Abstract
Neuronal aggregates involved in conscious awareness are not evenly distributed throughout the CNS but are comprised of key components referred to as the neural network correlates of consciousness (NNCC). A critical node in this network is the retrosplenial, posterior cingulate, and precuneal cortices (RSC/PCC/PrCC). The cytological and neurochemical composition of this region is reviewed in relation to the Brodmann map. This region has the highest level of brain glucose metabolism and cytochrome c oxidase activity. Monkey studies suggest that the anterior thalamic projection likely drives RSC and PCC metabolism and that the midbrain projection to the anteroventral thalamic nucleus is a key coupling site between the brainstem system for arousal and cortical systems for cognitive processing and awareness. The pivotal role of RSC/PCC/PrCC in consciousness is demonstrated with posterior cingulate epilepsy cases, midcingulate lesions that de-afferent this region and are associated with unilateral sensory neglect, observations from stroke and vegetative state patients, alterations in blood flow during sleep, and the actions of anesthetics. Since this region is critically involved in self reflection, it is not surprising that it is similarly a site for the NNCC. Interestingly, information processing during complex cognitive tasks and during aversive sensations such as pain induces efforts to terminate self reflection and result in decreased processing in PCC/PrCC. Finally, anatomical relations between the neural correlates of mind and NNCC in the cingulate gyrus do not appear to overlap and suggests that mental function and conscious awareness may be mediated by two neural networks.
Keywords: cingulate gyrus, medial parietal cortex, metabolism, anesthetics, sleep, anterior thalamus
Introduction
Consciousness is a multifaceted concept that can be divided into two major components: the level of consciousness (i.e., arousal, wakefulness or vigilance) and the content of consciousness (i.e., awareness of the environment and its relations to self). Although the entire forebrain is engaged to some extent in conscious information processing, there may be specific areas that are particularly crucial to consciousness. The midbrain reticular formation and its projections to the thalamic intralaminar nuclei establish and maintain wakefulness (Kinomura et al., 1996; Steriade, 1996) and postmortem assessments of the vegetative state show that damage to the dorsolateral midbrain and thalamus is a common feature in these cases (Kinney et al., 1994; Adams et al., 2000) and severe damage around the third ventricle that blocks this projection can produce coma (e.g., case 13, Malamud, 1967). The more difficult problem is the extent to which any forebrain region is necessary for conscious cognitive information processing.
In one sense, the entire cerebral cortex is necessary for awareness of numerous sensory and motor events. In this context, the null hypothesis states that no part of the forebrain plays a disproportionate role in consciousness or information processing during wakefulness. According to the null hypothesis, the neural correlates of consciousness are fully distributed throughout the forebrain. We will attempt to reject the null hypothesis by showing first that parts of the cingulate gyrus are necessary for conscious experience, although they may not be sufficient, and second that there is a critical linkage between the brainstem arousal system and posterior cingulate (PCC) and retrosplenial (RSC) cortices that assures a close coupling between arousal and awareness. This view and its supporting facts can be extended to a few other regions that form a critical neural network correlates of consciousness (NNCC) that is both necessary and sufficient for conscious cognitive information processing.
We propose that PCC and precuneus cortex (PrCC) together are pivotal for conscious information processing. Support for this hypothesis is derived from postmortem assessments of epilepsy, stroke, monkey and human cingulotomy lesions, and vegetative state cases, functional imaging during tasks requiring consciousness for sensorimotor processing, metabolic studies including those of sleep states and anesthetic responses, and vegetative state, coma, dementia and amnesia. We also emphasize a critical linkage between brainstem generated arousal and processing in the PCC/PrCC region and this dual function assures that PCC/PrCC is a key node in the NNCC.
Anatomical Overview of Posterior Cingulate Gyrus & Precuneal Cortex
Many observations are available from human imaging studies of the PCC and medial parietal lobe region; however, standardized software packages continue to use Brodmann’s anatomical observations from a century ago. Figure 1 provides a context for this region according to recent cytological observations. Figure 1A is a co-registration of Brodmann’s map with a postmortem case for which we have extensive histology. The histological case is a left hemisphere that was flipped horizontally to match the familiar Brodmann map. All numbers refer to our recent observations (Vogt et al., 2003; 2005) except for the hand drawn numbers for areas 31 and 23 that are original to Brodmann as are the symbolic designations for each area. Four numbered arrows mark critical locations and issues about the cytological organization of the cingulate gyrus as follows: 1. The border between our area p24′ and area 23d is quite caudal to the original border that Brodmann placed between his areas 24 and 23 which was approximately at the vertical plane of the anterior commissure (VCA). 2. Area 31 does extend dorsally and caudally around area 23; however, we observe a dysgranular area rostral to the arrow and do not agree that area 31 with its very thick layer IV extends as far rostrally as Brodmann placed it. 3. The third arrow marked “7m” refers to the border between medial parietal area 7m and area 31 which we have identified slightly more ventral than Brodmann. Area 7m is PrCC and we use the terms interchangeably. 4. This is the level at which the section immunoreacted for neuron-specific nuclear binding protein was taken for higher magnification in B. This antibody stains mainly neurons, thus removing glial, vascular, and neuropil elements from the analysis and providing a more precise view of cortical lamination patterns and differences among two or more areas. Since Brodmann reconstructed his map onto the convoluted brain surface, he was unable to show the exact placement of RSC area 29 and 30 in the callosal sulcus on the ventral bank of the cingulate gyrus. For this reason, he extended the retrosplenial areas caudally and ventrally, and in the process, he moved the “apparent” border of .area 23 quite caudal on the caudomedial lobule. This reduction in the distribution of area 23 was transposed to the atlas of Talairach and Tournoux (1988) and has produced continuing confusion in the human functional imaging literature as to the true extent of area 23 and the location of RSC. This issue has been thoroughly considered in another context (Vogt et al., 2001).
