Abstract
Swarming and the expression of phase polyphenism are defining characteristics of locust species. Increases in local population density mediate morphological, physiological and behavioural changes within individuals, which correlate with mass marching of juveniles in migratory bands and flying swarms of adults. The Australian plague locust (Chortoicetes terminifera) regularly forms migratory bands and swarms, but is claimed not to express phase polyphenism and has accordingly been used to argue against a central role for phase change in locust swarming. We demonstrate that juvenile C. terminifera express extreme density-dependent behavioural phase polyphenism. Isolated-reared juveniles are sedentary and repelled by conspecifics, whereas crowd-reared individuals are highly active and are attracted to conspecifics. In contrast to other major locust species, however, behavioural phase change does not accumulate across generations, but shifts completely within an individual's lifetime in response to a change in population density.
Keywords: phenotypic plasticity, behaviour, migration, phase polyphenism, insect
1. Introduction
At low population densities locusts express ‘solitarious’ phase characteristics, whereas high population densities induce the expression of ‘gregarious’ phase phenotypes from the same genotype. Although phase change typically involves many different morphological and physiological traits (Simpson & Sword 2008, 2009; Pener & Simpson in press), the change in behaviour that occurs in response to crowding is considered to be the principal driver underlying group formation and mass movement by marching bands of juveniles and flying swarms of adults (Simpson et al. 1999; Simpson & Sword 2009). Solitarious individuals are relatively sedentary and repelled by conspecifics, but high population densities induce individuals to become more active and attracted to one another. The spatial distribution of resources in locust habitats has been shown to be critical in initially bringing solitarious phase locusts together and promoting phase change (Babah & Sword 2004), while the ensuing directional mass movement of gregarious locusts at high population densities is collectively determined and influenced by cannibalistic interactions among individuals (Bazazi et al. 2008).
The Australian plague locust, Chortoicetes terminifera (Acrididae: Oedipodinae) frequently outbreaks and invades agricultural areas (Hunter 2004). Although C. terminifera forms characteristic migratory bands and swarms, it appears not to express density-dependent changes in colour or morphology as seen in other major locust species (Uvarov 1977). Accordingly, C. terminifera is widely assumed not to express phase polyphenism (Hunter 2004), an assertion that has been used to question the importance of phase polyphenism in locust swarm formation (Key 1950). However, early field observations of C. terminifera juvenile behaviour clearly described what appears to be behavioural phase change (Clark 1949).
Here, we quantify behavioural phase polyphenism in C. terminifera and show that it expresses density-dependent behavioural changes very similar to those of other major swarming locust species.
2. Material and methods
Chortoicetes terminifera rearing and behavioural analysis were modified from protocols for the desert locust, Schistocerca gregaria (Roessingh et al. 1993, Simpson et al. 1999; see the electronic supplementary material). Field-collected locusts were reared under crowded conditions for multiple generations. From these, individuals were reared in isolation for one, two and three generations. To test for the expression of behavioural phase change, the behaviour of individual mid-final instar nymphs reared either continuously crowded or isolated for three generations was assayed, using an automated video-tracking behavioural assay designed to quantify individual locomotory and position-related responses to a stimulus group of conspecifics.
Behavioural differences between isolated- and crowd-reared locusts were compared using binary logistic regression (BLR) modelling (see the electronic supplementary material). The resulting logistic regression model, which successfully discriminated between solitary-reared (solitarious) and crowd-reared (gregarious) locusts, was used to calculate the probability of solitarious phase group membership (Psolitarious), providing a quantitative measure of individual behavioural phase state for use as a dependent variable in subsequent experiments.
The presence of transgenerational epigenetic transfer of phase state, as known in other locust species (Miller et al. 2008), was assessed using group and pairwise comparisons of the Psolitarious values of fifth-instar nymphs from cohorts reared in isolation for one, two and three generations, and those from the continuously crowded gregarious culture (see the electronic supplementary material).
To test for an effect of isolation across multiple nymphal stages on the loss of gregarious behaviour, referred to as behavioural solitarization, crowd-reared third- and fourth-instar nymphs were isolated and assayed approximately one week later in the final (fifth) nymphal instar. Psolitarious scores of locusts removed from the crowd were compared with those of continuously crowded fifth-instar nymphs. Solitarization due to shorter term isolation within a single instar was tested by comparing the Psolitarious values of continuously crowded fifth-instar nymphs (24 hours post-ecdysis) with those of similar locusts isolated for 72 hours (see the electronic supplementary material).
