Synopsis
Cancer surveillance is an increasing part of everyday practice in gastrointestinal endoscopy due to the identification of high risk groups from genetic and biomarker testing, genealogic and epidemiologic studies, and the increasing number of cancer survivors. An efficient surveillance program requires a cost-effective means for image-guided cancer detection and biopsy. A laser-based tethered-capsule endoscope with enhanced spectral imaging is introduced for unsedated surveillance of the lower esophagus. An ultrathin version of this same endoscope technology provides a 1.2-mm guidewire with imaging capability and cannula-style tools are proposed for image-guided biopsy. Advanced 3D cell visualization techniques are described for increasing the sensitivity of early cancer diagnosis from hematoxylin-stained cells sampled from the pancreatic and biliary ducts.
Keywords: endoscope, biopsy, image-guided intervention, 3D cytology, cancer surveillance
Cancer surveillance is an increasing part of everyday practice in gastrointestinal (GI) endoscopy due to the identification of high risk groups from genetic and biomarker testing, genealogic and epidemiologic studies, and the increasing number of cancer survivors. For colorectal cancer, Winawer et al., [1] reports that there is growing demand of colonoscopy resources away from screening and more toward post-polypectomy surveillance. In the setting of pancreatic cancer, there is a need to shift emphasis from diagnosis of symptomatic pancreatic cancer to screening (and surveillance) of asymptomatic individuals at high risk [2]. For example, surveillance strategies developed for patients with hereditary pancreatic cancer can be used as a model for other high risk groups [3]. Thus, a major goal for the endoscopist and biomedical engineer is to move toward more pre-emptive and preventative procedures that ultimately will reduce GI cancer mortality rates [4].
The challenge for the next generation of endoscopic imaging is to improve the efficiency, sensitivity, and accuracy of disease detection and site selection for biopsy and cell sampling. Since many of the tissue and cellular features of pre-cancer and early-stage cancers are not visible using standard endoscopic tools, new technologies will need to be developed. These new endoscopic tools should be developed and tested not just for the main GI tract, but also for the smaller pancreatic and biliary ducts. In this technology report on the state of ultrathin and flexible laser scanning endoscopy, new endoscopic and cytologic tools are introduced for cost-effective cancer surveillance of the esophagus as well as the smaller pancreaticobiliary ducts reached by endoscopic retrograde cholangiopancreatography (ERCP). These proposed tools are expected to be widely applicable to clinical practice outside of gastroenterology, such as cancer screening and surveillance within fields of gynecology, pulmonology, and urology.
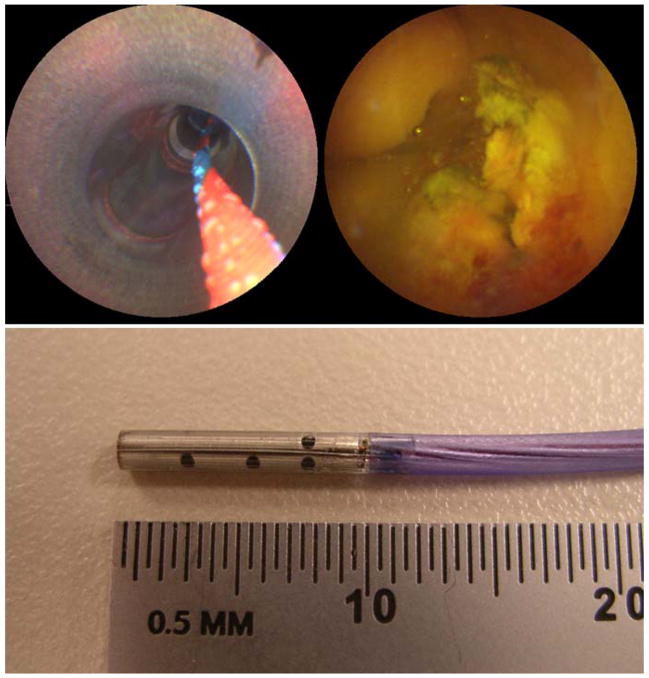
