Abstract
Reading is a complex skill that is not mastered by all children. At the age of five, on the cusp of pre-reading development, many factors combine to influence a child’s future reading success, including neural and behavioural factors such as phonological awareness and the auditory processing of phonetic input, and environmental factors, such as socioeconomic status (SES). We investigated the interactions between these factors in five-year old children by administering a battery of standardised cognitive and linguistic tests, measuring SES with a standardised scale, and using fMRI to record neural activity during a behavioral task, rhyming, that is predictive of reading skills. Correlation tests were performed, and then corrected for multiple comparisons using the False Discovery Rate (FDR) procedure. It emerged that only one relationship linking neural with behavioural or environmental factors survived as significant after FDR-correction: a correlation between SES and the degree of hemispheric specialisation in the left inferior frontal gyrus (IFG), a region which includes Broca’s area. This neural-environmental link remained significant even after controlling for the children’s scores on the standardised language and cognition tests. In order to investigate possible environmental influences on the left IFG further, grey and white matter volumes were calculated. Marginally significant correlations with SES were found, indicating that environmental effects may manifest themselves in the brain anatomically as well as functionally. Collectively, these findings suggest that the weaker language skills of low-SES children are related to reduced underlying neural specialisation, and that these neural problems go beyond what is revealed by behavioural tests alone.
1 Introduction
Many factors interact to shape children’s language development and reading ability (Goswami, 2006; Rayner et al., 2001). Behavioural tests have been a rich source of insights into children’s language and reading skills, but they cannot, on their own, reveal the mechanisms underlying them. Moreover, it can be difficult to determine whether problems on a language task are due to limitations of competence or of performance. Performance factors such as attention, motivation and response-speed can result in lower scores, even if the neural information processing necessary to carry out the task is successfully taking place. Neuroimaging studies offer an opportunity to probe the brain’s language areas directly, provided that a given task engages them sufficiently to produce measurable activation. Thus, neuroimaging may potentially allow us to tease competence and performance apart.
Environmental factors also play a major role in shaping children’s language and reading skills. One set of such factors that can be quantified, albeit far from perfectly, is socioeconomic status, or SES (Hollingshead, 1975). SES has consistently been found to have a major effect on children’s language skills (Bowey, 1995; Hoff, 2003; Huttenlocher et al., 2002; Noble et al., 2007; Walker et al., 1994). For example, Noble et al. (2007) recently found that SES explained 32% of the variance in children’s scores on phonological and vocabulary tests.
In the present study, we set out to uncover which out of a broad set of neural, behavioural and environmental variables are the most important in contributing to reading-related language skills. Our subjects were children aged five years old, not yet in kindergarten. Each child was given a battery of standardised cognitive and linguistic tests: the Peabody Picture Vocabulary Test (PPVT) (Dunn & Dunn, 1997), the Clinical Evaluation of Language Fundamentals test (CELF) (Semel et al., 1995), the Wechsler Preschool and Primary Scale of Intelligence (WPPSI) (Wechsler, 1989), and the Phonological Awareness Test (PAT) (Robertson & Salter, 1995). The subcomponents of these tests are listed in the Methods, below. In addition, the children’s socioeconomic status (SES) was recorded using the Hollingshead index (Hollingshead, 1975), which is the most broadly used SES measure, and which assesses factors such as parental education-level and occupation to produce an overall SES score.
To investigate neural factors, we scanned the children with fMRI while they were performing a rhyme-judgment task of auditorily presented monosyllabic words and non-words (Richards et al., 1999), with a control task of auditory tone discrimination. Rhyme tasks have been found to be sensitive measures of phonological awareness, which is strongly predictive of subsequent reading ability (Bryant et al., 1989).
2 Materials and methods
2.1 Subjects
Our subjects were children five years of age, and were all in preschool but not yet in kindergarten. Before any fMRI-scan visits, each child was given a session in a mock scanner, in order to familiarise them and also to screen out those who were not comfortable with the procedure. Out of 30 children who tried the mock scanner, 20 went on to try the real scanner, 15 completed the fMRI scan, and 14 yielded usable fMRI data. Of these 14, seven were female, all but one were right-handed, the age-range was 5 years 0 months to 5 years 8 months, and the average age was 5 years 4 months. The University of Washington Human Subjects Institutional Review Board approved this study, and each subject (as well as parent/guardian) gave written, informed consent.
2.2 Standardised tests
SES was calculated for each child’s family using the Hollingshead index (Hollingshead, 1975). The standardised tests administered were the Peabody Picture Vocabulary Test (Dunn & Dunn, 1997), the Phonological Abilities Test (PAT: rhyming, segmentation, isolation, deletion, blending. Robertson & Salter, 1995) the Clinical Evaluation of Language Fundamentals test (CELF: core language, receptive, expressive, language content, language structure, recall sentence context. Semel et al., 1995) and the Wechsler Preschool and Primary Scale of Intelligence (WPPSI: Verbal IQ, Performance IQ, Processing Speed IQ, Full Scale Intelligence Score, General Language Score. Wechsler, 1989).
2.3 MRI
The fMRI was carried out in a 1.5T GE Signa scanner. Twenty one axial slices (6mm thick, 1mm gap) were collected using a gradient-echo echo-planar pulse sequence: repetition time, TR = 3000ms; echo time, TE = 50ms, field of view = 240mm, matrix size 64 × 64. Each child performed one fMRI run, consisting of 114 TRs, lasting 5 mins 42 secs in total. The first three volumes were discarded, to allow the scanner time to equilibrate.
The tasks carried out during the scan were judgment of whether auditorily presented monosyllabic words and non-words rhymed, and a control task of auditory tone discrimination (Richards et al., 1999). The tasks were presented in alternating blocks, four blocks of each task, with each block lasting 30s and containing five trials. Six seconds before each block, the children received visual and auditory instructions telling them which task was about to follow.
High-resolution T1-weighted scans were also acquired, for anatomical localisation. These scans used a 3D fast spoiled gradient echo pulse sequence. Imaging parameters were as follows: TR = 11.1 ms, TE = 2.2 ms, flip angle 25 degrees, field of view = 240 mm, with 124 sagittal slices, slice thickness 1.4 mm, in-plane voxel size 0.9375 × 0.9375 mm. The entire acquisition time was 4 min 36s. While the structural scan was being carried out, the children watched a DVD movie of their choice.
