Abstract
All allogeneic (allo) and autologous (auto) hematopoietic stem cell transplantation (HSCT) recipients at St. Jude Children’s Research Hospital undergo pre-HSCT computed tomography (CT) of the sinuses, chest, and abdomen because they are at significant risk for opportunistic infections. We studied whether this extensive routine imaging is warranted to detect infection despite the risk of additional radiation exposure. We reviewed the medical records of all children receiving allo- and auto-HSCT at St. Jude in 2004 and 2005. Of the 184 eligible patients who received 187 transplants, 131 received allografts and 56 autografts. Solid tumors and lymphomas were removed from the final analysis of the chest and abdomen CT as this imaging is typically warranted as part of disease restaging; thus 111 allogeneic participants were included in this analysis. Both auto and allo-recipients were evaluated by sinus CT and included in this final analysis. Most allo- and auto-HSCT recipients (≥ 80%) did not have sinus, pulmonary, cardiac, or gastrointestinal symptoms; >85% of the evaluable allo-recipients had no prior fungal infection. Eighty-eight allo- and 31 auto-HSCT recipients had abnormal sinus CT findings, all unrelated to the underlying disease. Sixty-two (55.9%) of the allo-recipients had normal chest CT and 85 (76.6%) had normal abdominal CT. Of the 18 allo-recipients who began new therapy based on these findings, only 2 (11.1%) were related to chest CT findings and the other 16 were related to sinus findings. Our findings suggest that pre-HSCT routine CT imaging of the abdomen may not be warranted in a subset of allogeneic recipients who are asymptomatic and without previous infectious findings. Thus, these patients may be spared unnecessary radiation exposure. Recipients undergoing auto-HSCT or allo-HSCT for lymphomas or solid tumors will routinely undergo chest and abdominal CT imaging as part of their disease evaluation. The decision to perform chest CT should be made judiciously based on a careful history and physical examination. Sinus imaging, which was frequently abnormal, may be justified in all patients to plan post-HSCT care.
Keywords: Hematopoietic stem cell transplantation (HSCT), autologous HSCT, allogeneic HSCT, computed tomography (CT), infection, pediatrics
Introduction
Patients undergoing hematopoietic stem cell transplantation (HSCT) are at significant risk for toxicity following the procedure [1]. Bacterial, viral, and fungal pathogens frequently infect these immunocompromised patients [1–4]; therefore, clinicians may feel the need to screen patients for preexisting infections to reduce the risk of post-HSCT infections. However, the routine use of pre-HSCT radiographic imaging has not been adopted by many. At St. Jude Children’s Research Hospital, we have incorporated computed tomography (CT) imaging of the sinuses, chest, and abdomen into our pre-HSCT evaluation. Although this imaging may detect an infection, it increases the patient’s radiation exposure and complicates the scheduling of the pre-HSCT evaluation. Moreover, this radiological modality may produce imaging artifacts, which may lead to unnecessary medical evaluations or delays in HSCT. It is especially important to compare the benefits and risks of pre-HSCT radiographic imaging in children, who are at a rapid stage of growth and development and are particularly sensitive to the current and late effects of radiation [5, 6]. To determine the utility of clinical screening of the chest, abdomen, and sinuses by CT, we retrospectively reviewed a large group of consecutive pediatric and adolescent patients who underwent autologous (auto) or allogeneic (allo) HSCT at St. Jude from January 2004 to December 2005.
Patients and Methods
The study was approved by the St. Jude Institutional Review Board (IRB). We retrospectively reviewed the records of 195 consecutive children who underwent HSCT at St. Jude between January 1, 2004, and December 31, 2005. All patients were treated on institutional protocols approved by the IRB or non-protocol standard therapies. Written informed consent was obtained from the patient, parent, or legal parent before initiating therapy.
Medical records were reviewed for the date of CT imaging; information regarding sinus, respiratory, cardiac, and gastrointestinal symptoms at the time of CT imaging; history of pre-transplant fungal infection; underlying disease or indication for transplant; disease status at the time of transplant; absolute neutrophil count at the time of CT imaging; imaging results; date of transplant; and therapeutic interventions based on these findings prior to transplant. Imaging findings were categorized by the investigators as related if they were due to the disease (or its treatment) for which transplant was being performed. Otherwise, the findings were categorized as unrelated.