At the University of Washington, a new endoscopic imaging technology has been developed for two GI applications, the tethered-capsule endoscope (TCE) for esophageal imaging and the scanning fiber endoscope (SFE) for small duct imaging. Both endoscopes produce images in the same, but unique fashion [5,6]. The distal tip of a single illumination optical fiber is scanned in a spiral pattern. Narrow bands of red, green, and blue (RGB) laser light are scanned over the tissue at wide field of view (100 degrees). The collected light from this flying-spot scan is detected in time series, one-pixel-at-a-time from the light collected by a ring of optical fibers that surround the micro-optical scanner. Unlike video chip endoscopes, the number of pixels within an image is not determined by the number of camera sensor elements or collection optical fibers. The number of pixels in the TCE and SFE images is a user-controlled feature ultimately limited by optical performance of the lens system. Current TCE and SFE prototypes operate at 30 Hz for 500-line color images or 15 Hz for 1000-line color images. The size of the SFE is currently 1.2 mm in overall diameter with a rigid tip length of less than 10 mm (Figure 1). The capsule size of the TCE is 18 mm in length and 6.4 mm in overall diameter which is a standard size for over-the-counter pills (Figure 2), while the soft supple tether is 1.4 mm in diameter. The capsule design was implemented to facilitate swallowing. As the SFE and TCE probes are composed of relatively low cost components, these probes could be manufactured at high volume for single use, through reprocessing is currently under investigation.
Figure 1.
SFE images during in vivo imaging in a pig bile duct using ERCP procedure, top-left shows the 4.2-mm working channel and guidewire and top-right shows a SFE image frame. Photograph of SFE probe with 9-mm rigid tip length and 1.2-mm overall diameter is the lower image. Reprinted from [7] Seibel, E.J., Melville, C.D., Johnston, R.S., et al., Bile duct imaging with ultrathin laser scanning catheterscope in a swine model, Gastrointest Endosc 2008;67(5):AB133–134, with permission from Elsevier.
Figure 2.
Photograph of TCE probe with comparison ibuprofen gel capsule (Advil, Wyeth) alongside for size comparison. Photo credit is to RobWatters/The Daily of the University of Washington, Seattle, WA.
Proposed esophageal surveillance using TCE with image contrast endhancement
Barrett’s esophagus is a pre-cancerous change in the esophagus associated with gastroesophageal reflux. Screening and surveillance for Barrett’s esophagus is performed to look for dysplasia, the histologic change that precedes cancer. The determination of dysplasia is quite difficult with conventional endoscopic technology because dysplastic tissue is rarely visible [8]. Surface mucosal treatments of chomoendoscopy have been developed to increase the image contrast of dysplastic mucosal patterns with some successes [9], but an accessory channel is required for topical application of dye. Instead of modifying the tissue, the endoscope can be modified for higher intensity blue light illumination to detect surface mucosal thickening that is correlated with neoplasia in patients with Barrett’s esophagus [10]. Alternatively, the spectral content of the endoscopic illumination can be varied to increase contrast of both mucosal patterns and vascular patterns [11], but magnification can be a requirement to achieve detection rate advantages over white light imaging [12].
