Marmota marmota, Cohas et al. 2006). More generally, living with potential sexual competitors (subordinate or breeding males) should facilitate extra-pair paternities since it not only promotes male–male competition but also offers higher opportunities for female choice. The high percentage of extra-pair paternities in species where females are associated with several males (35.7% reported in Clutton-Brock & Isvaran 2006) supports this conclusion.
Acknowledgements
Thanks to Sébastien Devillard and Jean-Michel Gaillard for their very helpful comments and Elizabeth Yohannes for editing the English. Financial support was received from the Agence Nationale de La Recherche (ANR-08-BLAN-0214-03) and the CNRS (France).
Supplementary Material
Bibliographic records presenting extra-pair young proportion estimates for socially monogamous mammal species
References
- Akcay E., Roughgarden J. Extra-pair paternity in birds: review of the genetic benefits. Evol. Ecol. Res. 2007;9:855–868. [Google Scholar]
- Bates, D., Maechler, M. & Dai, B. 2008 lme4: linear mixed-effects models using S4 classes, v.0999375-25.
- Clutton-Brock T.H., Isvaran K. Paternity loss in contrasting mammalian societies. Biol. Lett. 2006;2:513–516. doi: 10.1098/rsbl.2006.0531. doi:10.1098/rsbl.2006.0531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn A. Mating systems and sexual conflict. In: Koenig W.D., Dickinson J., editors. Ecology and evolution of cooperative breeding in birds. Cambridge University Press; Cambridge, UK: 2003. pp. 81–101. [Google Scholar]
- Cohas A., Yoccoz N.G., Da Silva A., Goossens B., Allainé D. Extra-pair paternity in the monogamous alpine marmot (Marmota marmota): the roles of social setting and female mate choice. Behav. Ecol. Sociobiol. 2006;59:597–605. doi:10.1007/s00265-005-0086-8 [Google Scholar]
- Duncan R.P., Forsyth D.M., Hone J. Testing the metabolic theory of ecology: allometric scaling exponents in mammals. Ecology. 2007;88:324–333. doi: 10.1890/0012-9658(2007)88[324:ttmtoe]2.0.co;2. doi:10.1890/0012-9658(2007)88[324:TTMTOE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Freckleton R.P., Harvey P.H., Pagel M. Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 2002;160:712–726. doi: 10.1086/343873. doi:10.1086/343873 [DOI] [PubMed] [Google Scholar]
- Gowaty P.A. Battles of the sexes and origins of monogamy. In: Black J.M., editor. partnerships in birds: the study of monogamy. Oxford University Press; Oxford, UK: 1996. pp. 21–52. [Google Scholar]
- Griffith S.C., Owens I.P.F., Thuman K.A. Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol. Ecol. 2002;11:2195–2212. doi: 10.1046/j.1365-294x.2002.01613.x. doi:10.1046/j.1365-294X.2002.01613.x [DOI] [PubMed] [Google Scholar]
- Hoi-Leitner M., Hoi H., Romero-Pujante M., Valera F. Female extra-pair behaviour and environmental quality in the Serin (Serinus serinus): a test of the ‘constrained female hypothesis’. Proc. R. Soc. B. 1999;266:1021–1026. doi:10.1098/rspb.1999.0738 [Google Scholar]
- Jennions M.D., Petrie M. Why do females mate multiply? A review of the genetic benefits. Biol. Rev. (Camb.) 2000;75:21–64. doi: 10.1017/s0006323199005423. doi:10.1017/S0006323199005423 [DOI] [PubMed] [Google Scholar]
- Johnsen A., Lifjeld J.T., Rohde P.A., Primmer C.R., Ellegren H. Sexual conflict over fertilizations: female bluethroats escape male paternity guards. Behav. Ecol. Sociobiol. 1998;43:401–408. doi:10.1007/s002650050507 [Google Scholar]
- Nagelkerke N.J.D. A note on a general definition of the coefficient of determination. Biometrika. 1991;78:691–692. doi:10.1093/biomet/78.3.691 [Google Scholar]
- Pagel M. Inferring the historical patterns of biological evolution. Nature. 1999;401:877–884. doi: 10.1038/44766. doi:10.1038/44766 [DOI] [PubMed] [Google Scholar]
- R Development Core Team 2008 R: a language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing.
- Reeve H.K. Game theory, reproductive skew, and nepotism. In: Dugatkin R.H., editor. Theory and animal behaviour. Oxford University Press; Oxford, UK: 1998. pp. 118–145. [Google Scholar]
- Trivers R.L. Parental investment and sexual selection. In: Campbell B., editor. Sexual selection and the descent of man, 1871–1971. Aldline; Chicago, IL: 1972. pp. 136–179. [Google Scholar]
- Westneat D.F., Stewart I.R.K. Extra-pair paternity in birds: causes, correlates and conflict. Annu. Rev. Ecol. Syst. 2003;34:365–396. doi:10.1146/annurev.ecolsys.34.011802.132439 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bibliographic records presenting extra-pair young proportion estimates for socially monogamous mammal species