Abstract
Trade-offs are central to life-history theory but difficult to document. Patterns of phenotypic and genetic correlations in rhesus macaques, Macaca mulatta—a long-lived, slow-reproducing primate—are used to test for a trade-off between female age of first reproduction and adult survival. A strong positive genetic correlation indicates that female macaques suffer reduced adult survival when they mature relatively early and implies primate senescence can be explained, in part, by antagonistic pleiotropy. Contrasts with a similar human study implicate the extension of parental effects to later ages as a potential mechanism for circumventing female life-history trade-offs in human evolution.
Keywords: life history, quantitative genetics, genetic correlation, maturation, senescence, primate
1. Introduction
Trade-offs among traits that contribute to lifetime fitness are ubiquitous features of models of life-history evolution (Stearns 1989). Trade-offs occur when organisms pay a fitness cost, such as delaying sexual maturation, to gain a fitness benefit, such as increased chances of survival later in life. A genetic correlation results when alleles at a locus influence both traits involved in a trade-off (Roff 2002). Despite the importance of trade-offs to life-history theory, they have been notoriously difficult to document (Roff & Fairbairn 2007). One reason for this problem is that although trade-offs should result from the partitioning of finite resources, environmental variation in resource acquisition can mask the genetic patterns expected from trade-offs (van Noordwijk & de Jong 1986). Phenotypic correlations between life-history traits are thus less likely to reflect trade-offs than their genetic correlations (Reznick 1985).
The characteristic ability of humans to control the resources available within social groups (Kaplan & Robson 2002; Lee 2008) complicates the empirical exploration of trade-offs in human populations. Also, quantitative genetic approaches have rarely been applied to human life-history variation and only in recent agricultural or industrialized settings (e.g. Westendorp & Kirkwood 1998; Kirk et al. 2001; Pettay et al. 2005). Furthermore, the kinds of trade-offs often explored and the non-human taxa in which they have been studied may have little to do with the important trade-offs that have affected females during human evolution (Hawkes & Paine 2006). For these reasons, I applied quantitative genetic techniques to explore female life-history trade-offs in a population of rhesus macaques (Macaca mulatta).
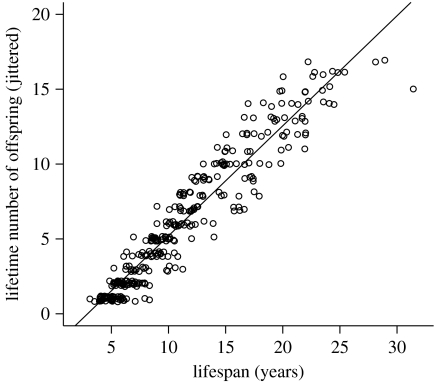
The clearest set of trade-offs for female primates are between current reproduction and survival or, more generally, current and future reproduction. The primate emphasis on extended juvenility, long lifespan and reduced reproductive rate implies that much of their ability to attain high fitness depends on their continued survival from year to year (Martin 1990; Kappeler & Pereira 2003). More than 90 per cent of variation in lifetime number of offspring in female rhesus macaques is explained by adult lifespan (figure 1). Simple demographic models of primate life histories also demonstrate that lifetime fitness (λ, finite rate of increase) is most responsive to changes in adult survival rates (Heppell et al. 2000).
Figure 1.
Regression of lifetime number of offspring on lifespan for female rhesus macaques at Cayo Santiago (p<0.0001, R2=0.91). Small random deviations (jittering) are added to number of offspring to expose overlapping points.
Trade-offs between current reproduction and survival or future reproduction may act over a variety of time scales. If costs of reproduction have long-term additive or multiplicative consequences, it is comparisons of distant life-history events that will reveal trade-offs (Rose & Charlesworth 1994). Indeed, Williams's (1957) antagonistic pleiotropy theory of ageing hypothesizes the existence of trade-offs mediated by loci having opposing fitness effects on these early and late life-history variables. Genetic correlations between the variables can be used to index these pleiotropic effects and assess the strength of proposed trade-offs. This model has received broad support in experimental investigations and some naturalistic studies (Charlesworth 1994; Hughes et al. 2002).
I focus on the relationship between adult survival and female age of first reproduction (AFR)—a key early life-history variable for female primates (Bercovitch & Berard 1993; Charnov & Berrigan 1993; Altmann & Alberts 2005). Furthermore, female AFR is known to be heritable in the study population, making genetic covariance with other life-history traits plausible (Blomquist 2009). A life-history trade-off will be indicated by a positive relationship between AFR and adult survival, because when all other factors are held constant, decreasing AFR will increase lifetime fitness (Roff 2002).