To test for an effect of crowding across multiple nymphal stadia on behavioural gregarization, second-generation solitary-reared third- and fourth-instar locusts were crowded until assayed approximately one week later in the fifth instar. Psolitarious values of the recently crowded locusts were compared with those of continuously isolated fifth-instar controls. Gregarization due to crowding within a single instar was tested by comparing the Psolitarious values of second-generation solitary-reared fifth instars with those of similar locusts crowded for 72 hours (see the electronic supplementary material).
3. Results
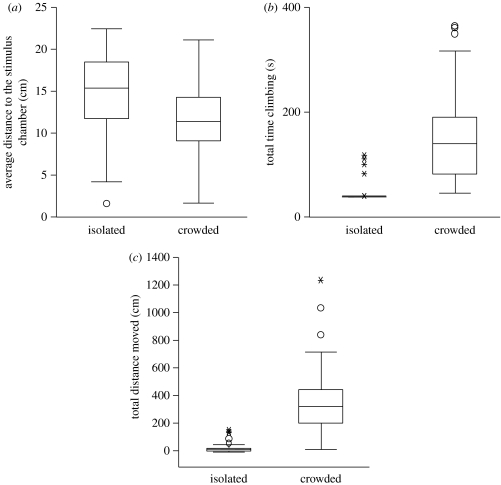
The behaviour of crowd-reared C. terminifera nymphs differed significantly from that of solitary-reared individuals. A forward-conditional stepwise BLR model classified 100 per cent of solitary-reared and 97.4 per cent of crowd-reared locusts into their correct rearing category, with an overall accuracy of 98.7 per cent (table 1). Crowd-reared nymphs moved more, spent more time climbing and spent more time near the stimulus group than did solitary-reared individuals (table 1; figure 1).
Table 1.
Behavioural variable coefficients retained in the most parsimonious forward-conditional logistic regression model derived from 78 crowd-reared and 78 third-generation isolated-reared fifth-instar C. terminifera locusts. (Negative coefficients indicate that the magnitude of response was greater in crowd-reared (gregarious) as opposed to solitary-reared (solitarious) individuals. The Wald statistic indicates the significance of variable contributions to the model. The Hosmer and Lemeshow goodness-of-fit test was not significant (p=0.859, d.f.=8). (Model: η=β0+β1X1+β2X2+⋯+βkXk, with Psolitarious=eη/(1+eη).))
| variable | coefficient β | coefficient β, s.e. | Wald statistic | significance of Wald statistic |
|---|---|---|---|---|
| average distance to the stimulus chamber | 0.438 | 0.155 | 7.956 | 0.005 |
| distance moved | −0.054 | 0.019 | 8.147 | 0.004 |
| climb time | −0.074 | 0.27 | 7.523 | 0.006 |
| constant | −0.359 | 1.32 | 0.074 | 0.785 |
Figure 1.
Box plots representing (a–c) the behavioural variables retained in the logistic regression model from 78 crowd-reared and 78 third-generation isolated-reared C. terminifera fifth-instar nymphs. Each box displays the median value, with the ends of boxes representing the 25th and 75th percentiles and the ends of the whiskers representing the 10th and 90th percentiles. Circles, outliers; asterisks, extreme outliers.
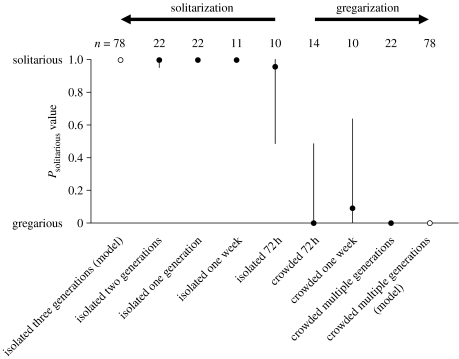
Isolation of gregarious phase insects for a single generation resulted in a complete loss of gregarious behaviour, with no evidence of epigenetic transmission across generations (Kruskal–Wallis test, K=30.76, p<0.0001, d.f.=3). Pairwise tests were significant between the long-term crowd-reared group and all three solitary-reared groups with no differences between first-, second- and third-generation solitary-reared groups (Mann–Whitney U-test, all p<0.05; figure 2).
Figure 2.
Summary of the time course of behavioural gregarization and solitarization in C. terminifera. Filled circles represent median (±95% CI) Psolitarious values of final instar juveniles from the isolated and crowded treatment groups of different durations. Open circles depict the Psolitarious values of the long-term crowded and third-generation isolated locusts used to construct the logistic regression model (table 1). Note that both gregarization and solitarization are achieved after 72 hours, and that the behavioural state attained after 72 hours is equivalent to that attained after many generations of either crowded or isolated rearing. Confidence intervals were obtained using the Resampling Stats add-in for Excel (Resampling Stats, Inc. 2006).