Figure 1.
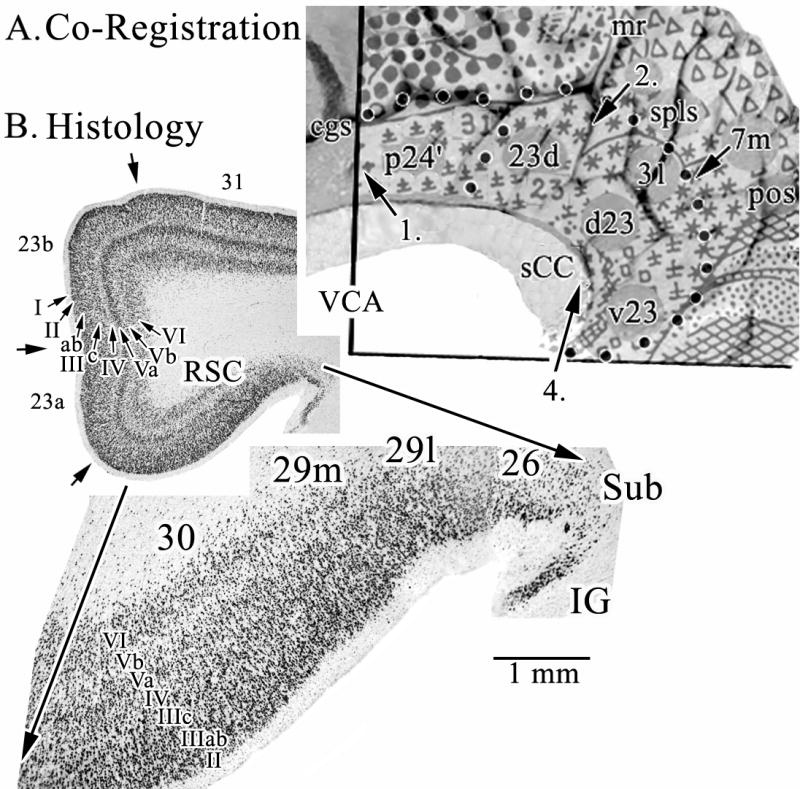
A. Co-registration of Brodmann’s map of posterior cingulate and precuneal cortices and a postmortem case for joint histological assessment. Stroked dots outline the border of cingulate cortex and four numbered arrows refer to locations of particular Brodmann areas discussed in the text. Brodmann’s actual numbers 23 and 31 are shown in the co-registration, while all other numbers refer to Vogt et al. (2005). The arrow at 4. points to the level of the coronal histological section taken for B. A critical issue at 4. is the manner in which Brodmann extended retrosplenial areas 29 and 30 onto the gyral surface where area 23 is located. RSC comprises the ventral bank of the cingulate gyrus in the callosal sulcus and is not exposed as suggested in his map. Abbreviations: cgs, cingulate sulcus; IG, indusium griseum; mr, marginal ramus of the cgs; pos, parieto-occipital sulcus; sCC, splenium of the corpus callosum; Sub, dorsal subiculum; VCA, vertical plane at the anterior commissure;
The PCC is comprised of areas 23 and 31. These areas have well-differentiated layers IIIc, IV, and Va as seen in Figure 1B. Only area 23d has a dysgranular layer IV that can form neuronal aggregates rather than a single and continuous layer. Area 7m (not shown) has vertically oriented clumps of dendrites in layers III and IV, a thicker layer IV, and relatively larger layer IIIc neurons than those in layer Va (the opposite of cingulate architecture). In contrast to PCC and PrCC, the RSC has poorly differentiated layers IIIc, IV, and Va as shown in the pullout magnification of RSC in the figure. In fact, like area 23d, area 30 has a dysgranular layer IV which is hard to observe in both photographs. Finally, area 26 is ectosplenial cortex and, along with the subiculum (Sub) and indusium griseum (IG) form the fasciolate gyrus. Since no human in vivo imaging modality has the resolution necessary to identify these latter three areas, they are not considered further here.
Epilepsy, Stroke & Vegetative State
According to Mazars (1970), the first episode of a cingulate epilepsy discharge is associated with “absences that are often mistaken for inattention. Twenty of our patients never had convulsive seizures and their ‘absences’ did not differ much from the short spells of loss of consciousness of ‘petit mal’… the time to regain normal consciousness was longer, and a less abrupt recovery of consciousness was associated with an outburst of temper which could be so marked as to obscure the preceding ‘absence.’ Photic stimulation did not precipitate the absences but emotion did so effectively.” Devinsky and Luciano (1993) reviewed many studies of cingulate epilepsies and it is interesting to note that anterior cingulate seizures spread quickly to frontal cortex and are most often associated with motor arrest, staring and subsequent automatic behaviors, while seizures localizing to PCC took longer to spread to the dorsal convexities. This could reflect generally weaker connections with the superior frontal gyrus (Vogt and Pandya, 1987). Finally, Archer et al. (2003) used spike-triggered fMRI to evaluate alterations in cerebral activity in adult patients with idiopathic generalized epilepsy and frequent spike and slow-wave discharges. Four of their 5 subjects had significant reductions in PCC activation and they concluded that altered PCC activation may play a role in the “absence” features of generalized spike and slow-wave discharges. Thus, differences between anterior and posterior cingulate epilepsies suggest that abnormal discharges in PCC spread slowly to superior frontal areas and can be associated with a loss of consciousness.
