Abstract
Among osteichthyans, basal actinopterygian fishes (e.g. paddlefish and bowfins) have paired fins with three endoskeletal components (pro-, meso- and metapterygia) articulating with polybasal shoulder girdles, while sarcopterygian fishes (lungfish, coelacanths and relatives) have paired fins with one endoskeletal component (metapterygium) articulating with monobasal shoulder girdles. In the fin–limb transition, the origin of the sarcopterygian paired fins triggered new possibilities of fin articulation and movement, and established the proximal segments (stylopod and zeugopod) of the presumptive tetrapod limb. Several authors have stated that the monobasal paired fins in sarcopterygians evolved from a primitive polybasal condition. However, the fossil record has been silent on whether and when the inferred transition took place. Here we describe three-dimensionally preserved shoulder girdles of two stem sarcopterygians (Psarolepis and Achoania) from the Lower Devonian of Yunnan, which demonstrate that stem sarcopterygians have polybasal pectoral fin articulation as in basal actinopterygians. This finding provides a phylogenetic and temporal constraint for studying the origin of the stylopod, which must have originated within the stem sarcopterygian lineage through the loss of the propterygium and mesopterygium.
Keywords: sarcopterygians, fin–limb transition, stylopod, shoulder girdle, polybasal fin articulation
1. Introduction
Crown sarcopterygians include all living lungfish, coelacanths and tetrapods and all the extinct descendants of their last common ancestor. Stem sarcopterygians are fossil forms phylogenetically basal to crown sarcopterygians and often provide clues to the origin of evolutionary novelties in crown sarcopterygians. Whether stem sarcopterygians have monobasal or polybasal paired fins is critical for studying the origin of the stylopod, the proximal segment of the metapterygial endoskeleton in the paired fins of sarcopterygian fishes and in tetrapod limbs. Speculations on the origin of the sarcopterygian fin (Rosen et al. 1981; Janvier 1996; Mabee 2000; Coates 2003; Davis et al. 2004; Friedman et al. 2007) have mainly been based on inference from chondrichthyans and basal actinopterygians and have not been tested with fossil findings.
No paired fin endoskeleton is known for stem sarcopterygians. However, the endoskeletal fin pattern can be inferred if the articular surface of the endoskeletal shoulder girdle is available. Among stem sarcopterygians, no shoulder girdle material was available when Achoania and Meemannia were first described (Zhu et al. 2001, 2006), while the reported shoulder girdle of Psarolepis (Zhu et al. 1999) was based on an internal mould, and the articulation surface was unknown. The shoulder girdles reported here represent the first fossil evidence bearing on the fin articulation in stem sarcopterygians.
2. Material and methods
The new shoulder girdles (figure 1) come from the Xitun Formation (Late Lochkovian, Lower Devonian) in association with the type specimens of Psarolepis (Yu 1998) and Achoania (Zhu et al. 2001). The specimens were prepared mechanically and are housed in the Institute of Vertebrate Paleontology and Paleoanthropology (IVPP), Beijing. The position of Psarolepis and Achoania as stem sarcopterygians, as well as the assignment of disarticulated specimens to each taxon first established on the basis of cranial material, is corroborated by the recent finding of a closely allied basal form from the Ludlow of the Silurian (Zhu et al. in press). The phylogenetic framework used here is based on Janvier (1996), Zhu et al. (1999, 2006, in press), Shubin et al. (2006) and Friedman (2007).
Figure 1.