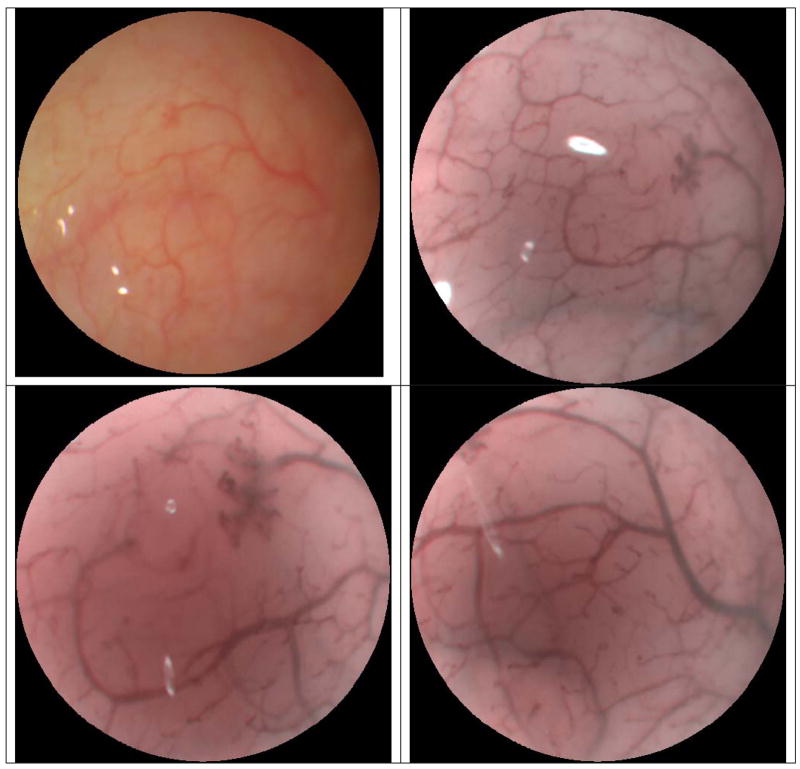
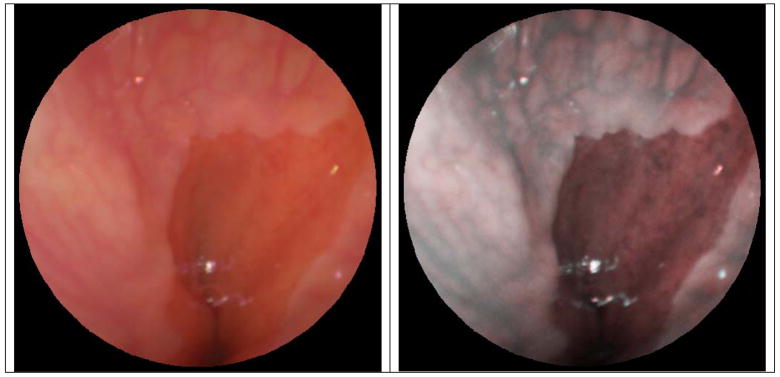
The combination of magnification and enhanced spectral imaging (ESI) is a standard imaging feature of the tethered-capsule endoscope that uses narrow laser bands for illumination. The unique aspect of the TCE is that the ESI image contrast enhancement can be applied in real-time with a user-controlled switch or from post-processing the digitally stored color video images. In Figures 3 and 4, both types of ESI enhancements are shown from single 500-line video frames acquired during 30 Hz operation (no image averaging). In Figure 3, a TCE image of normal contrast can be compared to TCE video frames with the ESI feature taken from approximately the same region of the human inner cheek at different magnifications and different locations. In Figure 4, a TCE image taken at the lower human esophagus during normal operation has been digitally stored and ESI contrast has been post-processed for comparison. Currently the TCE magnification is limited to approximately 130x of real magnification, corresponding to resolving features of 15 microns. The light exposure of the combined RGB laser illumination is equivalent to the standard illumination levels from white-light endoscopy, roughly the same as using three continuously scanned laser pointers.
Figure 3.
TCE images showing video frame in normal operation (top-left) and subsequent video frames captured with Enhanced Spectral Imaging (ESI) feature applied (top-right). TCE w/ESI is demonstrated with increased magnification within the previous field of view (bottom-left), and in a new region of the human cheek (bottom-right).
Figure 4.
TCE video frame acquired and digitally stored during human subjects examination of the lower esophagus. Normal TCE contrast is displayed on the left, and the same TCE video frame is postprocessed with enhanced spectral imaging (right).
A tethered capsule is not considered a new device for gastroenterology, as they have been used for over 30 years for obtaining tissue biopsies from the small intestine of children and adults [13]. Often these biopsies from tethered capsules provide equivalent histological results to endoscopy, while the endoscopic procedure is considered advantageous due to a faster procedure, direct visualization of the bowel, and collection of many biopsy samples [14]. The TCE with high-quality video now provides the direct visualization and a fast procedure time for imaging the lower esophagus perorally, without the use of any anesthetic or sedation. Recently an air channel was added to the TCE tether and comfortably swallowed, allowing the user to control the distention of the esophagus and to remove bubbles [15]. Thus, with the existence of a micro-optical scanner for video-rate imaging and a side channel, the ability to add image-guided biopsy and simple interventions may be forthcoming.