Unilateral lesions in monkey midcingulate cortex produce a contralateral neglect as demonstrated with a somatosensory discrimination task (Watson et al., 1973). As shown below, such lesions de-afferent the PCC/PrCC and the neglect could reflect a selective loss of awareness of the contralateral sensory space. Interestingly, topographic disorientation is produced by a large right hemisphere lesion of RSC, PCC, and PrCC (Takahashi et al., 1997) and a unilateral, left hemispheric lesion of RSC and the fornix produced profound amnesia (Valenstein et al., 1987). In none of these latter cases, however, was there a loss of consciousness. This could be because the lesions were restricted to one hemisphere and/or because this region is part of a larger NNCC.
A frequent postmortem finding in traumatic vegetative state cases is diffuse axonal injury with focal lesions in the corpus callosum (Adams et al., 2000). Although the frequency of this finding may lead to speculation about its critical role in clinical outcomes (Kampfl et al., 1998), large splenial tumors (Yamamoto et al., 1990; Rudge and Warrington, 1991) and large unilateral cingulate tumors that greatly deform this region (Malamud, 1967) fail to alter consciousness. In view of these latter findings, it is doubtful that the vegetative state is a consequence of splenial and PCC damage. This does not mean, however, that PCC/PrCC are not relevant to conscious information processing; once again, functional lesion sizes and locations are difficult to interpret. Activity in the medial parietal and adjacent PCC also seems to differentiate minimally conscious from vegetative patients (Laureys et al., 2003).
Cerebral Metabolism
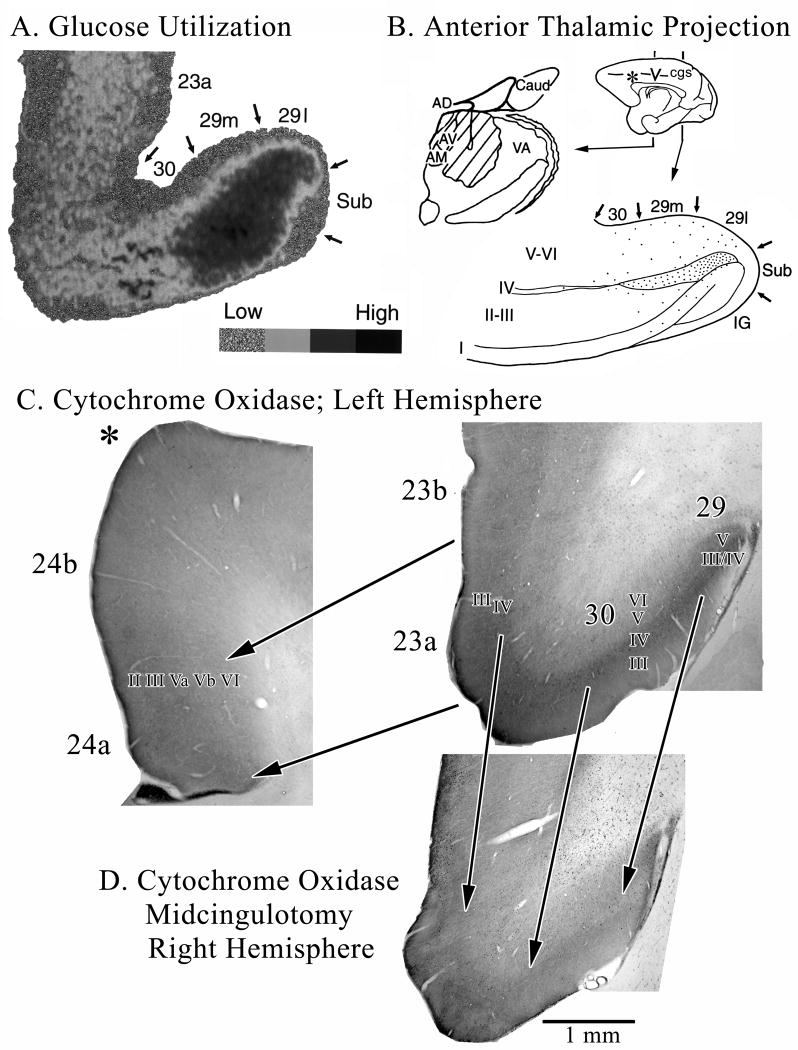
The highest level of cerebral glucose metabolism occurs in PCC and RSC. The highest level of basal glucose metabolism in the monkey brain is in RSC and the anterior thalamus and these levels are elevated during performance of a delayed-response task (Matsunami et al., 1981). We have discussed the close laminar association of both high levels of glucose metabolism determined with the 2-deoxy-D-glucose method and anterior thalamic projections assessed with tritiated-amino acid injections (Vogt et al., 1997). Figure 2A and B repeat these latter findings and provide the context of high cytochrome oxidase activity (Fig. 2C & D). Thalamic termination is greatest to the granular layer of area 29 (undifferentiated layer III/IV) and layers III-IV of area 30. Projections to area 23 arise mainly from the medial pulvinar and lateroposterior nuclei and these were not included in the amino acid injection shown in Figure 2B; hence thalamocortical connections to area 23 are not demonstrated in this case.
Figure 2.