Radiation Dose Estimation
At St. Jude, sinus CT is performed without IV contrast material by acquiring 2.5 mm-thick axial images at 5 mm intervals, using 4 detectors (4 × 1.25 mm), 120 kilovoltage peak (kVp), 200 milliamperes (mA), a tube rotation time of 0.5 s, and a 25 cm field of view. Chest and abdomen CTs are obtained with IV contrast material and helical acquisition of 5 mm-thick images, using 120 kVp and mA modulated by body part to a preset noise level of 5. Using these scanning parameters we determined radiation exposure in a 16 cm diameter phantom with a 100 mm long (3 cc active volume) ionization chamber (Radcal 9095 dosimetry system, RADCAL Inc., Monrovia, CA). The exposure measurement geometry captures radiation from the primary beam falling on the ionization chamber, as well as scatter radiation contribution from the phantom media, over the active length of the ionization chamber. Exposure readings were obtained in the middle and at the periphery of the phantom by inserting the ionization chamber into holes drilled into it. Exposure readings were then multiplied by exposure to absorbed dose conversion factors and length of the chamber and divided by the collimation employed for measurement. This provides the CT radiation absorbed dose descriptor Computed Tomography Dose Index over 100 mm length of ionization chamber (CTDI100). To obtain a weighted average of peripheral and central contributions to dose we calculated the Weighted Computed Tomography Dose Index (CTDIw). Next, we corrected the CTDIw for clinical technique factors such as table incrementation/pitch to obtain the Volume Computed Tomography Dose Index (CTDIvol). Finally, the effective dose was obtained by applying age specific conversion factors as suggested by Shrimpton and Wall [9]. This method is approved for pediatric CT accreditation by the American College of Radiology [10].
The radiation dose associated with a particular exam can be assessed by comparing it to background radiation as a standard. In the United States, the average effective background radiation dose per year is approximately 3.0 millisievert (mSv) [11, 12].
Statistical Methods
The demographic, clinical abnormalities, and signs and symptoms for allo- and auto-HSCTs were summarized using frequency tables. Logistic regression, with one covariate at a time, was used to evaluate the predictors of abnormal CT findings of the chest, abdomen and sinus for allo- and auto-HSCTs. P-values ≤ 0.05 were considered statistically significant. All analyses were performed by using SAS® v9.1 (SAS® Institute Inc., Cary, NC).
Results
Patient Characteristics
Of the 195 eligible patients, 11 were excluded from the final analysis: 1 had received a tandem transplant, 3 did not have pre-HSCT CTs, and 7 had undergone imaging evaluation more than 2 mo before HSCT. Nine patients received multiple transplants: 6 had 2 allo-HSCTs, 2 had 1 allo- and 1 auto-HSCT, and 1 had 2 allo- and 1 auto-HSCT. For those receiving multiple HSCTs, the median duration from one HSCT to another was 6.5 mo (range, 1.8–22.7 mo). Of the 184 patients included in the final analysis, 187 transplants were performed consisting of 131 (70.1%) allo-HSCT and 56 (29.9%) auto-HSCT. Three patients included in the final analysis had 1 autologous HSCT followed by an allogeneic HSCT. Of the 131 allo-HSCT recipients, 51 (39%) were female; 25 (45%) of the auto-HSCT recipients were female. The median age of allo-HSCT recipients was 10.9 yr (range, 0.5–26.6 yr) and the median age of auto-HSCT recipients was 7.3 yr (range, 1.5–26 yr). Most patients receiving an allo-HSCT had a hematologic malignancy (78.6%) whereas those undergoing an auto-HSCT predominantly had solid tumors (71.4%). All patients were included in the sinus CT findings; patients with solid tumors and lymphomas were excluded from CT chest and abdomen analyses as these patients routinely undergo this imaging as a part of disease evaluation. Thus, 111 allo-recipients, comprised of those with lymphoid (n=46) and myeloid (n=52) hematologic malignancies, and genetic disorders (n=13), were included in the chest and abdomen CT analyses and the characteristics regarding these patients are outlined in Table 1. Median absolute neutrophil count at the time of CT imaging was 1400/mm3.
Table 1.