Proposed small duct surveillance using SFE as a catheterscope
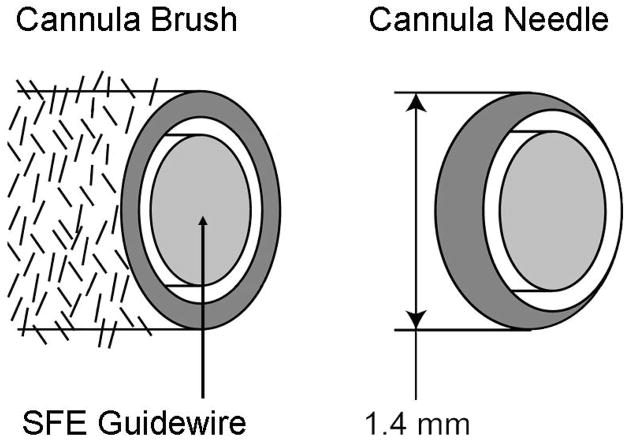
The SFE provides excellent image quality from 1-mm diameter catheter-style endoscopes which are analogous to a “guidewire with eyes.” In the standard ERCP procedure, the SFE with less than 10 mm rigid tip length can be introduced into either the biliary or pancreatic duct through the working channel of a side-viewing therapeutic duodenoscope (Figure 1, [7[). However, a steerable SFE would be preferable to navigate through the pancreatobiliary ducts and to reduce the forces applied to the walls of the ducts. Similar to a cardiovascular catheter, the capability for tip deflection can be added without additional size of the rigid tip and flexible shaft. Replacing the central wire within a cardiovascular catheter with the single illumination optical fiber of the SFE as the central compression element allows tip deflection by pulling on a pair of peripheral wires or the optical fibers used for light collection (Figure 5). The new endoscopic tool is a catheterscope with 4-way tip bending or steerable “guidewire with eyes.” Along this guidewire, cannula-style tools (brush, needle/tube, forceps) can be introduced into small ducts for image-guided interventions. Previously, a multimodal optical fiber has been incorporated into the forceps for point spectroscopic measurements of GI cancers prior to biopsy [16]. In our case, the annular gap that surrounds the proposed 1-mm SFE can be used for extracting cells and fluid samples from ducts smaller than 2 mm in diameter using a brush (Figure 6). Tissue core samples and aspirants may be extracted using a needle with SFE as the central plunger. Custom 2-mm diameter mini-pancreatoscopes are preferred over larger endoscopes as sphincterotomy is not required with the smaller scope [17]. Future clinical testing will be necessary to determine if small duct surveillance could be performed in a modified ERCP procedure that may not require fluoroscopy.
Figure 5.
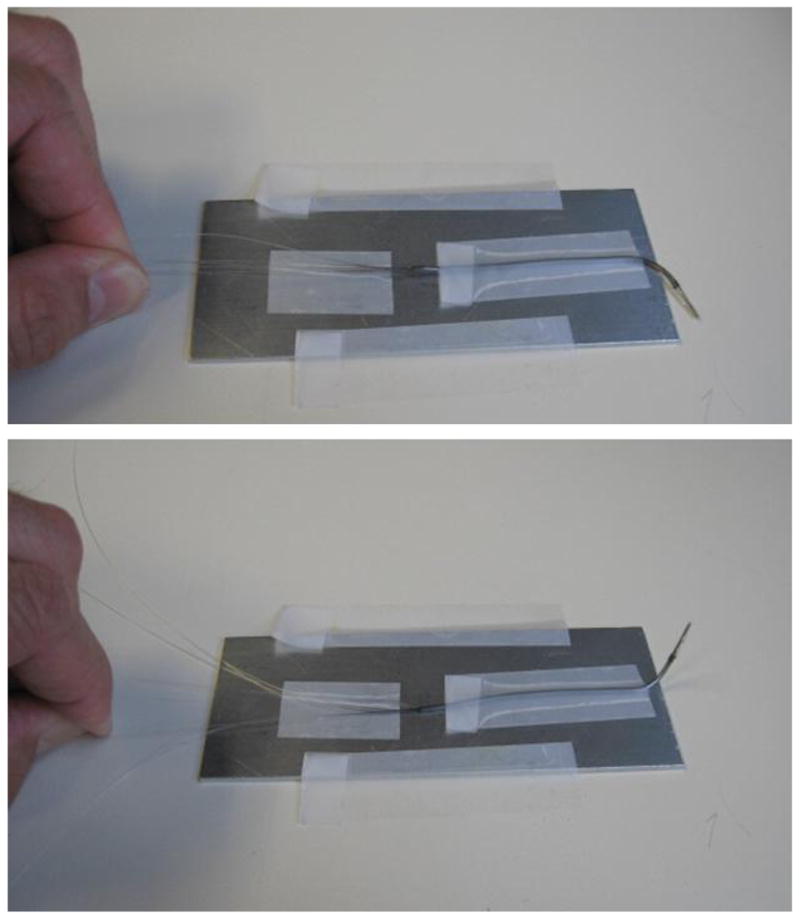
Photographic demonstration of tip bending in a 1.5 mm diameter SFE probe during manufacture.