Morphological context of metabolic activity in PCC in monkeys. A. 2-deoxy-D-glucose utilization coded for four levels of utilization and thalamic projections to RSC shown with a tritiated-amino acid injection (hatched) into the anterior thalamic nuclei and a coronal section through RSC areas 29 and 30. The close relationship between high glucose metabolism and thalamic afferents are obvious. Interestingly, high levels of the mitochondrial enzyme cytochrome c oxidase also occur in the granular layer of RSC and in layers III-IV of areas 30 and 23. The asterisk in B shows where the section through ACC in C. was taken. Notice that ACC has much less cytochrome c oxidase activity than does area 23 (shown with the pair of arrows delineating these areas). A midcingulotomy lesion (D.; at coronal level shown with “v” on medial surface in B.) that removes thalamic afferents to PCC/RSC as well as frontal lobe inputs shows a massive reduction of activity in the thalamoreceptive layers as predicted from selective thalamic lesions in rat. There is about a 20% volumetric reduction in the posterior cingulate gyrus and reductions in enzyme activity are emphasized with three arrows from layer III/IV in area 29 and layers III and IV in areas 30 and 23c. Thus, high metabolic activity in PCC, RSC, and PrCC is driven primarily by thalamic afferents.
High basal glucose metabolism overlaps with these latter layers on the ventral bank of the cingulate gyrus. Cytochrone c oxidase is a mitochondrial enzyme involved in oxidative phosphorylation and the density of activity generated by this enzyme is related to the density of mitochondria in a layer (Carroll and Wong-Riley, 1984). Axon terminals arising from neurons in the anterior thalamic nuclei in rat have a large diameter, are very dense with mitochondria, and they form asymmetric, excitatory synapses in RSC (Vogt et al., 1981). Moreover, thalamic lesions greatly reduce this enzyme’s activity in thalamoreceptive layers I–IV of RSC (Van Groen et al., 1993) suggesting that thalamic driving is critical to metabolic processes in RSC.
The rodent findings are extended for monkey in Figure 2(C, D). The highest level of cytochrome c oxidase activity is in RSC and adjacent area 23. A section of area 24 is provided at the asterisk in B for comparative purposes and it shows that ACC has only about 40% of the cytochrome c oxidase activity of areas 23 and 30. Area 7m (not shown) has a level of activity similar to that of area 23b. This figure also shows the effects of a midcingulotomy lesion in the contralateral hemisphere on cytochrome c oxidase activity (Fig. 2D). Notice in this section the elevated gliosis in the white matter, general shrinkage of the ventral cingulate gyrus and massive reduction in cytochrome c oxidase activity. Reduced cytochrome activity is most prominent in the thalamorecipient layers III/IV of areas 29, 30, and 23 as emphasized with the arrows in the figure. Thus, midcingulotomy lesions de-afferent PCC and RSC and this includes sectioning of thalamic afferents that travel through the cingulum bundle to terminate in the PCC.
The PCC/PrCC has the highest level of metabolism in human brain (Andreasen et al., 1995; Maquet et al., 1997; Minoshima et al., 1997). We evaluated rCMRGlu in 32 resting control subjects in regions of interest selected from the medial surface and three levels of the thalamus as shown in Table 1. Although the highest level of activity was in the PrCC, it was also quite high in PCC and the three samples from thalamus. The ACC and MCC were much lower and that in RSC was strikingly low. In view of the observations above for monkey, the latter observation for human RSC either reflects a substantial species difference or the limits of PET resolution in defining RSC have been reached. The latter is suspected both because this is a small region and it winds around the splenium and includes a variable amount of white matter that is likely proportionately greater than for the other regions and reduces the apparent level of glucose metabolism (i.e., partial volume effect bias). The slide-autoradiographic measure of glucose uptake in the monkey has a much greater precision and demonstrates laminar localizations that are not possible with human PET imaging. The most important conclusion from this histochemical analysis is that RSC likely has a similar or even higher level of glucose metabolism to PCC/PrCC and the RSC provides a critical link between the brainstem arousal system and cortical mechanisms of conscious awareness as discussed in the next section.
Table 1.
rCMRGlu*
| Cingulate Cortex | Retrosplenial & Precuneal Cortices | Thalamus | |||
|---|---|---|---|---|---|
| ACC | 7.8 ± 2 | RSC | 5.4 ± 2 | Anterior | 8.1 ± 1.9 |
| MCC | 7.6 ± 1.7 | PrCC | 10.4 ± 2.4 | Medial | 8.9 ± 2.4 |
| PCC | 9.3 ± 2.1 | Posterior | 8.7 ± 2.0 | ||
[18F]-fluorodeoxyglucose measurements by region of interest in 32 healthy volunteers; mg/100g/min; mean ± SD
Analysis of rCMRGlu in the vegetative state shows reduced activity in a large region of prefrontal cortex and a posterior, medial reduction in PCC/PrCC that might include RSC. In spite of the large prefrontal reductions, the crucial connection with this region that was disrupted in vegetative state was in PCC. As the relevant area overlaps the callosal sulcus, it is quite possible that the critically impaired connection is actually with RSC. Additionally, the thalamus also has reduced rCMRGlu in vegetative state (S. Laureys, unpublished observations) and this emphasizes further the critical linkage between the thalamus and PCC/PrCC from brainstem arousal systems.
Anteroventral Thalamic Link Between Conscious Wakefulness & Cognitive Awareness
Since the intralaminar thalamic nuclei are particularly important to wakefulness (Kinomura et al., 1996; Steriade, 1996), their projections might be expected to be a primary driver of information processing in critical parts of the NNCC. At first blush, this does not seem to be the case. Although PCC does receive a small projection from midline and intralaminar thalamic nuclei, the major projections of these nuclei are to MCC and ACC (Vogt et al., 1987). Although it is possible that a small projection from these nuclei might be effective in driving the entire NNCC, it is surprising that the rostral parts of the cingulate gyrus are not a critical part of this network based on the midline and intralaminar thalamic projections.