Patient demographics for allogeneic (N= 111) hematopoietic stem cell transplantation (HSCT) recipients. Patients receiving an allogeneic HSCT for lymphoma or solid tumor or an autologous HSCT were excluded from this analysis.
| Characteristics | N (%) |
|---|---|
| Gender | |
| Female | 42 (37.8%) |
| Male | 69 (62.2%) |
|
| |
| Race | |
| Caucasian | 58 (52.3%) |
| Black | 22 (19.8%) |
| Others | 31 (27.9%) |
|
| |
| Age at Transplant (year) | |
| <3 | 16 (14.4%) |
| 3–10 | 41 (36.9%) |
| 11–17 | 37 (33.3%) |
| 18–27 | 17 (15.3%) |
| Mean (standard deviation) | 10.3 (6.1) |
| Median (range) | 10.7 (0.5 – 21.7) |
|
| |
| Product Type | |
| PBSC | 54 (48.6%) |
| BM | 57 (51.4%) |
|
| |
| Diagnosis | |
| CML | 10 (9.0%) |
| Genetic disease | 13 (11.7%) |
| Lymphoid malignancy | 46 (41.4%) |
| Myeloid malignancy | 42 (37.8%) |
|
| |
| Donor Type | |
| Matched related (6/6) | 23 (20.7%) |
| Mismatched related | 54 (48.7%) |
| Matched unrelated (6/6) | 33 (29.7%) |
| Mismatched unrelated | 1 (0.9%) |
|
| |
| Status | |
| Alive | 77 (69.4%) |
| Dead | 34 (30.6%) |
Note: PBSC, peripheral blood stem cells; BM, bone marrow; CML, chronic myelogenous leukemia
For 4 allogeneic patients, a history of fungal infections was not known. Of the remaining 107 allo-recipients, 95 (88.8%) had no history of fungal infection. Twelve recipients (11.2%) had a history of fungal infection, and of these patients, 9 (75%) had only 1 site of infection, 2 (16.7%) had 2 sites, and 1 (8.3%) had 3 sites of involvement. Eight patients (66.6%) had a previous pulmonary fungal infection while 2 (16.7%) had abdominal involvement and 2 (16.7%) had positive blood cultures.
Most patients did not have underlying sinus (86.5%, allo; 80%, auto), pulmonary (84.9%, allo; 94.6%, auto), cardiac (97.6%, allo; 96.4%, auto) or gastrointestinal (95.2%, allo; 98.2%, auto) signs and symptoms at the time of imaging.
CT Findings
Figures 1A and 1B outline chest and abdomen CT findings for 111 allo-recipients. Of these allo-HSCT patients, 62/111 (55.9%) had normal chest CT findings. Of this group, only 5/62 (8.1%) had pulmonary or cardiac symptomatology. Eighty-five of 111 (76.6%) patients had normal abdominal CT findings, and of this group, only 3/85 (3.5%) had gastrointestinal symptoms. There were abnormal sinus findings in most of the allo- (67.2%) and auto-HSCT (55.4%) recipients, which were unrelated to the underlying disease process. Of the 8 patients with prior fungal pulmonary infections, 6 (75%) had abnormal pre-HSCT chest imaging. Only 4 of these patients had pulmonary or cardiac symptoms at the time of imaging. One of 2 patients with prior abdominal fungal infections had abnormal abdominal imaging pre-HSCT; only one had gastrointestinal symptoms at the time of CT. No auto-HSCT recipient had a history of fungal infection.
Figure 1.
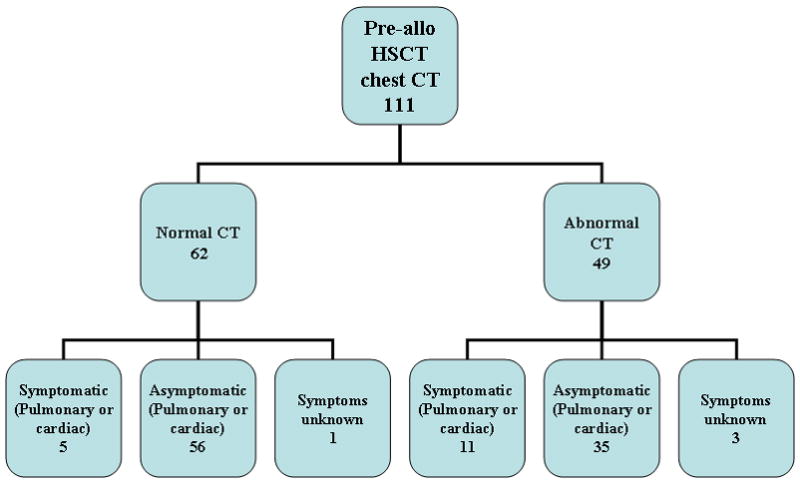
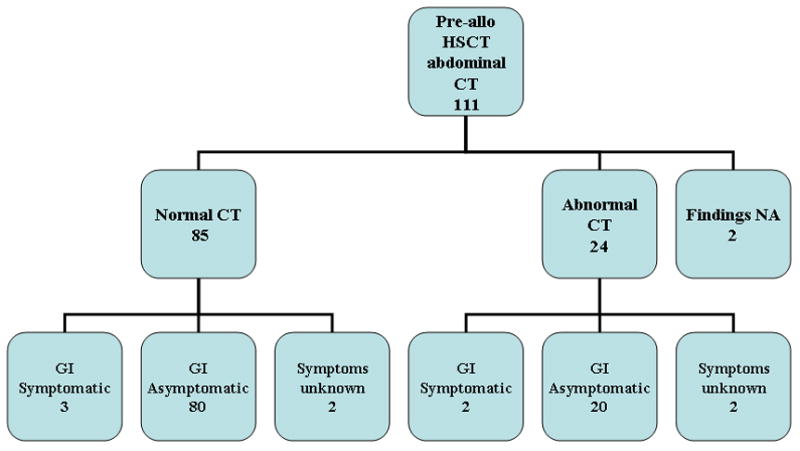
Number of allogeneic (allo) patients categorized by pre-HSCT chest (A) and abdominal (B) CT findings: normal versus abnormal CT imaging and classified further by symptomatology.