2.4 Voxel-based morphometry
Segmentation of the T1 anatomical scans into grey and white matter was performed using SPM5, with the addition of Christian Gaser’s VBM5 Toolbox for SPM5, version 1.03 (Gaser, 2007).
2.5 Statistical analysis
To look for correlations between brain, behaviour and the environment, average fMRI activation was calculated for each task condition and brain hemisphere, using anatomically-defined regions-of-interest (ROIs) from a standard atlas (Tzourio-Mazoyer et al., 2002). For our ROIs, we chose regions known to be involved in language processing: Heschl’s gyrus, inferior frontal gyrus (opercular, orbital and triangular parts separately, and also all three together), inferior parietal cortex, middle temporal gyrus, superior temporal gyrus, supramarginal gyrus, and the angular gyrus. For each ROI, we calculate the activations for each condition, and also the following derived measures: rhyme-task minus tone-task, left minus right hemisphere, and a hemispheric laterality index. The laterality index was calculated using the formula (L − R)/(|L| + |R|).
The calculated correlations were excluded if they were driven by outliers, defined as lying more than two standard deviations from the mean. The False Discovery Rate procedure was implemented in the standard manner: the p-values were sorted in ascending order, p1, p2, …, pi, … pV where V is the total number of correlations in the matrix, and then the threshold p-value was the largest pi such that pi ≤ q × i/V, where q is the desired corrected probability value, in this case 0.05 (Benjamini & Hochberg, 1995; Genovese et al., 2002).
Preprocessing and statistical analysis of the data were performed using SPM2 software (Wellcome Dept of Cognitive Neurology). Preprocessing included motion correction, normalization to the MNI305 stereotactic space (interpolating to 3mm cubic voxels) and spatial smoothing with an 8mm isotropic Gaussian kernel. Statistical analysis was performed using the general linear model. Global signal scaling was not applied, in order to prevent spurious deactivations. Comparisons of interest were implemented as linear contrasts. This analysis was performed individually for each subject, and contrast images for each subject were used in a second-level analysis treating subjects as a random effect.
3 Results
3.1 Subject diversity
The SES scores in our subjects ranged from 31.5 (the parents’ professions were fire-fighter and shop-assistant, and they did not have education past Community College) to 66 (one or both parents had graduate degrees and worked in a skilled professional job, e.g. aeronautical engineer). Thus, although our efforts to recruit subjects from a broader range of socioeconomic strata than are typically included in university studies were successful, the spread could best be described as spanning from “blue collar” to “white collar”. Similarly, the children’s scores on standardised tests tended to be slightly above the value of 100 that is meant to capture the population mean, but the overall variability of scores was sufficient to span a workable range. For example, the mean CELF Core Language score in our sample was 109.8, but the spread ranged from 86 to 127. Thus, our sample spanned a reasonable range of SES and standardised test scores; ultimately, however, a broader range would be desirable. Future studies testing the generalisability of the results presented here should aim to include even greater diversity.
3.2 ROI analysis
The fMRI activation levels were calculated in a set of regions-of-interest, or ROIs, covering brain areas known to be important for language (see Methods, below). For each ROI, we calculated the activation for each task, and also the following derived measures: rhyme-task minus tone-task, left minus right hemisphere, and a hemispheric laterality index. The fMRI scans also provided us with additional behavioural variables: each child’s average head motion, and the percentage correct for the rhyme and tone tasks during the practice and test runs.
3.3 False-Discovery Rate multiple comparisons correction
The large number of factors interacting to shape children’s language development raises a serious challenge for research with this sort of design. A study can focus narrowly on just one or two factors, but then risk missing key mechanisms, or it can investigate a broader set of measures, thereby incurring the statistical problem of needing to separate out, from amongst multiple comparisons, the meaningful results from those arising just by chance. Classical multiple-comparison tests such as Bonferroni-correction tend to come at a great cost to statistical power, and therefore reintroduce the risk of missing important effects that using the broader set of measures was supposed to avoid.
The recently developed statistical technique of calculating a “False Discovery Rate,” or FDR (Benjamini & Hochberg, 1995), solves this problem by providing a rigorous and easily-implemented multiple-comparisons correction procedure, without excessive loss of statistical power. The FDR method, which has recently started to be used widely in functional neuroimaging (Genovese et al., 2002) and also in genetics research (Broberg, 2005), provides a statistical threshold such that for any individual finding there is only a specified probability (e.g., p<0.05) of it being a false positive. In contrast, traditional, more conservative approaches such as Bonferroni correction yield thresholds for which the chance is p<0.05 that there is even a single false positive result anywhere in the study sample. In the Supplementary Material, the implementation of the FDR procedure is described and graphically illustrated (Supp. Fig. 1), and the strengths and limitations of the present FDR test are discussed in detail.
3.4 FDR-corrected correlation matrix
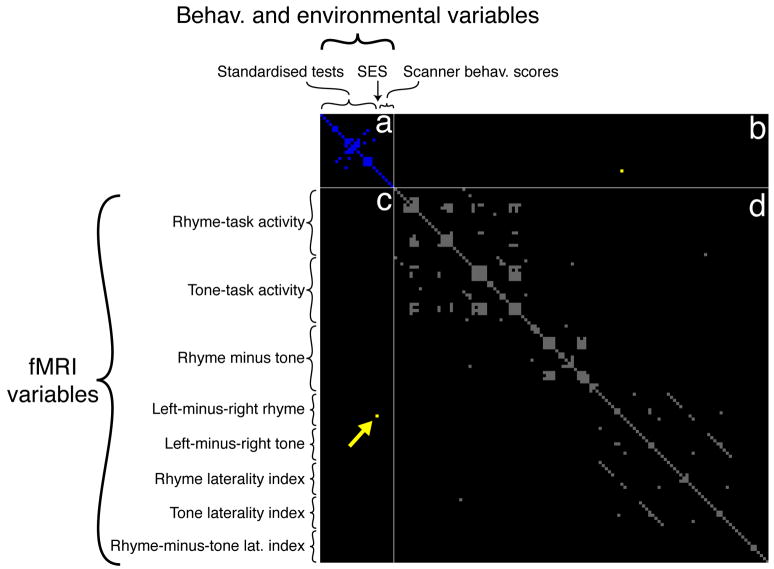
We applied the FDR method to our set of neural, behavioural and environmental variables, in order to find the most important interactions between them. The matrix of correlations between the variables was calculated, was then statistically corrected by removing the spurious outlier-driven correlations, and finally had a statistical threshold applied to it, corresponding to a false-discovery rate of p<0.05 (equal to an uncorrected value of p<0.00108). The resulting thresholded correlation matrix, showing the significant correlations between the 145 different variables, is shown in Fig. 1. An unthresholded version of this same correlation matrix, plotting the raw p-values on a continuous gray-scale colour-map, is shown in Supplementary Figure 2.