2. Material and methods
I used individual life-history data from a large population of free-ranging rhesus macaques that were transplanted to the 15.2 ha island of Cayo Santiago, Puerto Rico from India in 1938. Monkeys are fed commercial monkey chow and provided water ad libitum but forage on natural vegetation and live in naturally formed social groups (Rawlins & Kessler 1986).
Age of first reproduction (AFR) and survival rates from AFR to four different ages were measured (11, 16, 21 and 26 years). Data were selected to ensure precision of age estimates, omit potentially pathological individuals and avoid biasing survival rates to older ages by including recent birth cohorts in which only young individuals could have died (see the electronic supplementary material for details of data selection and analysis). Pearson's correlations among the life-history variables were calculated in R (R Development Core Team 2007).
Quantitative genetic analyses used univariate and bivariate ‘animal models’ (Kruuk 2004), relying on a large pedigree of the study population, which included paternities determined by microsatellite variation (Nurnberg et al. 1998). Additive genetic and residual (co)variance components and their standard errors were estimated in VCE 5.1 (Groeneveld & Kovac 1990). These are reported as genetic or residual correlations and heritabilities. The significance of a correlation or heritability from zero was tested by comparing the ratio of the value and its standard error to a t-distribution with degrees of freedom equal to the number of observations (Kruuk et al. 2000). Where multiple heritability estimates were available, their mean is reported in table 1.
Table 1.
Phenotypic (rP), genetic (rA) and residual (rR) correlations between female age of first reproduction (AFR) and adult survival rates to age x (sx). Standard errors are given in parentheses and p-values in italics below the correlation (*p<0.10, **p<0.05, ***p<0.01).
| s11 | s16 | s21 | s26 | |
|---|---|---|---|---|
| rP | 0.1094* | 0.1323** | 0.1008 | 0.0834 |
| 0.065 | 0.032 | 0.141 | 0.288 | |
| rA | 0.5900* | 0.5945*** | 0.4763*** | 0.7062** |
| (0.320) | (0.215) | (0.170) | (0.283) | |
| 0.067 | 0.006 | 0.006 | 0.013 | |
| rR | 0.0229 | −0.0281 | −0.0234 | −0.0231 |
| (0.063) | (0.068) | (0.082) | (0.076) | |
| 0.716 | 0.680 | 0.775 | 0.761 |
3. Results
Phenotypic correlations between AFR and each survival rate are all positive but very weak (range: 0.083–0.132). The largest of these correlations are for the younger survival rates (11 and 16 years) and both are statistically significant or nearly so (table 1). The implications of these small positive correlations are that any trade-off between early reproduction and survival is very weak and may only affect survival over the first decade after maturation.
Quantitative genetic analysis indicates modest heritabilities of AFR and survival rates (0.128–0.386). All of these are significantly greater than zero except the smallest, for survival to age 11 (table 2). Genetic correlations are all large and positive (0.476–0.706), indicating a strong trade-off between age of first reproduction and later life survival (table 1). None of the residual correlations between AFR and survival rates are significant, although most of them are weakly negative, implying that environmental effects that promote early maturation will raise later life survival. It is this combination of strong positive genetic correlations and weak negative residual correlations that results in the weak positive phenotypic values.
Table 2.
Descriptive statistics, coefficients of additive genetic variation (CVA) and heritabilities (h2) of female life-history traits. The p-values are for tests of the null hypothesis h2=0.
| n | range | mean | s.d. | CVA | h2±s.e. | p-value | |
|---|---|---|---|---|---|---|---|
| AFR | 1067 | 3–6 | 4.27 | 0.5728 | 0.91 | 0.1285±0.034 | <0.001 |
| s11 | 286 | 0.28–1 | 0.82 | 0.2192 | 0.74 | 0.1279±0.094 | 0.177 |
| s16 | 264 | 0.19–1 | 0.69 | 0.2630 | 2.58 | 0.2627±0.092 | 0.005 |
| s21 | 214 | 0.15–1 | 0.58 | 0.2683 | 4.67 | 0.3861±0.121 | 0.002 |
| s26 | 164 | 0.12–1 | 0.45 | 0.2369 | 3.86 | 0.3206±0.106 | 0.003 |
4. Discussion
The strong positive genetic correlation between female AFR and adult survival identifies an important genetically mediated constraint on the reproductive decision making of female primates. Heavy investment in offspring production early in life imposes a cost of reduced lifespan for female macaques. Using the estimated genetic variances and covariances of AFR and survival rate to age 16, the cost of maturing 1 year earlier is a loss of approximately 11 months of adult life (see the electronic supplementary material). The weaker phenotypic pattern may result from variation in resource abundance or psychosocial stress, which causes earlier maturation and higher survival or later maturation coupled with reduced survival (van Noordwijk & de Jong 1986; Roff & Fairbairn 2007).