We determined whether specific variables – gender, race, age at transplant, underlying diagnosis, graft product recipient was to receive, and type of donor – predicted the risk of finding abnormalities on pre-HSCT scans. Upon univariate analysis of the 111 allo-graft recipients, age at the time of transplant was statistically significant for abnormal chest findings [odds ratio 0.91 (0.85-0.79); P= 0.005]. Age was further categorized as <3 vs. ≥ 3 and incorporated as a covariate in the logistic model, and patients < 3 years of age were more likely to have abnormal chest CT findings than patients ≥3 years of age (P= 0.002). No variable showed statistical significance in those allo-recipients who had abdominal CT scans performed.
Therapy was initiated based on pre-transplant imaging in a small fraction of patients. While 18 of 111 allogeneic recipients with abnormal chest or abdominal CT findings had new therapy, only 2 were based on chest findings (11.1%). Of these 2 patients, 1 had a history of previous pulmonary fungal disease and new therapy was initiated based on this pre-HSCT finding (Figures 2A and 2B). Predominantly, treatments were initiated based on abnormal sinus findings (22/24 allo recipients; 7/8 auto recipients). No patient had therapy initiated based on abdominal findings.
Figure 2.
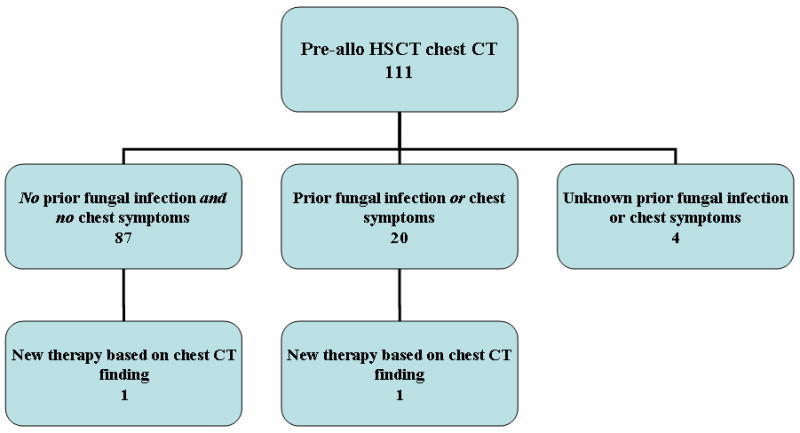
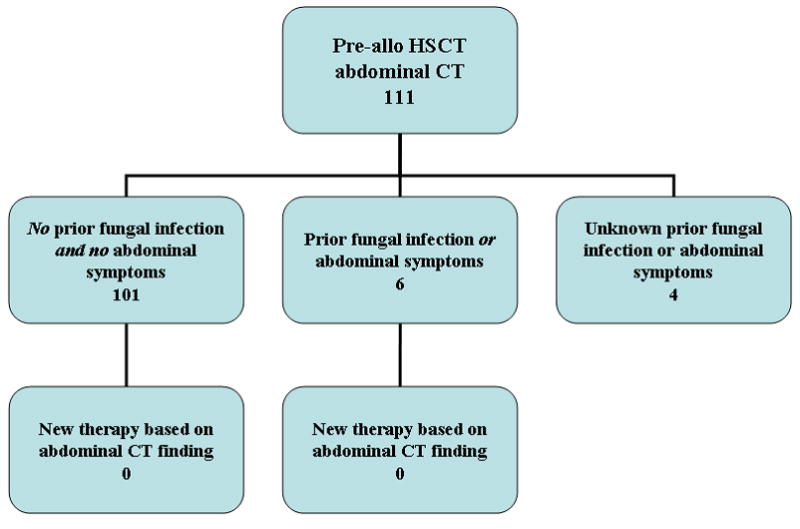
Organizational diagrams of allogeneic (allo) HSCT recipients who underwent pre-transplant chest (A) and abdominal (B) CT imaging. Patients were further characterized by history of fungal infection and symptomatology, followed by the initiation of new therapy based on CT findings. Only 2 new therapies were started based on chest CT findings and none on abdominal findings.