Figure 1.
The matrix of correlations between the 145 neural, behavioural and environmental variables. The correlations were statistically thresholded at a multiple-comparisons corrected p-value of p<0.05, using the False Discovery Rate (FDR) method (Benjamini & Hochberg, 1995; Genovese et al., 2002). The behavioural and environmental variables are grouped together, as are the fMRI variables. As a result of this grouping, quadrant (a) contains significant correlations within the behavioural and environmental variables, shaded in blue; quadrant (d) contains significant correlations within the fMRI variables, shaded in gray; quadrants (b) and (c), which are symmetrically identical, contain the significant correlations between the behavioural/environmental variables and the fMRI data, shaded in yellow. Only one such correlation survives FDR-correction: the correlation between SES and the hemispheric asymmetry of rhyme-task activation in the inferior frontal gyrus. This correlation is marked by the yellow arrow in quadrant (c). Note that the thresholded correlation matrix shown here indicates only whether a given entry in the correlation matrix passes or fails the FDR-significance test, and hence it does not depict actual correlation-values or p-values themselves. An unthresholded version of this same correlation matrix, plotting the raw p-values on a continuous gray-scale colour-map, is shown in Supplementary Figure 2.
The data reveal many statistically significant correlations within the behavioural and environmental variables, shaded in blue, and even more within the fMRI variables, shaded in gray. However, only one correlation between the behavioural/environmental variables and the fMRI data survives FDR-correction: the correlation between SES and rhyme-task left-minus-right activation in the inferior frontal gyrus (henceforth referred to as the IFG-asymmetry). The data underlying this correlation from all fourteen children are shown in the scatterplot in Fig. 2b.
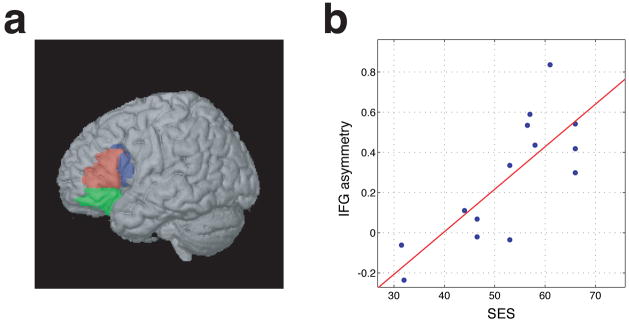
Figure 2.
(a): The three parts of the inferior frontal gyrus (IFG) studied here, using anatomically defined regions-of-interest (ROIs) from a standard atlas (Tzourio-Mazoyer et al., 2002) The orbital part is green, the triangular part is red, and the opercular part is blue. (b): The correlation between socioeconomic status (SES) and the left-minus-right rhyme-task activity in the inferior frontal gyrus (termed IFG-asymmetry). Each dot represents one child. Considered individually, the correlation is significant at p<0.001 (r = 0.78, p = 0.00098, uncorrected). Considered as part of the 145 × 145 matrix of correlations between all the measured variables, it is significant after FDR multiple-comparisons correction at p<0.05.
3.5 Correlations with SES of the left IFG, right IFG, and left-minus-right IFG
An interesting and at first sight somewhat counter-intuitive aspect of our data is that although the left-minus-right IFG activation in the rhyme task correlates strongly with SES, the activations in the left and right hemispheres considered individually are much less strongly correlated with SES. This is illustrated in Supplementary Figure 3, which shows scatterplots of the correlations of the individual hemispheres and also the left-minus-right hemispheric activation difference.
A likely explanation for this result is that baseline fMRI activation values often show large variability across subjects. The average activation with a single ROI from a given subject will reflect this variability. However, by taking two measurements from within each subject and computing the difference between them, much of this inter-subject baseline-variability is canceled out. The left-minus-right activation is of course just such a within-subject subtraction. This left-minus-right measure should therefore suffer from much less baseline-variability noise than the single-hemisphere activations, thus allowing correlations with environmental variables such as SES to manifest themselves more cleanly.
3.6 Correlations of subparts of IFG
Although Broca’s area occupies much of the left inferior frontal gyrus, the two are not identical. Broca’s area is defined by its function and cytoarchitecture, rather than by gross anatomical landmarks, and it varies significantly in location from person to person (Amunts et al., 1999). Therefore, in order to measure activation in a consistent way across our subjects, we used predefined anatomical regions-of-interest (ROIs), from a standard anatomical atlas (Tzourio-Mazoyer et al., 2002). For sensory or motor areas, separate functional localiser scans are sometimes used in order to try to identify the region’s location in each individual subject (Pulvermuller et al., 2006). However, higher-level brain areas do not lend themselves to isolated stimulation of this sort. Moreover, for young children scanning time must be kept brief. Therefore, atlas-based anatomical ROIs offered the best method of consistent across-subject region selection.
The ROIs that we selected for study were the main brain areas known to be involved in speech and language processing: Heschl’s gyrus, the superior temporal gyrus, the middle temporal gyrus, the supramarginal gyrus, the angular gyrus, the inferior parietal cortex, and the inferior frontal gyrus (orbital, opercular, and triangular parts), in both the left and right hemispheres in all cases. Broca’s area is generally considered to be approximated by the combination of the opercular and triangular parts (Brodmann’s areas 44 and 45, respectively). Given the existence of intersubject anatomical and functional variability and the imperfect fit of adult atlases to children’s brains, predefined anatomical ROIs can at most hope to capture as many of the individual subjects’ Broca’s areas as possible.
To investigate the relations between SES and Broca’s area in more detail, we calculated the average signal in each of the three IFG regions individually, in the union of the opercular and triangular parts, and in all three together. The locations of these regions in the brain, and the relationship between SES and IFG-asymmetry, are shown in Figure 2.