While there are a number of phenotypic studies that have suggested associations between early reproduction and longevity in humans (Le Bourg 2007), the only previous estimate of a genetic correlation between AFR and indicators of lifespan or survival for any primate is from pre-industrial Finns (Pettay et al. 2005). The correlation reported was also strongly positive, but it should be regarded with caution because AFR had a non-significant heritability. The macaque results are consistent with this single human estimate. However, AFR in Finns was strongly influenced by maternal effects, while these are very small for female macaques (Blomquist 2009). An intriguing interpretation of this difference is that humans have evolved mechanisms to circumvent life-history trade-offs that are expressed in other female primates, possibly through the deliberate control and redistribution of resources within social groups, perhaps along kin lines, that results in the extension of parental effects to later ages (Cheverud & Moore 1994; Hawkes et al. 1998; Kaplan & Robson 2002; Lee 2008). Similar investigations of human groups under different subsistence modes and residence system may resolve this question (Marlowe 2005; Quinlan 2008).
Acknowledgements
Cayo Santiago is part of the Caribbean Primate Research Center (CPRC), which is supported by the University of Puerto Rico, Medical Sciences Campus and the National Institutes of Health. The genetic database from which paternity data were provided was originally created by J. Berard, F. Bercovitch, M. Kessler, M. Krawczak, P. Nürnberg and J. Schmidtke. The National Science Foundation, Harry Frank Guggenheim Foundation, University of Berlin, Deutsche Forschungsgemeinschaft, Medizinische Hochschule Hannover, NIH and CPRC funded the creation of the genetic database. Additional funding for this research came from the University of Illinois Graduate College. M. Gerald, J. Cant, T. Kensler, B. Hallgrímsson and J. Turnquist were all helpful resources while working with CPRC materials. A. Figueroa, E. Davila and E. Maldonado must be credited for the completeness and upkeep of the demographic records on Cayo Santiago. S. Leigh, P. Garber, C. Roseman, R. Stumpf and J. Cheverud all provided helpful insights into this project. The comments of three anonymous referees improved the manuscript.
Supplementary Material
References
- Altmann J., Alberts S.C. Growth rates in a wild primate population: ecological influences and maternal effects. Behav. Ecol. Sociobiol. 2005;57:490–501. doi:10.1007/s00265-004-0870-x [Google Scholar]
- Bercovitch F.B., Berard J.D. Life history costs and consequences of rapid reproductive maturation in female rhesus macaques. Behav. Ecol. Sociobiol. 1993;32:103–109. doi:10.1007/BF00164042 [Google Scholar]
- Blomquist G.E. Fitness-related patterns of genetic variation in rhesus macaques. Genetica. 2009;135:209–219. doi: 10.1007/s10709-008-9270-x. doi:10.1007/s10709-008-9270-x [DOI] [PubMed] [Google Scholar]
- Charlesworth B. 2nd ed. Cambridge University Press; New York, NY: 1994. Evolution in age-structured populations. [Google Scholar]
- Charnov E.L., Berrigan D. Why do female primates have such long lifespans and so few babies? Or life in the slow lane. Evol. Anthropol. 1993;1:191–194. doi:10.1002/evan.1360010604 [Google Scholar]
- Cheverud J.M., Moore A.J. Quantitative genetics and the role of the environment provided by relatives in behavioral evolution. In: Boake C.R.B., editor. Quantitative genetic studies of behavioral evolution. University of Chicago Press; Chicago, IL: 1994. pp. 67–100. [Google Scholar]
- Groeneveld E., Kovac M. A generalized computing procedure for setting up and solving mixed linear models. J. Dairy Sci. 1990;73:513–531. [Google Scholar]
- Hawkes K., Paine R.R., editors. The evolution of human life history. School of American Research Press; Santa Fe, NM: 2006. [Google Scholar]
- Hawkes K., O'Connell J.F., Jones N.G., Alvarez H., Charnov E.L. Grandmothering, menopause, and the evolution of human life histories. Proc. Natl Acad. Sci. USA. 1998;95:1336–1339. doi: 10.1073/pnas.95.3.1336. doi:10.1073/pnas.95.3.1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppell S.S., Caswell H., Crowder L.B. Life histories and elasticity patterns: perturbation analysis for species with minimal demographic data. Ecology. 2000;81:654–665. doi:10.1890/0012-9658(2000)081[0654:LHAEPP] 2.0.CO;2 [Google Scholar]