Figure 6.
Cannula-style tools of brush and needle depicted around a future 1-mm SFE probe as a steerable guidewire with eyes.
3D cytology of cell samples from proposed SFE cannula tools
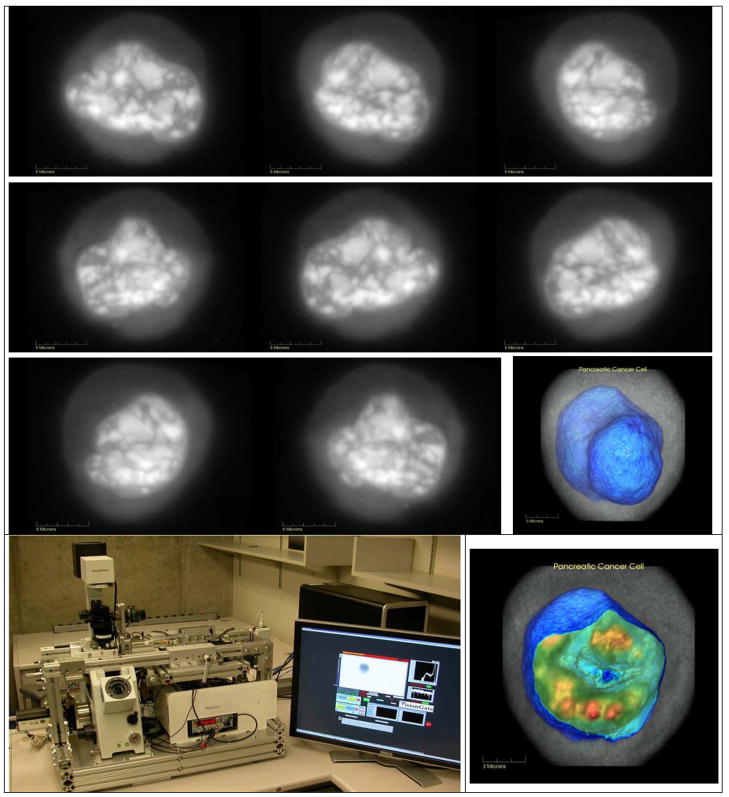
Although biomarker detection of disease within the GI tract is an ultimate goal, cell/tissue removal is required for diagnosis. Smaller cellular samples are less invasive, but without tissue architecture, standard cytology has proven to lack sensitivity. At the University of Washington a new optical microscope has been co-developed with VisionGate Inc., (Gig Harbor, WA) which can provide three-dimensional (3D) images for cytological analysis [18]. Hematoxylin stained and fixed cells are imaged in a transmission-like standard cytological examination. However, these cells are then rotated and re-imaged from 250 perspectives within 360 degrees to produce 3D images of submicron isometric resolution. Reconstructed 3D images are generated in the same manner that 3D x-ray images are generated using Computed Tomography (CT). Representative Cell-CT™ images of a cultured pancreatic cancer cell (PaTu1) are shown in grayscale in a series of perspectives, along with two images having pseudocolor thresholds set for highlighting the nuclear membrane and internal nuclear chromatin-dense structures (Figure 7). In a controlled comparison test on the same human cultured cells (A549 lung adenocarcinoma and SAEC airway epithelial), the use of a classifier using 3D cytological features versus standard cytology reduced the false negative rate by 3 fold at the same 96% specificity [19]. The improved sensitivity of 3D cytological imaging and analysis in this preliminary study provides an incentive for the long term success of small duct surveillance using less invasive micro-biopsy tools.