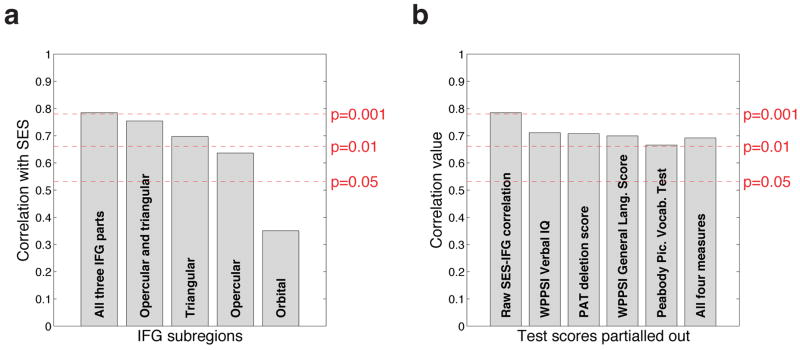
The correlation between SES and the degree of asymmetry (calculated by left-minus-right rhyme-task activation) was computed separately for each of these three IFG subregions, as shown in Fig. 3a. As can be seen from that figure, the union of all three IFG regions together correlates best with SES, followed by the triangular-opercular combination, and then the individual IFG subregions: triangular, then opercular, and lastly orbital. It might at first sight seem surprising that the union of all three IFG regions correlates better with SES than the triangular-opercular combination, which should notionally correspond more closely to Broca’s area. A likely explanation for this is that, due to anatomical and functional variability, some of the children’s individual Broca’s areas extend beyond the more restrictive triangular-opercular ROI, and that the union of all three IFG regions therefore succeeds better at encompassing all of the individual subjects’ actual Broca’s areas, albeit at the cost of also including some non-Broca’s tissue.
Figure 3.
(a): The correlations of the different subparts of the IFG with socioeconomic status. (b): The partial correlations between SES and IFG-asymmetry, after removing the effects of various standardised test scores. The test that was partialled out is marked on each bar, with the left-most bar showing the correlation without any partialling, and the right-most bar showing the effect of partialling out the four test scores simultaneously.
3.7 Partial correlations to factor out language skills
This strong correlation between SES and specialisation in the left IFG, emerging as the only significant correlation between the neural and the environmental and behavioural variables, raises three key questions. Is the SES/left-IFG link merely what would be predicted from known correlations between SES and behavioural language skills? Is the fMRI revealing a relationship between SES and language-processing that purely behavioural tests are unable to reveal? Finally, is there a causal connection between SES and the left IFG that operates via non-linguistic mechanisms?
If the SES/left-IFG link goes no further than what would be predicted from known correlations between SES and behavioural language skills, then partialling out the effect of scores on standardised language tests should abolish, or at least greatly weaken, the link. However, this is not what we observed. In fact, the SES/left-IFG link remains significant, even after correcting for the effects of all the language-score measures that are most strongly correlated with either SES or IFG-asymmetry.
To carry out the partial correlation analysis, we selected all the standardised test scores that correlated at p<0.05 (uncorrected) with either or both of those measures. These were: WPPSI Verbal IQ, WPPSI General Language Score, PAT phoneme deletion, and the Peabody Picture Vocabulary Test. The results of partialling out these variables are shown in Figure 3b. The correlation between SES and IFG-asymmetry remains significant at p<0.05, regardless of which of these four measures is partialled out. Indeed, in the worst case, namely partialling out the Peabody Picture Vocabulary Test, the correlation just barely misses the significance level of p<0.01. Moreover, the correlation remains significant at p<0.05 even when all four of the above measures are partialled out simultaneously.
Two measures that might, a priori, have been expected to correlate with SES and IFG-asymmetry were percentage-correct in the scanner rhyming task, and the PAT Rhyming score. However, these measures were found to correlate only weakly or not at all with SES and IFG-asymmetry, and hence would have been unable to act as confounding factors. (Scanner task percentage-correct: corr. with SES, r = 0.35, p>0.2, corr. with IFG, r = 0.065, p>0.8; PAT rhyming score: corr. with SES, r = −0.029, p>0.9, corr. with IFG, r = −0.030, p>0.9). Although the PAT Rhyming score did not correlate with SES or the IFG, it did show a relation (r = 0.68, p = 0.011 uncorrected) to rhyme-task activation asymmetry in a different language area, the supramarginal gyrus, which is known to be important in reading and dyslexia (Simos et al., 2000).
The low correlation between the children’s behavioural performance in the scanner and SES or IFG-asymmetry is likely due to the fact that some of the children seemed to press the response button randomly when in the machine, despite having been familiarised with the task before-hand, and also despite showing good understanding of rhyming outside of the scanner in the standardised tests. In five-year old subjects, this noncompliance is perhaps unsurprising. Our fMRI results suggest that the language areas of such subjects were indeed successfully processing the rhyme-task, but that this did not always translate into correct button-presses. Handedness was also not a factor. Only one of the children was left-handed, and that child had left-minus-right IFG activity greater than the group average. Age was also not a factor: the children’s ages did not correlate with SES (r = −0.05, p>0.85) or with IFG-asymmetry (r = 0.15, p>0.6), and the spread of the age-range was small: 5 years to 5 years 8 months.
Returning to our three questions above, our results, as shown in Fig. 3b, answer the first one in the negative: the SES/left-IFG link is not merely what would be predicted from known correlations between SES and behavioural language skills. Although some of the behavioural measures correlate well with SES and also well with the left IFG, the removal of the effect of any of them individually, or even of all of them together, fails to rob the SES/left-IFG link of statistical significance.
3.8 Further exploration of the SES/left-IFG link: correlations with neuroanatomy
The primary purpose of this study was to investigate relations between the environment, behaviour, and language-related neural processing in children. However, neuroanatomical differences across individuals can also correlate with variations in linguistic ability, and hence are also of potential interest, although static anatomy tends to reflect processing differences to a less degree than does functional activation.