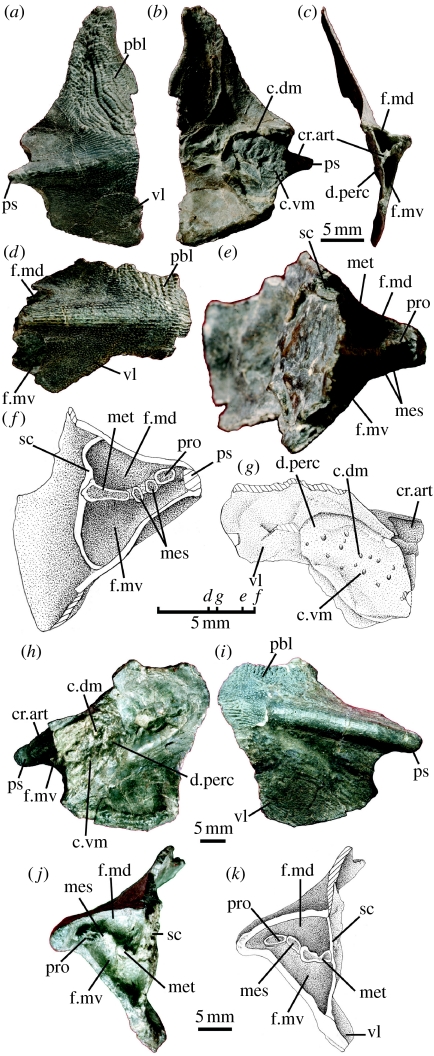
Polybasal shoulder girdles of (a–g) Psarolepis and (h–k) Achoania with articular facets for multiple pterygial components. (a–c) Right cleithrum with scapulocoracoid (Psarolepis, IVPP V15544.1) in (a) lateral, (b) medial and (c) posterior views. (d,e) Right cleithrum with scapulocoracoid (Psarolepis, V15544.2) in (d) lateral and (e) posteromedial views. (f,g) Illustrative drawings of V15544.2 to emphasize structures in (f) posteromedial and (g) medial views. (h–j) Left cleithrum with scapulocoracoid (Achoania, V15545) in (h) medial, (i) lateral and (j) posterior views. (k) Illustrative drawing of V15545 in posterior view. c.dm, dorsomedial canals for pterygial nerves and vessels; c.vm, ventromedial canals for pterygial nerves and vessels; cr.art, articular crest of scapulocoracoid; d.perc, pericardial depression; f.md, dorsal muscle fossa; f.mv, ventral muscle fossa; mes, articular facet for mesopterygium or mesopterygial radial(s); met, articular facet for metapterygium; pbl, postbranchial lamina of cleithrum; pro, articular facet for propterygium; ps, pectoral spine of cleithrum; sc, scapulocoracoid; vl, ventral lamina of cleithrum.
3. Results
(a) Shoulder girdle of Psarolepis
Specimens IVPP V15544.1 and V15544.2 (figure 1a–g) show the same morphology and large-pore cosmine ornamentation as those in Psarolepis (Zhu et al. 1999). The scapulocoracoid is a massive, perichondrally ossified plate attached to the cleithrum by its entire surface, a condition similar to placoderms and different from the tripodal scapulocoracoid in crown sarcopterygians (Janvier 1996). The internal face of the scapulocoracoid is pierced by two rows of openings for blood vessels and nerves (c.dm and c.vm; figure 1b,g) as in placoderms (Stensiö 1959). The articular crest is a horizontal raft-like plate, which divides the fossa for the origin of the pectoral fin muscle group into two parts, respectively, corresponding to the supraglenoid foramen and the supracoracoid foramen in crown sarcopterygians (f.md and f.mv; figure 1c,e,f; Jarvik 1980).
The articular crest carries four well-defined facets comparable with those in chondrichthyans and basal actinopterygians (Rosen et al. 1981; Gardiner 1984). The medial-most facet is elongated and significantly wider than other facets and is probably for the metapterygium, the facet close to the pectoral spine is small and is probably for the propterygium, and the two facets in between are the smallest and are probably for the mesopterygium or mesopterygial radials (met, pro and mes; figure 1e,f). These articular facets demonstrate that Psarolepis has pro-, meso- and metapterygia as in basal actinopterygians and chondrichthyans, a condition previously unknown in sarcopterygians (Mabee 2000; Friedman et al. 2007). The enlarged medial-most facet indicates that the metapterygium is larger than pre-metapterygial radials, a condition different from those in basal actinopterygians and consistent with the position of Psarolepis as a stem sarcopterygian.