Figure 7.
Cell-CT instrument and transmission images of human pancreatic cancer cell (PaTu1, alcohol fixed, hematoxylin stained) shown in grayscale in series of different rotational perspectives. Same cell is visualized using Volview software with thresholding set to opacify the irregular nuclear envelope (blue) and highlight chromatin-dense bodies within slice of nucleus (red).
Summary
The scanning fiber endoscope technology is being demonstrated as a platform technology on which future clinical protocols will be developed for cancer surveillance. In the esophagus, the tethered-capsule endoscope with enhanced spectral imaging can be used without sedation which lowers the cost and reduces barriers for esophageal cancer screening and surveillance. In the pancreatic and biliary ducts, the SFE technology with cannula tools can provide high quality image guidance for biopsy while being of a smaller size and greater flexibility than current endoscope technology. Finally, the anticipated increase in sensitivity from 3D cytological analysis of cell samples from these small ducts should provide the necessary clinical tools required for a successful endoscopic cancer surveillance program of the pancreas and bile ducts.
Acknowledgments
Engineering of the new endoscopic tools was provided by Dave Melville, Rich Johnston, and Cameron Lee from the Human Photonics Lab. The new cytological instrument and 3D visualization was engineered by Michael Meyer, Richard Rahn, Florence Patten, Mark Fauver, Thomas Neumann, and Alan C. Nelson of VisionGate Inc., Gig Harbor, WA. Cell-CT is a trademark registered by Visiongate Inc. Technical support was received by David Crispin on the cultured pancreatic cancer cells. The first author gratefully thanks attendees of the ASGE course, The Next Generation in Endoscopic Imaging, Dallas, TX, who provided information on tethered capsule devices for small bowel biopsy.
Funding
As principle investigator, Eric Seibel received funding in support of this project from the following sources: NIH/NCI (CA094303), NSF (CBET-0809070), PENTAX (HOYA Corp., Tokyo, Japan), and VisionGate Inc., (Gig Harbor, WA). Jason Dominitz is supported by an ASGE Career Development Award and NIH/NCI grant CA128231.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Eric J. Seibel, Research Associate Professor Mechanical Engineering, Adjunct Bioengineering, Human Photonics Laboratory, University of Washington, Box 352600, Seattle, WA 98195, USA, voice: (206) 616-1486, fax: (206) 685-8047, eseibel@u.washington.edu, http://www.hpl.washington.edu.
Teresa A. Brentnall, Professor, Department of Medicine, University of Washington, 1959 NE Pacific St, Box 356424, Seattle, WA 98115, phone 206-543-3280, fax 206-685-9478, teribr@u.washington.edu.
Jason A. Dominitz, Associate Professor of Medicine, Assistant Chief for Academic Affairs, Division of Gastroenterology, University of Washington School of Medicine, Director, Northwest Hepatitis C Resource Center, VA Puget Sound Health Care System, 1660 S. Columbian Way (111-Gastro) Seattle, WA 98108, voice: (206) 764-2285, fax (206) 764-2232, Jason.Dominitz@va.gov.