An alternative explanation suggests that the cholinergic mesopontine projections to the anteroventral thalamic nucleus are involved in cortical arousal and provide a linkage with conscious cognitive processing. The lateral tegmental reticular formation, parabrachial and laterodorsal tegmental nuclei contain cholinergic neurons that project to the anteroventral thalamic nucleus (Sofroniew et al., 1985). Electrical stimulation of these nuclei evokes cholinergically mediated, muscarinic, long-lasting excitatory potentials in the anteroventral thalamic nucleus that could mediate arousal (Curro et al., 1991). As noted above, the anteroventral nucleus has a prominent projection to RSC and this is an excitatory connection that likely mediates a high level of glucose metabolism in RSC. This need not be an isolated RSC circuit, however, because area 23 is heavily and reciprocally connected with RSC (Vogt and Pandya, 1987). Alteration in a prefrontal/RSC connection in vegetative state around the callosal sulcus suggests this region mediates a pivotal link with arousal brainstem systems.
Thus, although there are two aspects to consciousness (arousal/wakefulness and awareness), they have a critical conjunction in the RSC and PCC region. This linkage between the two systems suggests that the posterior cingulate gyrus is one of the most important regions for awareness and is a pivotal player in the NNCC.
Sleep
A region critical to conscious information processing might be expected to be inactivated during sleep and area 31 of PCC has reduced blood flow during the rapid-eye movement (REM) phase of sleep (Maquet et al., 1996). This does not prove that area 31 quiescence is pivotal to REM sleep, however, it is supportive of that conclusion because there was also a massive bilateral prefrontal inactivation during REM. Figure 3 provides measures of rCBF in PrCC and PCC during wakefulness and each stage of sleep and shows that, although REM has a profound reduction in both regions, there is also a substantial reduction in blood flow in both regions during the preceding stages of sleep. In view of the massive and reciprocal connections between PCC and prefrontal cortex, it is possible that PCC/PrCC is shut off by prefrontal cortex according to the above noted functional interaction between these regions in vegetative state cases. Thus, the PCC/PrCC decrease could be a primary or secondary event and it still suggests the relevance of this area in conscious processing. Additionally, thalamic involvement is not a mechanism of reducing area 31 blood flow, since the left thalamus had increased blood flow during REM (Maquet et al., 1996).
Figure 3.
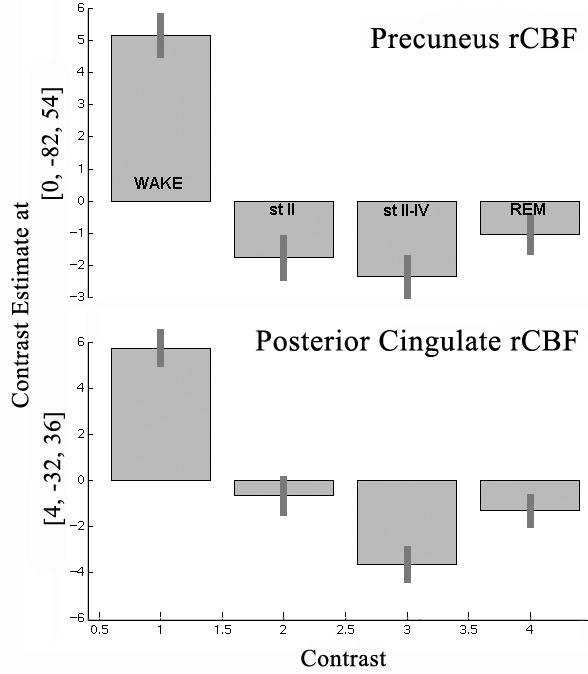
Estimates of rCBF in precuneal and posterior cingulate cortices during wakefulness and three stages of sleep. The pattern is similar for both cortices with a high level of rCBF during wakefulness and reductions during sleep. The one main difference between these areas is that PCC during stage II sleep has a higher level of activity than during REM than is the case for PrCC. Thus, the transition to sleep involves greater reductions in rCBF in PrCC than in PrCC.
Anesthetic Sensitivity
Although anesthetics have general mechanisms of action such as at the GABAA receptor and these likely reduce neuron activity throughout the brain, there are also particularly susceptible regions and modulation of their activity may be most closely associated with conscious functions. In the rat, the tuberomamillary nucleus is particularly sensitive to anesthetics that act at GABAA receptors including propofol (Nelson et al., 2002). Although the rat has a well differentiated RSC, it does not have areas 23, 31, or 7m (Vogt et al., 2004) and the tuberomamillary nucleus does not project to RSC in rat or monkey brain. Thus, other mechanisms must be considered in relation to anesthetic actions and human consciousness.
In keeping with its general actions at the GABAA receptor, propofol produces marked global reductions in rCBF, however, there are a few particular regions with relatively large reductions in rCBF at the lowest levels of mean alveolar concentrations. These include the thalamus and PCC/PrCC (Fiset et al., 1999; Kaisti et al., 2002). Although individual changes in rCBF did not correlate with propofol concentrations and the differences between three levels of propofol were not significant for any areas (Kaisti et al., 2002), this latter study did show that the lowest concentration of propofol assessed produced a profound average reduction in PCC/PrCC and Fiset et al., (1999) showed a strong negative correlation between rCBF in the thalamus and PCC/PrCC and propofol concentration. Furthermore, the close correlation of activity under propofol in the thalamus and midbrain in this latter study confirms the relevance of the reticular projections to thalamus as mediating arousal and initiation of consciousness. Halothane also decreases rCMRGlu in PCC/PrCC (Alkire et al., 1999) suggesting that many anesthetics target this region as an endpoint to induce a loss of consciousness.