(b) Shoulder girdle of Achoania
Specimen V15545 (figure 1h–k) resembles the shoulder girdle of Psarolepis in general, but differs in the shorter length of the pectoral spine, as well as in the larger size and more robust shape of the cleithrum. These differences in size and stoutness resemble those observed between Psarolepis and Achoania in cranial and mandibular materials (Yu 1998; Zhu et al. 1999, 2001; Zhu & Yu 2004). The pore size on the cosmine surface resembles more closely that of Achoania than that of Psarolepis and other large-pored sarcopterygians (Styloichthys and Meemannia; Zhu et al. 2006). Based on these features, V15545 is tentatively referred to Achoania. As in Psarolepis, the scapulocoracoid is a massive plate pierced internally by two rows of openings (c.dm and c.vm; figure 1h). The articular crest bears three facets, instead of four as in Psarolepis. The facets for the metapterygium and the propterygium (met and pro; figure 1j,k) resemble those in Psarolepis. However, only one facet (mes; figure 1j,k) for the mesopterygium or mesopterygial radial(s) can be identified.
4. Discussion
The discovery of the polybasal shoulder girdle in stem sarcopterygians has wide implications for studying the evolution of sarcopterygians in general and the fin–limb transition in particular.
The finding demonstrates that stem sarcopterygians have polybasal pectoral fin articulation as in chondrichthyans and basal actinopterygians such as Mimia, paddlefish and bowfins (figure 2). Consequently, the origin of the monobasal sarcopterygian fin must have occurred at a point crownward to Psarolepis and Achoania, instead of that at the split between sarcopterygians and actinopterygians. The notion that the monobasal, lobed fin characterizes all sarcopterygians (Romer 1955; Rosen et al. 1981; Janvier 1996) should be revised.
Figure 2.
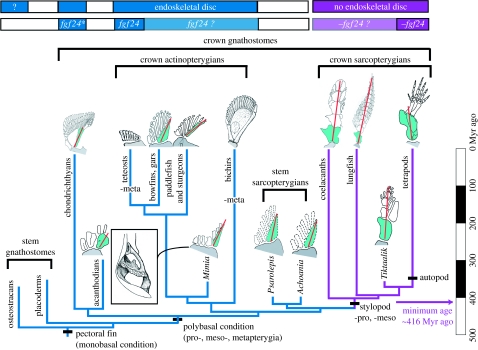
Composite cladogram showing pectoral fin–limb and girdle conditions among gnathostome groups. The monobasal lobe fins or stylopod originated in the internode between stem sarcopterygians (Psarolepis and Achoania) and crown sarcopterygians, with a minimum age of 416 Myr ago. Crown sarcopterygians (purple) lack pro- and mesopterygia. Lineages in blue possess pro- and mesopterygia. Red line indicates the metapterygial axis, green shading the metapterygium and grey shading the scapulocoracoid. Minus sign indicates loss of pterygial components. Inset shows the polybasal shoulder girdle of Mimia in posterior view (Gardiner 1984). Top bars summarize the distribution of the endoskeletal disc (first row) and of the fgf24 gene (second row): blue for the presence; purple for the absence; light blue or purple for the inferred presence or absence; and asterisk for the probable presence of fgf24 orthologue in shark (Draper et al. 2003). The likely presence of the endoskeletal disc in osteostracans is based on Janvier et al. (2004), suggesting that stem gnathostomes and crown sarcopterygians may have different developmental patterns for pectoral fins. Drawings adapted and redrawn from Jarvik (1980), Gardiner (1984), Janvier (1996), Mabee (2000), Clack (2002), Shubin et al. (2006) and Friedman et al. (2007).
The polybasal fin articulation in stem sarcopterygians adds more primitive features to the sarcopterygian morphotype (Zhu et al. 2001, 2006), and lends support to the hypothesis that the presence of three pterygial radials represents the primitive condition for osteichthyans (Janvier 1996), with subsequent loss of the metapterygium in advanced actinopterygians (teleosts; Mabee 2000) and loss of the propterygium and mesopterygium in crown sarcopterygians (including tetrapods).
Loss of the propterygium and mesopterygium signifies the origin of the stylopod, and is developmentally achieved through the loss of the endoskeletal disc, a chondrogenic plate of mesenchymal cells (Davis et al. 2004). The phylogenetic constraint provided by stem sarcopterygians will help the study of the stylopod origin to catch up with the study of the autopod origin (Wagner & Chiu 2001; Shubin et al. 2006; Davis et al. 2007; Johanson et al. 2007) by focusing more on phylogenetically appropriate forms in fossil and developmental studies.