Given the strong correlation between SES and inferior frontal cortex that emerged from the fMRI data, we investigated this area further by quantifying its neuroanatomy. Specifically, we wished to test the hypothesis that the left IFG region, whose activation’s hemispheric asymmetry was revealed to be related to SES in the above analyses, might also show a connection with SES at the anatomical level. Other studies of reading in children have demonstrated that combining structural and functional analyses can be a powerful approach (Hoeft et al., 2007). For the neuroanatomical analysis, we followed the same approach used in the fMRI analysis of using pre-defined anatomical ROIs from the Tzourio-Mazoyer et al. (2002) atlas, thereby ensuring uniformity in the anatomical parcellation across subjects and, by pooling across larger ROIs, helping to avoid finding spurious small local neural inter-subject differences. Specifically, the subjects’ anatomical scans were segmented into grey and white matter, and the volume of each tissue type within each ROI was calculated. Similar ROI-based volumetric approaches using the Tzourio-Mazoyer et al. (2002) atlas have been applied in studies of schizophrenia (Lee et al., 2007; Lopez-Garcia et al., 2006; Mitelman et al., 2007, 2005, 2003), of autism (Rojas et al., 2006) and of dementia (Rosano et al., 2005).
A potential difficulty for analysing the anatomical scans of young children is that the process of segmentation into grey and white matter can be quite sensitive to image noise and artifacts caused by head-motion. To address this problem, we used the VBM5 Toolbox for SPM by Christian Gaser (Gaser, 2007), which uses Hidden Markov Random Fields to reduce the occurrences of artifactual “speckles” of one tissue type inside another. Using this toolbox, we found that despite the presence of noise in several of the images, all the children’s T1 scans gave good quality segmentations. All fourteen segmentations are shown in Supplementary Figure 4.
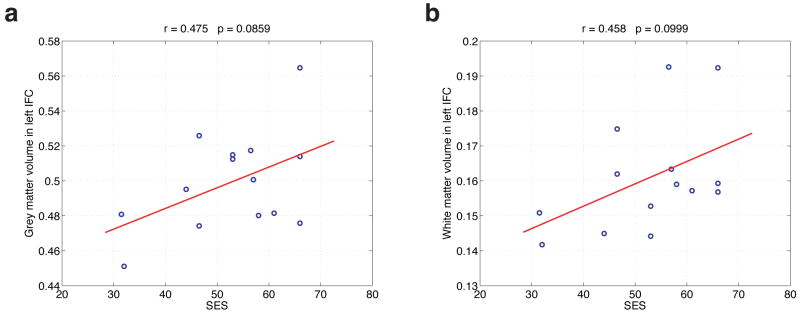
It was found that both grey matter volume and white matter volume in the left IFG showed marginally significant correlations with SES, as is shown in Figure 4. This therefore weakly confirms the above hypothesis that this region would be relatable to SES not only functionally but also anatomically. It seems likely that a larger sample of children might suffice to push the marginally significant correlation below the p < 0.05 threshold, and it would be interesting to explore this in future work.
Figure 4.
(a): The correlation of grey matter volume in the left IFG with SES. (b): The correlation of white matter volume in the left IFG with SES.
The functional MRI data did not generate comparable hypotheses about the other ROIs analysed in the study, but for control purposes we also calculated their grey and white matter volumes, the corresponding correlations with SES, and also the correlations for a left-minus-right derived volume measure. It was found that no ROI outside of the left IFG had a stronger correlation with SES in any of these measures. The only stronger correlations that emerged were from subparts of the left IFG itself: the grey matter in left IFG orbital part had a slightly stronger correlation, and for white matter the compound left opercular-triangular subpart was more strongly correlated with SES than was the whole IFG region comprising all three subparts. The right-hemisphere IFG correlation with SES was weaker, both for gray-matter (p = 0.16) and white-matter (p = 0.25), and the left-minus-right IFG measures were quite uncorrelated with SES (gray-matter: p > 0.7, white-matter: p > 0.35). Histograms showing the distribution of all the ROIs’ correlations and the positions of the left IFG results in that distribution are shown in Supplementary Figure 5.
In a study of the anatomical left-hemisphere versus right-asymmetry asymmetry of the temporal plane in 11-year old children, Eckert et al. (2001) found that greater asymmetry was predictive of increased phonological awareness skills, but that this anatomical measure was not related to SES. In our data, we also did not find any correlation between SES and the measures of temporal lobe ROIs. However, given the difference in our subject age-groups (5 years vs. 11 years) and the differences in anatomical measures (volume vs. asymmetry), it is unclear whether these two negative results are strongly connected.
4 Discussion
The present study investigated a broad range of behavioural, neural and environmental factors involved in five-year-old children’s language skills, and found that the strongest relationship amongst them was between SES and the degree of hemispheric specialisation in the left IFG. This neural-environmental link remained significant even after controlling for the children’s scores on the standardised language and cognition tests. Exploring the left IFG’s relation to SES further, we found that its grey and white matter volumes showed marginally significant correlations with SES, suggesting that the link may manifest itself anatomically as well as functionally.
These results highlight the key advantage gained by adding neuroimaging to purely behavioural measures. It is likely that many developmental processes affect particular brain areas, such as Broca’s, with specificity. However, in behavioural tests, we are testing the whole person; no matter how refined the test, the score will be some compound function of perception, cognition, attention and motor control. With fMRI, we have the luxury of being able to probe the response of the left IFG directly, without needing to pass through the complex filter of behavioural responses. From this, the answer to our second question follows: the fMRI is indeed revealing a relationship between SES and language-processing that the purely behavioural tests fail to show.
4.1 Possible causal links
The last of our three questions, however, remains so far unanswered: is there a causal connection between SES and the left IFG that operates via non-linguistic mechanisms? The fact that the SES/left-IFG link goes beyond what would be predicted from the language tests raises the possibility that SES may affect the brain not only via linguistic mechanisms, but also via extra-linguistic causal pathways, such as nutrition, quality of healthcare, and stress. In nutrition-related measures, at least, our data reveal no gross effects: the children’s weights did not correlate with SES (r = 0.10, p > 0.7). In order to account for our data, any extra-linguistic factors would have to affect the brain without impacting upon language test scores. Although perhaps unlikely, such a possibility cannot be ruled out a priori. It could also in principle be possible for such mechanisms, despite being non-linguistic, to impact more upon Broca’s than they do upon other brain areas; higher-level processing, such as is carried out in Broca’s area, is often especially vulnerable to environmental adversity.