Finally, although fentanyl has a complex action in mediating rCBF, it does reduce flow in the thalamus and PCC when compared to a placebo or during noxious heat stimulation (Adler et al., 1997). These studies taken together support the notion that PCC and PrCC are prominently impacted by low doses of anesthetics. The role of global changes and interactions with the thalamic/reticular activating system is not yet clear, however, the linkage between these systems points to a pivotal role of PCC/PrCC in consciousness when viewed in relation to the high level of metabolism of these areas.
Self Reflection & Complex Information Processing
The PCC has a major role in visuospatial orientation, topokinesis and navigation of the body in space (review; Vogt et al., 2004). Recent functional imaging suggests this function may be specifically related to self reflection (Johnson et al., 2002; Kelley et al., 2002) and autobiographical memory (Piefke et al., 2003) and this includes assessments of objects in space in terms of a first-person orientation (Vogeley et al., 2004). Although the previous studies all engaged PCC, precuneal cortex has also been involved in intentional, self processing (Kircher et al., 2002). The essential function of self reflection for PCC/PrCC is compatible with a pivotal role for this region in conscious awareness. Indeed, self reflection may be the primary substrate for conscious awareness as located in the PCC/PrCC.
This functional substrate may have implications for understanding brain activity during complex cognitive tasks that are not associated with self reflection and actually inhibit functions in PCC/PrCC. Analysis of cognitive skill learning is a means of analyzing the neural correlates of consciousness (Raichle, 2000). Contrasts generated by reading nouns minus viewing nouns in this latter study greatly decreased activity in PCC/PrCC. Also, this region is inactivated during a working memory task (Greicius et al., 2003) and during noxious thermal stimulation (Vogt et al., 1996). Raichle suggested that activity in this and associated regions must be suspended for proper task execution. During noxious thermal stimulation, subjects attempt to withhold conscious perception of the pain stimulation and terminate functions relating to self in PCC/PrCC. It is also possible that during complex sensory processing in other modalities, activity in this region must be suspended. Thus, conscious reflection on self is mediated by PCC/PrCC and this processing must be suspended and activity in this posterior cingulate region inactivated when engaging in complex cognitive and sensory information processing tasks. To the extent that PCC/PrCC is essential for conscious awareness, painful stimulation leads subjects to internally inactivate the region of cortex that underpins their conscious awareness.
Anatomical Relationships Between Mental and Conscious Information Processing
The disproportionately important role of RSC/PCC/PrCC in conscious awareness raises another issue: To what extent do the NNCC in the cingulate gyrus overlap with the neural correlates of mind (NCM)? The same view of consciousness has been argued previously for NCM (Vogt and Devinsky, 2001) to the extent that there is a part of cingulate cortex that plays a particularly important role in mental function based on lesion and functional imaging observations. The neuroscientific definition of mind in this context is those locations in the brain where mental activity has the highest probablility of causing an increase in neuronal and functional activation. Thus, intentions to move/willed actions, long-term storage of self-centered information, the logic of mental activity including inductive reasoning, and theory of mind tasks in which a subject is asked to interpret the mental state of another individual all activate only part of the cingulate gyrus rather than its full extent The conclusion from this assessment was that ACC and MCC along with adjacent frontal areas 8, 9, and 10 form a cingulofrontal convergence region that is a critical node in the NCM. It is striking that only a few studies engaged PCC as in theory of mind work (Fletcher et al., 1995) and this was attributed to its role in self-centered reflection as part of the paradigm rather than a pivotal role for PCC in the NCM.
It appears that the NNCC and NCM do not appreciably overlap in the cingulate gyrus. This conclusion raises doubts about the extent to which mental function in terms of cognitive processing and conscious awareness is subserved by the same neural substrates. Viewed in this manner, mental function is a higher order motor selection process, while conscious awareness involves a continual stream of self reflection and an effort to relate the self to its sensory environment. Surprisingly, both functional networks may operate essentially independent of each other but together form the essence of human brain function. Thus, human functional imaging provides an important context for resolving many of the essential philosophical questions regarding mind and consciousness raised over the past two millennia and it appears these issues will eventually be resolved via a thoughtful consideration of the structure, functions and pathologies of the cingulate gyrus.
Abbreviations
- ACC
anterior cingulate cortex (perigenual region)
- AD
anterodorsal thalamic nucleus
- AM
anteromedial thalamic nucleus
- AV
anteroventral thalamic nucleus
- Cgs
cingulate sulcus
- Caud
caudate nucleus
- IG
indusium griseum
- MCC
midcingulate cortex
- mr
marginal ramus of the cingulate sulcus
- NCM
neural correlates of mind
- NNCC
neural network correlates of consciousness
- PCC
posterior cingulate cortex
- PCG
posterior cingulate gyrus
- Pos
parieto-occipital sulcus
- PrCC
precuneus cortex
- rCBF
regional cerebral blood flow
- rCMRGlu
regional cerebral metabolic rate; l glucose
- RSC
retrosplenial cortex
- REM
rapid-eye-movement sleep
- sCC
splenium of the corpus calllosum
- Sub
dorsal subiculum
- VA
ventral anterior thalamic nucleus
- VCA
vertical plane at the anterior commissure
Footnotes
This research was supported by National Institute of Health grant #NS44222 (BAV).