The effective radiation dose estimates (in mSv) based on age of patient for CT of the sinuses were 2.10 (1 yr old), 1.64 (5 yr old), 1.23 (10 yr old), 1.39 (15 yr old), 1.62 (adult), and those for CT of the chest and abdomen were 4.90 (1 year old), 4.20 (5 yr old), 4.50 (10 yr old), 4.60 (15 yr old), and 8.90 (adult).
Discussion
We undertook this retrospective study to determine whether pre-transplant CTs of sinuses, chest, and abdomen are warranted in detecting asymptomatic infections in patients preparing to undergo auto- or allo-HSCT. We found that routine imaging of the abdomen in asymptomatic, allogeneic patients was unlikely to reveal any previously unknown abnormalities or abnormalities requiring alterations in treatment with respect to abnormal findings. Patients with solid tumors and lymphomas routinely undergo this radiologic modality as part of their disease re-evaluation before HSCT and therefore, were excluded from the chest and abdomen CT analysis. Several transplant recipients had unrelated chest abnormalities, such as pericardial fluid, serosal thickening, and atelectasis; however, only 2 patients received new therapy based on these CT abnormalities. Six patients in the allo-recipient cohort, ranging in age from 0.8–19.66 years, had atelectasis on chest CT suggesting that atelectasis is a cause of abnormal finding at any age. Although this finding suggests that many of the chest CT findings were incidental and did not change the patient’s course of therapy, a clinician should also consider the patient’s underlying disease, previous therapy, and concurrent risks for a pulmonary process when determining the necessity of performing a pre-HSCT chest CT. CT imaging of the sinuses was frequently abnormal, even in asymptomatic patients. In a previous report, severity of pre-HSCT sinusitis as determined by CT correlated with symptomatology and radiological findings after HSCT [13]. Thus, routine detection of sinusitis before HSCT may allow early initiation of antibiotic therapy, potentially leading to a reduction in significant post-transplant sinus disease.
There are no other published studies on the utility of pre-transplant chest or abdominal screening by CT for patients. Ours is the first study to document that routine imaging of the chest and abdomen is unlikely to detect clinically undetectable abnormalities or alter management prior to HSCT. It can be argued that a chest and abdomen CT before HSCT, particularly for an allogeneic recipient, provides a baseline for comparison when post-HSCT scans are obtained and abnormalities are noted. On the other hand, clinically insignificant findings may delay transplant while a work-up of the abnormalities is pursued. Children who are not able to lay still need to be sedated for the imaging and thus, anesthesia risks should also be considered. Furthermore, the benefits of a pre-HSCT chest and abdomen CT in asymptomatic patients may not outweigh the risks of additional radiation exposure. This is especially important when children and adolescents are being treated – at a time of rapid body development and organ growth, their bodies may be more sensitive to the damaging effects of radiation than those of adults [14]. Radiation carcinogenic effects resulting from diagnostic imaging procedures are stochastic. That is, there is no threshold dose at which a malignancy is induced. Rather, the probability of secondary malignancy increases with increasing radiation exposure and is cumulative over time. Radiation-induced malignancies, which may occur because of the additive effects of radiation therapy as well as multiple radiographic images, may have long latency periods, frequently spanning decades [12]. Thus, when considering the benefit to risk ratio of routine CT imaging examinations involving asymptomatic children, one should be careful in recommending this imaging procedure if it is unlikely to result in alterations in their clinical care. A more detailed statistical analysis of this project was limited due to small patient numbers, even at a large pediatric transplant center.