Within a single generation, long-term crowded individuals shifted rapidly to the solitarious behavioural condition upon isolation. The Psolitarious values of crowd-reared fifth-instar nymphs isolated from the third or fourth instar were significantly higher than those in the non-isolated control group (U=19, p=0.020; figure 2). Isolating crowd-reared nymphs for 72 hours resulted in the expression of solitarious phase behaviour, with a median Psolitarious value of 0.95 for the treatment group versus 0 for the controls (U=16, p=0.003; figure 2).
Crowding across multiple instars induced behavioural phase change in solitary-reared locusts. The Psolitarious values of solitary-reared fifth-instar locusts that had been crowded since either the third or fourth nymphal stadium were significantly lower than those of uncrowded controls (U=30, p=0.001; figure 2). Behavioural gregarization was induced by crowding for 72 hours, with significantly lower Psolitarious values among locusts in the crowded treatment group relative to the isolated controls (U=17, p=0.022; figure 2).
4. Discussion
The Australian plague locust expresses classic density-dependent behavioural phase polyphenism, which is both qualitatively and quantitatively similar to that reported in other major swarming locust species (Simpson et al. 1999; Pener & Simpson in press). Nymphs reared under isolated conditions were much more sedentary and repelled by conspecifics relative to those reared under crowded conditions, which were more active and attracted to other locusts (figure 1).
The lack of obvious density-dependent phenotypic changes in colour and morphology in C. terminifera fostered the notion that phase polyphenism might not play a role in the formation and mass movement of locusts in migratory bands and swarms during outbreaks (Key 1950; Hunter 2004). On the contrary, our results for C. terminifera are consistent with the predicted role of behavioural phase change as a driver of locust swarm formation and mass movement during outbreaks (Simpson & Sword 2009). Importantly, the elucidation of behavioural phase change in C. terminifera will enable the established understanding of the relationship between locust resource distribution patterns, gregarization and swarm formation to be applied to improve locust forecasting and management based on local habitat information (Babah & Sword 2004).
Although the behaviour of solitarious and gregarious phase C. terminifera nymphs is similar to that in other locusts, the time course of its expression within and across generations differs. Epigenetic transmission of phase traits has been found in other locust species, mediated by maternally produced gregarizing chemicals (Miller et al. 2008). In the desert locust, S. gregaria, whereas behavioural gregarization occurs within hours, three successive generations of isolated rearing are required before long-term crowded insects express fully solitarious behaviour (Roessingh et al. 1993). In C. terminifera, behavioural phase change was complete in either direction within days (figure 2). This result does not prove the absence of maternal inheritance of phase in C. terminifera, rather it shows that behavioural solitarization and gregarization proceed to completion so rapidly that behavioural phase state does not accumulate over successive generations. Perhaps this rapid time course of solitarization reflects ecological differences between locust species in the autocorrelation of local population densities across generations, or, possibly, C. terminifera lacks genetic variation at loci critical for the transmission of epigenetic effects (e.g. Kucharski et al. 2008).
Locust swarming and the expression of phase polyphenism appear to have arisen independently numerous times, which gives rise to two questions (Song 2005; Lovejoy et al. 2006; Simpson & Sword 2009): (i) what is the role of genetics versus the environment in locust swarming and (ii) have unique mechanisms underlying the expression of phase polyphenism independently evolved several times, or are the differences among species due to modifications of the same gene regulatory pathways? Functional genomics resources for Locusta migratoria (Kang et al. 2004; Ma et al. 2006) offer tools for unravelling the molecular genetic mechanisms underlying locust phase change. Locusta migratoria and C. terminifera are in the same subfamily (Oedipodinae), but L. migratoria expresses extreme phase changes in multiple traits including coloration and morphology, whereas C. terminifera appears to change only in its behaviour. Thus, comparative gene expression studies hold potential for identifying the suite of genes underlying behavioural phase change in these two locusts. Similarities and differences between the two will serve as a basis for broader phylogenetic studies of the genetic and regulatory mechanisms underlying locust phase change, thereby providing insights into the evolution of phenotypic plasticity and development of new pest management approaches.
Acknowledgements
This study was funded by Australian Research Council Linkage Project grant LP0669080, in partnership with the Australian Plague Locust Commission. We thank Laury McCulloch, Martin Steinbauer, Tim Dodgson and Naz Soran for their assistance.