Although extra-linguistic mechanisms linking SES to Broca’s development may well exist, there is no shortage of linguistic causal pathways that could also connect the two. Language development is strongly affected by the richness of the linguistic environment in which a child is raised. One of the key variables measured in the Hollingshead SES scale is the education level of the child’s parents, and greater education is predictive of richer parental vocabulary and syntax, of more books in the home, of more help at home with reading, and of several other factors that could all contribute to a child’s language development (Bowey, 1995; Hoff, 2003; Huttenlocher et al., 2002; Noble et al., 2007; Walker et al., 1994). Although the children in our study spanned a fairly large range of the socioeconomic spectrum, it would be desirable for follow-up studies to include an even broader range, especially at the lower end of the scale, where language-difficulties are likely to be more pronounced.
In order to explore hypothesised causal connections properly, future follow-up studies would need to be both longitudinal and interventional (Bradley & Bryant, 1983). An interesting possible manipulation would to be select children whose test scores fall into a narrow range but who span a broad spectrum of SES. Any differences in brain activation observed across these children would therefore presumably be attributable to variations in SES, rather than performance. Conversely, by selecting children from a narrow range of SES but whose test scores vary widely, the effect of SES on brain activation could be experimentally controlled for. In the present study, partial correlation analyses attempt to achieve similar controls using post-hoc statistical methods. However, with sufficiently large and diverse sample sizes, future studies may be able to achieve these direct and more powerful empirical controls instead.
4.2 Development of asymmetry in Broca’s area
In the present study, we examined the average activity in several language-related areas (see the Methods section for a full list), and considered the activation for each area and each task individually, as well as looking at left-minus-right and rhyme-minus-tone contrasts. It is therefore notable that it was the left-minus-right activity that emerged as significantly correlating with a behavioural or environmental measure, as opposed to the plain activity level of any area considered individually. Moreover, of all the brain areas included in our study, it is Broca’s area for which the left-right asymmetry has been best established as being a meaningful indicator, and in our data it was the left IFG whose asymmetry emerged as significant. More specifically still, only the rhyme-task activation-asymmetry emerged as meaningful; the tone-task asymmetry did not correlate significantly with any of the scores on standardised tests.
Cytoarchitectural studies of Broca’s area by Amunts, Zilles and colleagues have shown that its asymmetry increases with age, peaking at around five years of age in area 45 (the triangular part of the IFG) and at around eleven years in area 44 (the opercular part) (Amunts et al., 2003). Their findings fit perfectly with the functional asymmetry observed in the five-year old children in our study, raising the question of whether cytoarchitectural changes may in fact be underlying the differences in neural activation. Unfortunately, in vivo methods for investigating cytoarchitecture have yet to be developed, but future technical advances may allow this structure/function hypothesis to be tested.
Developmental studies of the neuroanatomy of Broca’s area also fit with our finding of marginally significant correlations between SES and both grey and white matter volume in that region. An interesting hypothesis that could be explored in future work is whether the richer linguistic environments that children in higher-SES households are exposed to may lead to greater volume in Broca’s area, in addition to the increased asymmetry of functional activation observed in the present study. Recent studies have found evidence that extended training and practice does appear to induce measurable neuroanatomical changes in visual cortex (Draganski et al., 2004), the hippocampus (Draganski et al., 2006) and the cerebellum (Cannonieri et al., 2007), thus raising the possibility that stimulation from a child’s linguistic environment may induce analogous changes in Broca’s area.
4.3 Strengths and limitations of the present tests
The present study has usable fMRI data from 14 children (a subset of the larger group of 30 children who tried the mock-scanner as an initial practice step). Although this is quite a respectable number of subjects for a functional imaging study of five-year old children, any attempt to make a truly definitive statement about the population of children in this age group would require a larger study. Similarly, although we made efforts to recruit children from as broad a range of socioeconomic conditions as we could, our sample of families does not draw from the much lower-SES populations that also exist.
With regard to the statistical analyses, the strong SES/IFG correlation that we observed survives two types of tests which take into account the fact that several different data variables were collected and tested together: an FDR multiple-comparisons correction, and partial-correlation tests to remove the effects of related variables. In the Supplementary Material, the implementation of the FDR procedure is described and graphically illustrated, and its strengths and limitations are discussed in detail. Collectively, these tests strengthen our results, and draw attention to an empirical link between environmental and neural factors important to education. The results therefore provide a motivation and rationale for future investigations that explore the observed link.
4.4 Relation to previous studies
Our finding of a link between SES and the specialisation of Broca’s area is consistent with, but goes beyond, previous findings in the literature. Socioeconomic status has been found to have a major effect on children’s language skills (Bowey, 1995; Hoff, 2003; Huttenlocher et al., 2002; Noble et al., 2007; Walker et al., 1994), and our own behavioural data is consistent with that finding; two test scores correlated with SES significantly at an uncorrected p-value of p<0.05: the Peabody Picture Vocabulary Test (Dunn & Dunn, 1997) (r = 0.68, p<0.01), and the WPPSI (Wechsler, 1989) Verbal IQ (r = 0.54, p< 0.05). However, very few MRI studies of children’s language skills have included SES as a factor (Eckert et al., 2001; Noble et al., 2006; Shaywitz et al., 2003), and none has yet demonstrated any clear relationship between SES and neural activity. As far as we are aware, the only previous study to have shown a relationship between SES and neural activity is that of Noble et al. (2006). They found a complex modulatory role for SES, in which the higher the SES, the less typical were the children’s brain-behaviour relationships. In contrast, the present finding is quite direct: the higher the SES, the greater the degree of specialisation of the left IFG. One possible cause of the difference between our results and those of Noble et al. is that they used a word-reading task, which requires not only phonological processing but also visual decoding. In contrast, our task was rhyme-judgment, and used purely auditory presentation. It is possible that our task therefore tapped more specifically into processes of phonological manipulation, hence probing Broca’s area more directly. Other differences between the two studies are that Noble et al. specifically studied children with below-average phonological awareness scores, and that their children were slightly older (aged 6 to 9).