For instance, a fibroblast growth factor gene (fgf24) involved in fin outgrowth is present in zebrafish and possibly shark but absent in mouse, chick and human (Draper et al. 2003; Fischer et al. 2003; Mercader 2007). With the presence of fgf24 presumed to be a primitive gnathostome condition, subsequently lost after sarcopterygians diverged from actinopterygians (Draper et al. 2003), it is tempting to suggest that the loss of the propterygium and mesopterygium may have been correlated with the loss of fgf24 (figure 2), a molecular event that might have occurred crownward to Psarolepis and Achoania, with a minimum age of 416 Myr ago as established by the earliest record of crown sarcopterygians (Zhu & Fan 1995). The distribution pattern of fgf24 in paddlefish, bichirs, lungfish and coelacanths will provide a potential test of this suggestion.
Acknowledgements
We thank P. Janvier for advice and stimulating discussions, M. Chang for continuing support and providing specimen V15544.2, M. Yang for artwork and X. Lu for specimen preparation. This work was supported by the Major Basic Research Projects (2006CB806400) of MST of People's Republic of China, the Chinese Academy of Sciences (K2CX2-YW-156), the CAS/SAFEA International Partnership Program for Creative Research Teams and the Chinese Foundation of Natural Sciences. X.Y. thanks Kean University for faculty research support.
Supplementary Material
High-resolution image of figure 1
References
- Clack J.A. Indiana University Press; Bloomington/Indianapolis, IN: 2002. Gaining ground—the origin and evolution of tetrapods. [Google Scholar]
- Coates M.I. The evolution of paired fins. Theory Biosci. 2003;122:266–287. doi:10.1007/s12064-003-0057-4 [Google Scholar]
- Davis M., Shubin N., Force A. Pectoral fin and girdle development in the basal actinopterygians Polyodon spathula and Acipenser transmontanus. J. Morphol. 2004;262:608–628. doi: 10.1002/jmor.10264. doi:10.1002/jmor.10264 [DOI] [PubMed] [Google Scholar]
- Davis M.C., Dahn R.D., Shubin N.H. An autopodial-like pattern of Hox expression in the fins of a basal actinopterygian fish. Nature. 2007;447:473–476. doi: 10.1038/nature05838. doi:10.1038/nature05838 [DOI] [PubMed] [Google Scholar]
- Draper B.W., Stock D.W., Kimmel C.B. Zebrafish fgf24 functions with fgf8 to promote posterior mesodermal development. Development. 2003;130:4639–4654. doi: 10.1242/dev.00671. doi:10.1242/dev.00671 [DOI] [PubMed] [Google Scholar]
- Fischer S., Draper B.W., Neumann C.J. The zebrafish fgf24 mutant identifies an additional level of Fgf signaling involved in vertebrate forelimb initiation. Development. 2003;130:3515–3524. doi: 10.1242/dev.00537. doi:10.1242/dev.00537 [DOI] [PubMed] [Google Scholar]
- Friedman M. Styloichthys as the oldest coelacanth: implications for early osteichthyan interrelationships. J. Syst. Palaeontol. 2007;5:289–343. doi:10.1017/S1477201907002052 [Google Scholar]
- Friedman M., Coates M.I., Anderson P. First discovery of a primitive coelacanth fin fills a major gap in the evolution of lobed fins and limbs. Evol. Dev. 2007;9:329–337. doi: 10.1111/j.1525-142X.2007.00169.x. [DOI] [PubMed] [Google Scholar]
- Gardiner B.G. The relationships of the palaeoniscid fishes, a review based on new specimens of Mimia and Moythomasia from the Upper Devonian of Western Australia. Bull. Br. Mus. Nat. Hist. (Geol.) 1984;37:173–428. [Google Scholar]
- Janvier P. Clarendon Press; Oxford, UK: 1996. Early vertebrates. [Google Scholar]