References
- Adams JH, Graham DI, Jennett B. The neuropathology of the vegetative state after an acute brain insult. Brain. 2000;123:1327–1338. doi: 10.1093/brain/123.7.1327. [DOI] [PubMed] [Google Scholar]
- Adler LJ, Gyulai FE, Diehl DJ, Mintun MA, Winter PM, Firestone LL. Regional brain activity changes associated with fentanyl analgesia elucidated by positron emission tomography. Anesth Analg. 1997;84:120–126. doi: 10.1097/00000539-199701000-00023. [DOI] [PubMed] [Google Scholar]
- Alkaire MT, Pomfrett MT, Haier CJ, Gianzero RJ, Chan MV, Jacobson BP, Fallon JH. Functional brain imaging during anesthesia in humans: effects of halothane on global and regional cerebral glucose metabolism. Anesthesiology. 1999;90:701–709. doi: 10.1097/00000542-199903000-00011. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, Ponto LL, Hichwa RD. Remembering the past: Two facts of episodic memory explored with positron emission tomography. Am J Psychiatry. 1995;152:1576–1585. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- Archer JS, Abbott DF, Waites AB, Jackson GD. fMRI “deactivation” of posterior cingulate cortex during generalized spike and wave. NeuroImage. 2003;20:1915–1922. doi: 10.1016/s1053-8119(03)00294-5. [DOI] [PubMed] [Google Scholar]
- Bischof M, Bassetti CL. Total dream loss: A distinct neuropsychological dysfunction after bilateral PCA stroke. Ann Neurol. 2004 doi: 10.1002/ana.20246. in press. [DOI] [PubMed] [Google Scholar]
- Carroll EW, Wong-Riley MTT. Quantitative light and electron microscopic analysis of cytochrome oxidase-rich zones in the striate cortex of the squirrel monkey. J Comp Neurol. 1984;222:1–17. doi: 10.1002/cne.902220102. [DOI] [PubMed] [Google Scholar]
- Curro DR, Pare D, Steriade M. Short-lasting nicotinic and long-lasting muscarinic depolarizing responses of thalamocortical neurons to stimulation of mesopontine cholinergic nuclei. J Neurophysiol. 1991;65:393–406. doi: 10.1152/jn.1991.65.3.393. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Luciano D. Neurobiology of Cingulate Cortex and Limbic Thalamus. Birkhauser; Boston: 1993. The contributions of cingulate cortex to human behavior; pp. 527–556. [Google Scholar]
- Fiset P, Paus T, Daloze T, Plourde G, Meuet P, Bonhomme V, Haij-Ali N, Backman SB, Evans AC. Brain mechanisms of propofol-induced loss of consciousness in humans: A positron emission tomographic study. J Neurosci. 1999;19:5506–5513. doi: 10.1523/JNEUROSCI.19-13-05506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hyopothesis. Proc Natl Acad Sci. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Kaisti KK, Metsahonkala L, Teras M, Oikonen V, Aalto S, Jaaskelainen S, Hinkka S, Scheinin H. Effects of surgical levels of Propofol and Servoflurane anesthesia on cerebral blood flow in healthy subjects studied with positron emission tomography. Anesthesiology. 2002;96:1358–1370. doi: 10.1097/00000542-200206000-00015. [DOI] [PubMed] [Google Scholar]
- Kampfl A, Schutzhard E, Franz E, Pfausler B, Haring H-P, Ulmer H, Felber S, Golaszewski S, Aichner F. Prediction of recovery from post-traumatic vegetative state with cerebral magnetic-resonance imaging. The Lancet. 1998;351:1763–1767. doi: 10.1016/S0140-6736(97)10301-4. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cog Neurosci. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Korein J, Panigraphy A, Dikkes P, Goode R. Neuropathological findings in the brain of Karen Ann Quinlan, The role of the thalamus in the persistent vegetative state. NEJM. 1994;330:1469–1475. doi: 10.1056/NEJM199405263302101. [DOI] [PubMed] [Google Scholar]
- Kinomura S, Larsson J, Gulyas B, Roland PE. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science. 1996 doi: 10.1126/science.271.5248.512. [DOI] [PubMed] [Google Scholar]
- Kircher TTJ, Brammer M, Bullmore E, Simmons A, Bartels M, David AS. The neural correlates of intentional and incidental self processing. Neuropsychologia. 2002;40:683–692. doi: 10.1016/s0028-3932(01)00138-5. [DOI] [PubMed] [Google Scholar]
- Laureys S, Goldman S, Phillips C, Van Bogaert P, Aerts J, Luxen A, Franck G, Maquet P. Impaired effective cortical connectivity in vegetative state: Preliminary investigation using PET. NeuroImage. 1999;9:377–382. doi: 10.1006/nimg.1998.0414. [DOI] [PubMed] [Google Scholar]
- Laureys S. Differences in brain metabolism between patients in coma, vegetative state, minimally conscious state and locked-in syndrome. Eur J Neurol. 2003;10:224. [Google Scholar]
- Malamud N. Psychiatric disorder with intracranial tumors of limbic system. Arch Neurol. 1967;17:113–123. doi: 10.1001/archneur.1967.00470260003001. [DOI] [PubMed] [Google Scholar]
- Maquet P, Peters J-M, Aerts J, Delfiore G, Degueldre C, Luxen A, Franck G. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature. 1996;383:163–166. doi: 10.1038/383163a0. [DOI] [PubMed] [Google Scholar]