As children who struggle with reading become older, some of them develop compensatory strategies that can help to alleviate the difficulties, albeit without completely removing them. Both the Noble et al. (2006) and Shaywitz et al. (2003) studies suggest that these compensatory processes may act to recruit more right-hemisphere activation for reading, and that this recruitment takes place to a greater degree in higher SES groups, indicating that it may be partly fueled by the more resource-rich environments. Specifically, Shaywitz et al. (2003) found that adults who read well but who previously showed reading impairment showed more right-sided perisylvian and superior frontal activity during reading, whereas persistently poor readers did not, and moreover that it the accuracy-improved readers had attended more socially advantaged schools (measured by the proportion of pupils receiving subsidised meals) than the persistently poor readers. The Noble et al. (2006) found a result pointing in a similar direction: looking for brain areas whose activation correlated with phonological awareness scores, they found more left perisylvian activity in lower SES children, and more right perisylvian activity in children who were of higher SES.
In our study, the children were young (five years old) and were not pre-selected for having any language difficulties (indeed, children with a known history of language impairment were excluded). Thus, in this subject group, neither the need for compensatory strategies nor the time to develop them would so far be present. In these children, higher SES predicted greater left-hemisphere specialisation. In contrast, amongst the older children and adults of the Noble et al. (2006) and Shaywitz et al. (2003) studies, it appears that higher SES environments may facilitate compensatory processes that act in the opposite direction, namely by increasing right-hemisphere recruitment. An intriguing possible hypothesis is that during early development, greater environmental resources may help to drive the development of typical left-hemisphere language processing, but that later in life, better environmental resources may help children whose left-hemisphere language regions have not fully developed to devise compensatory strategies which act to recruit processing on the right. It is clear that future studies will be needed to unpick the complex neural and environmental processes shaping children’s developmental trajectories.
The correlation in our data between SES and the hemispheric specialisation of the left IFG remained significant, even after partialling out the results of standardised language tests. Thus, the fMRI is revealing a relationship between SES and language-processing that the purely behavioural tests fail to uncover. Whereas behavioural measures are obliged to test the whole person at once, fMRI can directly probe specific brain areas, thereby providing a sensitive measure of how the environment impacts upon cognitive and neural development.
4.5 Conclusion
SES has consistently been found to have a major effect on children’s language skills (Bowey, 1995; Hoff, 2003; Huttenlocher et al., 2002; Noble et al., 2007; Walker et al., 1994), and our behavioural data were consistent with these previous findings, showing moderate correlations between SES and the PPVT and WPPSI Verbal IQ. However, the correlation between SES and left-minus-right rhyme-task activation in the IFG was stronger than either of these behaviour-SES relations, and indeed remained significant even after the effects of those behavioural measures were partialled out.
Thus, our results demonstrate that the weaker language skills of low-SES children are not just a behavioural phenomenon, but more fundamentally are linked by the present study to weaker neural specialisation. This finding raises the question of what might underlie this linkage between SES and the development of language processing in the brain (Greenough et al., 1987; Ramey & Ramey, 1998). One candidate mechanism that we are currently investigating is the richness of the vocabulary and syntax to which a child is exposed. The education level of the children’s parents, along with other SES-related factors such as the number of books in the home, are important contributors to the richness of a child’s linguistic environment (Bowey, 1995; Hoff, 2003; Huttenlocher et al., 2002; Noble et al., 2007; Walker et al., 1994).
The children in this study were five years old and not yet in kindergarten. At this age crucial pre-reading skills are developing, and these subskills underpin later success when formal schooling in learning to read starts. Several of these key pre-reading skills were probed directly in subparts of the standardised tests that were conducted: for example, the PPVT tested vocabulary and the PAT measured the phonological skills. The measures of pre-reading subskills, environmental factors and language-related brain activation that were carried out in this study would be of the greatest benefit if they could serve as the starting point of a longitudinal study, tracking a cohort of children as they enter early formal education, and measuring the trajectory of their subsequent reading scores. This would allow one to assess the degree to which neural, behavioural and environmental measures at the age of five are able to predict later reading ability, and could perhaps even allow reading difficulties to be preempted.
As is the case for any study of correlation, the present data, while demonstrating a strong connection between SES and the specialisation of the left IFG, do not reveal a specific causal link. However, by revealing connections between SES and specific neural processes, this finding opens the door to future studies, addressing how a child’s environment shapes language processing in the brain. Many children grow-up in low-SES environments, and the present study suggests that such environmental conditions, and the learning opportunities that they do and do not provide, can be linked specifically to the left IFG. Future studies of the development of language in the brain should include socioeconomically diverse populations, and should make Broca’s area a target of investigation.
Supplementary Material
Acknowledgments
Support for this research was generously provided by the Delle Foundation and an NSF Science of Learning Center grant to the University of Washington’s LIFE Center. This work was facilitated by P30 HD02274 from the National Institute of Child Health and Human Development and supported by NIH UW Research Core Grant, University of Washington P30 DC04661.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings H, Zilles K. Broca’s region revisited: cytoarchitecture and intersubject variability. J Comp Neurol. 1999;412:319–41. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Ditterich A, Zilles K. Broca’s region: cytoarchitectonic asymmetry and developmental changes. J Comp Neurol. 2003;465:72–89. doi: 10.1002/cne.10829. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc B. 1995;57:289–300. [Google Scholar]
- Bowey JA. Socioeconomic status differences in preschool phonological sensitivity and first-grade reading achievement. J Educ Psychol. 1995;87:476–487. [Google Scholar]