Supplementary Material
References
- Babah M.A.O., Sword G.A. Linking locust gregarization to local resource distribution patterns across a large spatial scale. Environ. Entomol. 2004;33:1577–1583. [Google Scholar]
- Bazazi S., Buhl J., Hale J.J., Anstey M.L., Sword G.A., Simpson S.J., Couzin I.D. Collective motion and cannibalism in locust migratory bands. Curr. Biol. 2008;18:735–739. doi: 10.1016/j.cub.2008.04.035. doi:10.1016/j.cub.2008.04.035 [DOI] [PubMed] [Google Scholar]
- Clark L.R. Behaviour of swarm hoppers of the Australian plague locust (Chortoicetes terminifera Walker) CSIRO Bull. 1949;245:1–27. [Google Scholar]
- Hunter D.M. Advances in the control of locusts (Orthoptera: Acrididae) in eastern Australia: from crop protection to preventative control. Aust. J. Entomol. 2004;43:293–303. doi:10.1111/j.1326-6756.2004.00433.x [Google Scholar]
- Kang L., Chen X., Zhou Y., Liu B., Zheng W., Li R., Wang J., Yu J. The analysis of large-scale gene expression correlated to the phase changes of the migratory locust. Proc. Natl Acad. Sci. USA. 2004;101:17611–17615. doi: 10.1073/pnas.0407753101. doi:10.1073/pnas.0407753101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key K.H.L. A critique on the phase theory of locusts. Q. Rev. Biol. 1950;25:363–407. doi: 10.1086/397834. doi:10.1086/397834 [DOI] [PubMed] [Google Scholar]
- Kucharski R., Maleszka J., Foret S., Maleszka R. Nutritional control of reproductive status in honeybees via DNA methylation. Science. 2008;319:1827–1830. doi: 10.1126/science.1153069. doi:10.1126/science.1153069 [DOI] [PubMed] [Google Scholar]
- Lovejoy N.R., Mullen S.P., Sword G.A., Chapman R.F., Harrison R.G. Ancient trans-Atlantic flight explains locust biogeography: molecular phylogenetics of Schistocerca. Proc. R. Soc. B. 2006;273:767–774. doi: 10.1098/rspb.2005.3381. doi:10.1098/rspb.2005.3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z., Yu J., Kang L. LocustDB: a relational database for the transcriptome and biology of the migratory locust (Locusta migratoria) BMC Genomics. 2006;7:11. doi: 10.1186/1471-2164-7-11. doi:10.1186/1471-2164-7-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G.A., Islam M.S., Claridge T.D.W., Dodgson T., Simpson S.J. Swarm formation in the desert locust Schistocerca gregaria: isolation and NMR analysis of the primary maternal gregarizing agent. J. Exp. Biol. 2008;211:370–376. doi: 10.1242/jeb.013458. doi:10.1242/jeb.013458 [DOI] [PubMed] [Google Scholar]
- Pener, M. P. & Simpson, S. J. In press. Locust phase polyphenism: an update. Adv. Insect Physiol.
- Roessingh P., Simpson S.J., James S. Analysis of phase-related changes in behaviour of desert locust nymphs. Proc. R. Soc. B. 1993;252:43–49. doi:10.1098/rspb.1993.0044 [Google Scholar]
- Simpson S.J., Sword G.A. Locusts. Curr. Biol. 2008;18:364–366. doi: 10.1016/j.cub.2008.02.029. doi:10.1016/j.cub.2008.02.029 [DOI] [PubMed] [Google Scholar]
- Simpson S.J., Sword G.A. Phase polyphenism in locusts: mechanisms, population consequences, adaptive significance and evolution. In: Whitman D., Ananthakrishnan T., editors. Phenotypic plasticity of insects: mechanisms and consequences. Science Publishers Inc; Plymouth, UK: 2009. pp. 147–190. [Google Scholar]
- Simpson S.J., McCaffery A.R., Hägele B.F. A behavioral analysis of phase change in the desert locust. Biol. Rev. 1999;74:461–480. doi:10.1017/S000632319900540X [Google Scholar]
- Song H. Phylogenetic perspectives on the evolution of locust phase polyphenism. J. Orthopt. Res. 2005;14:235–245. doi:10.1665/1082-6467(2005)14[235:PPOTEO]2.0.CO;2 [Google Scholar]
- Uvarov B.P. Grasshoppers and locusts. vol. 2. Centre for Overseas Pest Research; London, UK: 1977. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.