- Hughes K.A., Alipaz J.A., Drnevich J.M., Reynolds R.M. A test of evolutionary theories of aging. Proc. Natl Acad. Sci. USA. 2002;99:14286–14291. doi: 10.1073/pnas.222326199. doi:10.1073/pnas.222326199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H.S., Robson A.J. The emergence of humans: the coevolution of intelligence and longevity with intergenerational transfers. Proc. Natl Acad. Sci. USA. 2002;99:10221–10226. doi: 10.1073/pnas.152502899. doi:10.1073/pnas.152502899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappeler P.M., Pereira M.E. University of Chicago Press; Chicago, IL: 2003. Primate life histories and socioecology. [Google Scholar]
- Kirk K.M., Blomberg S.P., Duffy D.L., Heath A.C., Owens I.P., Martin N.G. Natural selection and quantitative genetics of life-history traits in western women: a twin study. Evolution. 2001;55:423–435. doi: 10.1111/j.0014-3820.2001.tb01304.x. doi:10.1111/j.0014-3820.2001.tb01304.x [DOI] [PubMed] [Google Scholar]
- Kruuk L.E.B. Estimating genetic parameters in natural populations using the ‘animal model’. Phil. Trans. R. Soc. B. 2004;359:873–890. doi: 10.1098/rstb.2003.1437. doi:10.1098/rstb.2003.1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruuk L.E.B., Clutton-Brock T.H., Slate J., Pemberton J.M., Brotherstone S., Guinness F.E. Heritability of fitness in a wild mammal population. Proc. Natl Acad. Sci. USA. 2000;97:698–703. doi: 10.1073/pnas.97.2.698. doi:10.1073/pnas.97.2.698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourg E. Does reproduction decrease longevity in human beings? Ageing Res. Rev. 2007;6:141–149. doi: 10.1016/j.arr.2007.04.002. doi:10.1016/j.arr.2007.04.002 [DOI] [PubMed] [Google Scholar]
- Lee R. Sociality, selection, and survival: simulated evolution of mortality with intergenerational transfers and food sharing. Proc. Natl Acad. Sci. USA. 2008;105:7124–7128. doi: 10.1073/pnas.0710234105. doi:10.1073/pnas.0710234105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlowe F.W. Hunter–gatherers and human evolution. Evol. Anthropol. 2005;14:54–67. doi: 10.1016/j.jhevol.2014.03.006. doi:10.1002/evan.20046 [DOI] [PubMed] [Google Scholar]
- Martin R.D. Princeton University Press; Princeton, NJ: 1990. Primate origins and evolution: a phylogenetic reconstruction. [Google Scholar]
- Nurnberg P., et al. Paternity assessment in rhesus macaques (Macaca mulatta): multilocus DNA fingerprinting and PCR marker typing. Am. J. Primatol. 1998;44:1–18. doi: 10.1002/(SICI)1098-2345(1998)44:1<1::AID-AJP1>3.0.CO;2-#. doi:10.1002/(SICI)1098-2345(1998)44:1<1::AID-AJP1>3.0.CO;2-# [DOI] [PubMed] [Google Scholar]
- Pettay J.I., Kruuk L.E.B., Jokela J., Lummaa V. Heritability and genetic constraints of life-history trait evolution in preindustrial humans. Proc. Natl Acad. Sci. USA. 2005;102:2838–2843. doi: 10.1073/pnas.0406709102. doi:10.1073/pnas.0406709102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan R.J. Human pair-bonds: evolutionary functions, ecological variation, and adaptive development. Evol. Anthropol. 2008;17:227–238. doi:10.1002/evan.20191 [Google Scholar]
- R Development Core Team 2007 R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- Rawlins R.G., Kessler M.J., editors. The cayo santiago macaques: history, behavior, and biology. SUNY Press; Albany, NY: 1986. [Google Scholar]
- Reznick D. Costs of reproduction: an evaluation of the empirical evidence. Oikos. 1985;44:257–267. doi:10.2307/3544698 [Google Scholar]
- Roff D.A. Sinauer Associates; Sunderland, MA: 2002. Life history evolution. [Google Scholar]
- Roff D.A., Fairbairn D.J. The evolution of trade-offs: where are we? J. Evol. Biol. 2007;20:433–447. doi: 10.1111/j.1420-9101.2006.01255.x. doi:10.1111/j.1420-9101.2006.01255.x [DOI] [PubMed] [Google Scholar]
- Rose M.R., Charlesworth B. Genetics of life history in Drosophila melanogaster. I. Sib analysis of adult females. Genetics. 1981;97:173–186. doi: 10.1093/genetics/97.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns S.C. Trade-offs in life history evolution. Funct. Ecol. 1989;3:259–268. doi:10.2307/2389364 [Google Scholar]
- van Noordwijk A.J., de Jong G. Acquisition and allocation of resources: their influence on variation in life history tactics. Am. Nat. 1986;128:137–142. doi:10.1086/284547 [Google Scholar]
- Westendorp R.G., Kirkwood T.B. Human longevity at the cost of reproductive success. Nature. 1998;396:743–746. doi: 10.1038/25519. doi:10.1038/25519 [DOI] [PubMed] [Google Scholar]
- Williams G.C. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. doi:10.2307/2406060 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.