- Bradley L, Bryant PE. Categorizing sounds and learning to read: a causal connection. Nature. 1983;301:419–421. [Google Scholar]
- Broberg P. A comparative review of estimates of the proportion unchanged genes and the false discovery rate. BMC Bioinformatics. 2005;6:199. doi: 10.1186/1471-2105-6-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant PE, Bradley L, Maclean M, Crossland J. Nursery rhymes, phonological skills and reading. J Child Lang. 1989;16:407–28. doi: 10.1017/s0305000900010485. [DOI] [PubMed] [Google Scholar]
- Cannonieri GC, Bonilha L, Fernandes PT, Cendes F, Li LM. Practice and perfect: length of training and structural brain changes in experienced typists. Neuroreport. 2007;18(10):1063–6. doi: 10.1097/WNR.0b013e3281a030e5. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427(6972):311–2. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Buchel C, May A. Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci. 2006;26(23):6314–7. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn L, Dunn L. Peabody Picture Vocabulary Test – Third Edition. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- Eckert MA, Lombardino LJ, Leonard CM. Planar asymmetry tips the phonological playground and environment raises the bar. Child Dev. 2001;72:988–1002. doi: 10.1111/1467-8624.00330. [DOI] [PubMed] [Google Scholar]
- Gaser C. VBM Toolbox for SPM5. 2007 http://dbm.neuro.uni-jena.de/vbm/markov-random-fields/
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Goswami U. Neuroscience and education: from research to practice? Nat Rev Neurosci. 2006;7:406–11. doi: 10.1038/nrn1907. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Black JE, Wallace CS. Experience and brain development. Child Dev. 1987;58:539–59. [PubMed] [Google Scholar]
- Hoeft F, Ueno T, Reiss AL, Meyler A, Whitfield-Gabrieli S, Glover GH, Keller TA, Kobayashi N, Mazaika P, Jo B, Just MA, Gabrieli JDE. Prediction of children’s reading skills using behavioral, functional, and structural neuroimaging measures. Behav Neurosci. 2007;121(3):602–13. doi: 10.1037/0735-7044.121.3.602. [DOI] [PubMed] [Google Scholar]
- Hoff E. The specificity of environmental influence: socioeconomic status affects early vocabulary development via maternal speech. Child Dev. 2003;74(5):1368–1378. doi: 10.1111/1467-8624.00612. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. Yale University, Department of Sociology; 1975. [Google Scholar]
- Huttenlocher J, Vasilyeva M, Cymerman E, Levine S. Language input and child syntax. Cognit Psychol. 2002;45(3):337–374. doi: 10.1016/s0010-0285(02)00500-5. [DOI] [PubMed] [Google Scholar]
- Lee K, Farrow T, Parks R, Newton L, Mir N, Egleston P, Brown W, Wilkinson I, Woodruff P. Increased cerebellar vermis white-matter volume in men with schizophrenia. J Psychiatr Res. 2007;41(8):645–51. doi: 10.1016/j.jpsychires.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia P, Aizenstein H, Snitz B, Walter R, Carter C. Automated roi-based brain parcellation analysis of frontal and temporal brain volumes in schizophrenia. Psychiatry Res. 2006;147(2–3):153–61. doi: 10.1016/j.pscychresns.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Mitelman S, Brickman A, Shihabuddin L, Newmark R, Hazlett E, Haznedar M, Buchs-baum M. A comprehensive assessment of gray and white matter volumes and their relationship to outcome and severity in schizophrenia. Neuroimage. 2007;(null)(null) doi: 10.1016/j.neuroimage.2007.04.070. (null) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitelman S, Buchsbaum M, Brickman A, Shihabuddin L. Cortical intercorrelations of frontal area volumes in schizophrenia. Neuroimage. 2005;27(4):753–70. doi: 10.1016/j.neuroimage.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Mitelman S, Shihabuddin L, Brickman A, Hazlett E, Buchsbaum M. Mri assessment of gray and white matter distribution in brodmann’s areas of the cortex in patients with schizophrenia with good and poor outcomes. Am J Psychiatry. 2003;160(12):2154–68. doi: 10.1176/appi.ajp.160.12.2154. [DOI] [PubMed] [Google Scholar]
- Noble KG, McCandliss BD, Farah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev Sci. 2007;10(4):464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Noble KG, Wolmetz ME, Ochs LG, Farah MJ, McCandliss BD. Brain-behavior relationships in reading acquisition are modulated by socioeconomic factors. Dev Sci. 2006;9(6):642–654. doi: 10.1111/j.1467-7687.2006.00542.x. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F, Huss M, Kherif F, Moscoso del Prado Martin F, Hauk O, Shtyrov Y. Motor cortex maps articulatory features of speech sounds. Proc Natl Acad Sci U S A. 2006;103:7865–70. doi: 10.1073/pnas.0509989103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramey CT, Ramey SL. Early intervention and early experience 1998 [Google Scholar]
- Rayner K, Foorman BR, Perfetti CA, Pesetsky D, Seidenberg MS. How psychological science informs the teaching of reading. Psychol Sci. 2001;2:31–74. doi: 10.1111/1529-1006.00004.. [DOI] [PubMed] [Google Scholar]
- Richards TL, Dager SR, Corina D, Serafini S, Heide AC, Steury K, Strauss W, Hayes CE, Abbott RD, Craft S, Shaw D, Posse S, Berninger VW. Dyslexic children have abnormal brain lactate response to reading-related language tasks. AJNR Am J Neuroradiol. 1999;20:1393–8. [PMC free article] [PubMed] [Google Scholar]
- Robertson C, Salter W. Phonological Awareness Test. East Moline, IL: LinguiSystems; 1995. [Google Scholar]
- Rojas D, Peterson E, Winterrowd E, Reite M, Rogers S, Tregellas J. Regional gray matter volumetric changes in autism associated with social and repetitive behavior symptoms. BMC Psychiatry. 2006;6(null):56. doi: 10.1186/1471-244X-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C, Becker J, Lopez O, Lopez-Garcia P, Carter C, Newman A, Kuller L, Aizenstein H. Morphometric analysis of gray matter volume in demented older adults: exploratory analysis of the cardiovascular health study brain mri database. Neuroepidemiology. 2005;24(4):221–9. doi: 10.1159/000085140. [DOI] [PubMed] [Google Scholar]
- Semel E, Wiig E, Secord W. Clinical Evaluation of Language Fundamentals – Third Edition. San Antonio, TX: Psychological Corporation; 1995. [Google Scholar]
- Shaywitz SE, Shaywitz BA, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Pugh KR, Holahan JM, Marchione KE, Fletcher JM, Lyon GR, Gore JC. Neural systems for compensation and persistence: young adult outcome of childhood reading disability. Biol Psychiatry. 2003;54:25–33. doi: 10.1016/s0006-3223(02)01836-x. [DOI] [PubMed] [Google Scholar]
- Simos PG, Breier JI, Fletcher JM, Foorman BR, Bergman E, Fishbeck K, Papanicolaou AC. Brain activation profiles in dyslexic children during non-word reading: a magnetic source imaging study. Neurosci Lett. 2000;290:61–5. doi: 10.1016/s0304-3940(00)01322-7. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Walker D, Greenwood C, Hart B, Carta J. Prediction of school outcomes based on early language production and socioeconomic factors. Child Dev. 1994;65:606–21. [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Preschool and Primary Scale of Intelligence - Revised. San Antonio, TX: Psychological Corporation; 1989. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.