References
- 1.Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology. 2006;130(6):1872–85. doi: 10.1053/j.gastro.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Canto MI. Screening and surveillance approaches in familial pancreatic cancer. Gastrointest Endosc Clin N Am. 2008;18(3):535–553. doi: 10.1016/j.giec.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Brentnall TA. Management strategies for patients with hereditary pancreatic cancer. Curr Treat Options Oncol. 2005;6(5):437–445. doi: 10.1007/s11864-005-0046-6. [DOI] [PubMed] [Google Scholar]
- 4.Kelloff GJ, Sullivan DC, Baker H, et al. Imaging science development for cancer prevention and preemption. Cancer Biomark. 2007;3(1):1–33. doi: 10.3233/cbm-2007-3101. [DOI] [PubMed] [Google Scholar]
- 5.Seibel EJ, Carroll R, Dominitz JA, et al. Tethered capsule endoscopy, a low-cost and high-performance alternative technology for the screening of esophageal cancer and Barrett’s esophagus. IEEE Trans Biomed Eng. 2008;55(3):1032–1042. doi: 10.1109/TBME.2008.915680. [DOI] [PubMed] [Google Scholar]
- 6.Seibel EJ, Brown CM, Dominitz JA, et al. Scanning single fiber endoscopy: a new platform technology for integrated laser imaging, diagnosis, and future therapies. Gastrointest Endosc Clin N Am. 2008;18:467–478. doi: 10.1016/j.giec.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seibel EJ, Melville CD, Johnston RS, et al. Bile duct imaging with ultrathin laser scanning catheterscope in a swine model. Gastrointest Endosc. 2008;67(5):AB133–134. [Google Scholar]
- 8.Wilson BC. Detection and treatment of dysplasia in Barrett’s esophagus: a pivotal challenge in translating biophotonics from bench to bedside. J Biomed Optics. 2007;12(5):051401-1–22. doi: 10.1117/1.2795688. [DOI] [PubMed] [Google Scholar]
- 9.ASGE American Society for Gastrointestinal Endoscopy, Technical Committee. Chromoendoscopy. Gastrointest Endosc. 2007;66(4):639–649. doi: 10.1016/j.gie.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 10.Kara MA, Peters FP, ten Kate FJW, et al. Endoscopic video autofluorescence imaging may improve the detection of early neoplasia in patients with Barrett’s esophagus. Gastrointest Endosc. 2005;61(6):679–685. doi: 10.1016/s0016-5107(04)02577-5. [DOI] [PubMed] [Google Scholar]
- 11.Sharma P, Bansal A, Mathur S, et al. The utility of a novel narrow band imaging endoscopy system in patients with Barrett’s esophagus. Gastrointest Endosc. 2006;64(2):167–239. doi: 10.1016/j.gie.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 12.Friedland S. Narrow band imaging of the bile duct. Gastrointest Endosc. 2007;66(4):737–738. doi: 10.1016/j.gie.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 13.Dickey W, Porter KG. Perendoscopic Watson capsule biopsy of the jejunum: a simple, effective, safe method which does not require fluoroscopy. Gastrointest Endosc. 1995;41(1):81–82. doi: 10.1016/s0016-5107(95)70285-7. [DOI] [PubMed] [Google Scholar]
- 14.Gottrand F, Michaud L, Guimber D, et al. Comparison of fiberendoscopy and suction capsule for small intestinal biopsy in children with and without celiac disease. J Pediatr Gastroenterol Nutr. 1999;28(3):353. doi: 10.1097/00005176-199903000-00033. [DOI] [PubMed] [Google Scholar]
- 15.Seibel EJ, Melville CD, Lung JKC, et al. Swallowable capsule with air channel for improved image-guided cancer detection in the esophagus. SPIE Medical Imaging 2009; International Society for Optical Engineering (submitted); [Google Scholar]
- 16.Wong Kee Song LM, Marcon NE. Fluorescence and Raman spectroscopy. Gastrointest Endosc Clin N Am. 2003;13:279–296. doi: 10.1016/s1052-5157(03)00013-8. [DOI] [PubMed] [Google Scholar]
- 17.Kodama T, Tatsumi Y, Sato H, et al. Initial experience with a new peroral electronic pancreatoscope with an accessory channel. Gastrointest Endosc. 2004;59(7):895–900. doi: 10.1016/s0016-5107(04)01272-6. [DOI] [PubMed] [Google Scholar]
- 18.Fauver M, Seibel EJ, Rahn JR, et al. Three-dimensional imaging of single isolated cell nuclei using optical projection tomography. Opt Express. 2005;13(11):4210–4223. doi: 10.1364/opex.13.004210. [DOI] [PubMed] [Google Scholar]
- 19.Meyer MG, Fauver M, Rahn JR, et al. Automated cellular analysis in 2D and 3D: A comparative study. Pattern Recognition (in press) 2009;42(1):141–146. [Google Scholar]