- Maquet P, Degueldre C, Delfiore G, Aerts J, Peters JM, Luxen A, Frank G. Functional neuroanatomy of human slow wave sleep. J Neurosci. 1997;17:2807–2812. doi: 10.1523/JNEUROSCI.17-08-02807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunami KI, Kawashima T, Satake H. Mode of [14C]-2-deoxy-D-glucose uptake into retrosplenial cortex and other memory-related structures of the monkey during a delayed response task. Brain Res Bull. 1989;22:829–838. doi: 10.1016/0361-9230(89)90026-9. [DOI] [PubMed] [Google Scholar]
- Mazars G. Criteria for identifying cingulate epilepsies. Epilepsia. 1970;11:41–47. doi: 10.1111/j.1528-1157.1970.tb03865.x. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey K, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M. The sedative component of anesthesia is mediated by GABAA receptors in an endogenous sleep pathway. Nat Neurosci. 2002;5:978–984. doi: 10.1038/nn913. [DOI] [PubMed] [Google Scholar]
- Pifke M, Weiss PH, Zilles K, Markowitch HJ, Fink GR. Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain. 2003;126:650–668. doi: 10.1093/brain/awg064. [DOI] [PubMed] [Google Scholar]
- Raichle ME. The neural correlates of consciousness: An analysis of cognitive skill learning. In: Gazzaniga MS, editor. The New Cognitive Neurosciences. The MIT Press; Cambridge, MA: 2000. pp. 1305–1318. [Google Scholar]
- Rudge P, Warrington EK. Selective impairment of memory and visual perception in splenial tumors. Brain. 1991;114:349–360. doi: 10.1093/brain/114.1.349. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Priestley JV, Consolazione A, Eckenstein F, Cuello AC. Cholinergic projections from the midbrain and pons to the thalamus in the rat identified by combined retrograde tracing and choline acetyltransferase immunohistochemistry. Brain Res. 1985;329:213–223. doi: 10.1016/0006-8993(85)90527-x. [DOI] [PubMed] [Google Scholar]
- Steriade M. Arousal: Revisiting the reticular activating system. Science. 1996;272:225–226. doi: 10.1126/science.272.5259.225. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kawamura M, Shiota J, Kasahata N, Hirayama K. Pure topographic disorientation due to right retrosplenial lesion. Neurology. 1997;49:464–469. doi: 10.1212/wnl.49.2.464. [DOI] [PubMed] [Google Scholar]
- Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT. Retrosplenial amnesia. Brain. 1987;110:1631–1646. doi: 10.1093/brain/110.6.1631. [DOI] [PubMed] [Google Scholar]
- Van Groen T, Vogt BA, Wyss JM. Interconnection between the thalamus and retrosplenial cortex in the rodent brain. In: Vogt BA, Gabriel M, editors. Neurobiology of Cingulate Cortex and Limbic Thalamus. Birkhauser; Boston: 1993. pp. 123–150. [Google Scholar]
- Vogeley K, Ritzl MM, Falkai P, Zilles K, Fink GR. Neural correlates of first-person perspective as one constituent of human self-consciousness. J Cog Neurosci. 2004;16:817–827. doi: 10.1162/089892904970799. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Derbyshire SWJ, Jones AKP. Pain processing in four regions of human cingulate cortex localized with co-registered PET and MR Imaging. Eur J Neurosci. 1996;8:1461–1473. doi: 10.1111/j.1460-9568.1996.tb01608.x. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Devinsky O. Topography and relationships of mind and brain. Prog Brain Res. 2000;122:11–22. doi: 10.1016/s0079-6123(08)62127-5. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN. Cingulate cortex in rhesus monkey. II. Cortical afferents. J Comp Neurol. 1987;262:271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Rosene DL, Pandya DN. Cingulate cortex in rhesus monkey I. Cytoarchitecture and thalamic afferents. J Comp Neurol. 1987;262:256–270. doi: 10.1002/cne.902620207. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Rosene DL, Peters A. Synaptic termination of thalamic and callosal afferents in cinguate cortex of the rat. J Comp Neurol. 1981;201:265–283. doi: 10.1002/cne.902010210. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Farber NB. Cingulate cortex and disease models. In: Paxinos G, editor. The Rat Nervous System. 3. Elsevier; San Diego, CA: 2004. pp. 705–727. [Google Scholar]
- Vogt BA, Vogt L, Hof PR. Cingulate Gyrus. In: Paxinos G, Mai JK, editors. The Human Nervous System. 2. Elsevier; San Diego, CA: 2003. pp. 915–949. [Google Scholar]
- Vogt BA, Vogt LJ, Laureys S. Cytology and functionally correlated circuits of posterior cingulate areas. 2005 doi: 10.1016/j.neuroimage.2005.07.048. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Vogt LJ, Nimchinsky EA, Hof PR. Primate cingulate chemoarchitecture and its disruption in Alzheimer’s disease. In: Bloom FE, Björklund A, Hökfelt T, editors. The Primate Nervous System, Part 1. Vol. 13. Elsevier; New York: 1997. pp. 455–528. [Google Scholar]
- Watson RT, Heilman KM, Cauthen JC, King FA. Neglect aster cingulectomy. Neurology. 1973;23:1003–1007. doi: 10.1212/wnl.23.9.1003. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Kurobe H, Kawamura J, Hashimoto S, Nakamura M. Subacute dementia with nectrotizing encephalitis selectively involving the fornix and splenium. J Neurol Sci. 1990;96:159–172. doi: 10.1016/0022-510x(90)90129-b. [DOI] [PubMed] [Google Scholar]