Another limitation of this study is that it was performed retrospectively and interventions, whether diagnostic or therapeutic were at the discretion of the primary transplant attending. In most cases with radiological abnormalities, therapeutic interventions or further diagnostic evaluations including cultures were not performed; we do not know if this was the best decision, but a medical decision nonetheless. Primary attendings decided when to further investigate and the presence or absence of symptoms may have played a factor in when physicians decided to investigate abnormalities. Furthermore, due to the retrospective nature of this study, we do not know whether transplants were delayed based on CT findings. A delay proceeding to transplant could have occurred in patients with abnormalities, but this is not definitively known.
Our findings suggest that routine CT screening of the abdomen in asymptomatic patients undergoing allo-HSCT rarely identifies new abnormalities that are undetectable clinically and does not lead to significant changes in patient management. Performing chest CTs should be decided judiciously based on the individual patient’s history and current clinical status. Routine CT imaging of the sinuses frequently reveals abnormalities, and therefore may be considered in autologous and allogeneic patients before transplantation in concert with an otolaryngology consultation.
Acknowledgments
This work was supported in part by NIH Cancer Center Support grant P30CA 21765 and by the American Lebanese Syrian Associated Charities (ALSAC).
Ms. Nancy Wright for data collection, and Ms. Vani Shanker for her assistance with manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Afessa B, Peters SG. Major complications following hematopoietic stem cell transplantation. Semin Respir Crit Care Med. 2006;27:297–309. doi: 10.1055/s-2006-945530. [DOI] [PubMed] [Google Scholar]
- 2.Junghanss C, Marr KA, Carter RA, et al. Incident and outcome of bacterial and fungal infections following nonmyeloablative compared with myeloablative allogeneic hematopoietic stem cell transplantation: a matched control study. Biol Blood Marrow Transplant. 2002;8:512–520. doi: 10.1053/bbmt.2002.v8.pm12374456. [DOI] [PubMed] [Google Scholar]
- 3.Bjorklund A, Aschan J, Labopin M, et al. Risk factors for fatal infectious complications developing late after allogeneic stem cell transplantation. Bone Marrow Transplant. 2007;40:1055–1062. doi: 10.1038/sj.bmt.1705856. [DOI] [PubMed] [Google Scholar]
- 4.Kanne JP, Godwin JD, Franquet T, et al. Viral pneumonia after hematopoietic stem cell transplantation: high-resolution CT findings. J Thorac Imaging. 2007;23:292–299. doi: 10.1097/RTI.0b013e31805467f4. [DOI] [PubMed] [Google Scholar]
- 5.Leung W, Ahn H, Rose SR, et al. A prospective cohort study of late sequelae of pediatric allogeneic hematopoietic stem cell transplantation. Medicine. 2007;86:215–224. doi: 10.1097/MD.0b013e31812f864d. [DOI] [PubMed] [Google Scholar]
- 6.Uderzo C, Pillon M, Corti P, et al. Impact of cumulative anthracycline dose, preparative regimen and chronic graft-versus-host disease on pulmonary and cardiac function in children 5 years after allogeneic hematopoietic stem cell transplantation: a prospective evaluation on behalf of the EBMT Pediatric Diseases and Late Effects Working Parties. Bone Marrow Transplant. 2007;39:667–675. doi: 10.1038/sj.bmt.1705652. [DOI] [PubMed] [Google Scholar]
- 7.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HLA-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 8.National Cancer Institute’s (NCI) cancer therapy evaluation program (CTEP) common toxicity criteria manual: common toxicity criteria, version 2.0, June 1, 1999.
- 9.Shrimpton PC, Wall BF. Reference doses for paediatric computed tomography. Radiation Protection Dosimetry. 2000;90:249–252. [Google Scholar]
- 10.http://www.acr.org/accreditation/computed/qc_forms/Phantom_Testing_InstructionFinal.aspx.
- 11.Cameron JR. [May 22, 1998];Are X-rays safe? Available at: http://www.angelfire.com/mo/radioadaptive/jcameron1.html.
- 12.Brody AS, Frush DP, Huda W, Brent RL. Radiation risk to children from computed tomography. Pediatrics. 2007;120:677–682. doi: 10.1542/peds.2007-1910. [DOI] [PubMed] [Google Scholar]
- 13.Billings KR, Lowe LH, Aquino VM, Biavati MJ. Screening sinus CT in pediatric bone marrow transplant patients. Int J Pediatr Otorhinolaryngol. 2000;52:253–260. doi: 10.1016/s0165-5876(00)00296-2. [DOI] [PubMed] [Google Scholar]
- 14.Brenner DJ, Hall EJ. Computed tomography-an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]