- Janvier P., Arsenault M., Desbiens S. Calcified cartilage in the paired fins of the osteostracan Escuminaspis laticeps (Traquair 1880), from the Late Devonian of Miguasha (Québec, Canada), with a consideration of the early evolution of the pectoral fin endoskeleton in vertebrates. J. Vert. Paleontol. 2004;24:773–779. doi:10.1671/0272-4634(2004)024[0773:CCITPF]2.0.CO;2 [Google Scholar]
- Jarvik E. Basic structure and evolution of vertebrates. vol. 1. Academic Press; London, UK: 1980. [Google Scholar]
- Johanson Z., Joss J., Boisvert C.A., Ericsson R., Sutija M., Ahlberg P.E. Fish fingers: digit homologues in sarcopterygian fish fins. J. Exp. Zool. (Mol. Dev. Evol.) 2007;308B:757–768. doi: 10.1002/jez.b.21197. doi:10.1002/jez.b.21197 [DOI] [PubMed] [Google Scholar]
- Mabee P.M. Developmental data and phylogenetic systematics: evolution of the vertebrate limb. Am. Zool. 2000;40:789–800. doi:10.1668/0003-1569(2000)040[0789:DDAPSE]2.0.CO;2 [Google Scholar]
- Mercader N. Early steps of paired fin development in zebrafish compared with tetrapod limb development. Dev. Growth Differ. 2007;49:421–437. doi: 10.1111/j.1440-169X.2007.00942.x. [DOI] [PubMed] [Google Scholar]
- Romer A.S. Herpetichthyes, Amphibioidei, Choanichthyes or Sarcopterygii? Nature. 1955;176:126. doi:10.1038/176126a0 [Google Scholar]
- Rosen D.E., Forey P.L., Gardiner B.G., Patterson C. Lungfishes, tetrapods, paleontology, and plesiomorphy. Bull. Am. Mus. Nat. Hist. 1981;167:159–276. [Google Scholar]
- Shubin N.H., Daeschler E.B., Jenkins F.A.J. The pectoral fin of Tiktaalik roseae and the origin of the tetrapod limb. Nature. 2006;440:764–771. doi: 10.1038/nature04637. doi:10.1038/nature04637 [DOI] [PubMed] [Google Scholar]
- Stensiö E.A. On the pectoral fin and shoulder girdle of the arthrodires. K. Svenska Vetensk. Akadl. Hand. 1959;(8):1–229. [Google Scholar]
- Wagner G.P., Chiu C.-H. The tetrapod limb: a hypothesis on its origin. J. Exp. Zool. (Mol. Dev. Evol.) 2001;3291:226–240. doi: 10.1002/jez.1100. doi:10.1002/jez.1100 [DOI] [PubMed] [Google Scholar]
- Yu X.-B. A new porolepiform-like fish Psarolepis romeri gen. et sp. nov. (Sarcopterygii, Osteichthyes) from the Lower Devonian of Yunnan, China. J. Vert. Paleontol. 1998;18:261–274. [Google Scholar]
- Zhu M., Fan J.-H. Youngolepis from the Xishancun Formation (early Lochkovian) of Qujing, Yunnan. Geobios M.S. 1995;19:293–299. doi:10.1016/S0016-6995(95)80130-8 [Google Scholar]
- Zhu M., Yu X.-B. Lower jaw character transitions among major sarcopterygian groups—a survey based on new materials from Yunnan, China. In: Arratia G., Wilson M.V.H., Cloutier R., editors. Recent advances in the origin and early radiation of vertebrates. Verlag Dr Friedrich Pfeil; München, Germany: 2004. pp. 271–286. [Google Scholar]
- Zhu M., Yu X.-B., Janvier P. A primitive fossil fish sheds light on the origin of bony fishes. Nature. 1999;397:607–610. doi:10.1038/17594 [Google Scholar]
- Zhu M., Yu X.-B., Ahlberg P.E. A primitive sarcopterygian fish with an eyestalk. Nature. 2001;410:81–84. doi: 10.1038/35065078. doi:10.1038/35065078 [DOI] [PubMed] [Google Scholar]
- Zhu M., Yu X.-B., Wang W., Zhao W.-J., Jia L.-T. A primitive fish provides key characters bearing on deep osteichthyan phylogeny. Nature. 2006;441:77–80. doi: 10.1038/nature04563. doi:10.1038/nature04563 [DOI] [PubMed] [Google Scholar]
- Zhu, M., Zhao, W.-J., Jia, L.-T., Lu, J., Qiao, T. & Qu, Q.-M. In press. The oldest articulated osteichthyan reveals mosaic gnathostome characters. Nature (doi:10.1038/nature07855) [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
High-resolution image of figure 1