Abstract
Objectives
Our goal was to determine whether obstetrical outcomes and serum angiogenic factors are altered in women with gestational proteinuria without hypertension.
Methods
We performed a nested case-control study of 108 women with gestational proteinuria, comparing them to 1564 randomly selected normotensive women without proteinuria during pregnancy (controls) and to 319 women who developed pre-eclampsia.
Results
Women with gestational proteinuria had greater body-mass index and higher blood pressure at study enrollment. Adverse obstetrical outcomes were infrequent. Levels of PlGF were lower than controls beginning early in gestation. Compared to gestational-age matched controls, PlGF was reduced beginning 6 to 8 weeks before proteinuria. Although sFlt-1 and soluble endoglin concentrations were elevated 1 to 2 weeks before proteinuria, these elevations were modest and transient. After onset of proteinuria, angiogenic factor levels generally did not differ significantly from controls.
Conclusion
Gestational proteinuria in healthy nulliparous women appears to be a mild variant of pre-eclampsia.
Keywords: gestational proteinuria, pre-eclampsia, angiogenic factor, soluble fms-like tyrosine kinase 1, soluble endoglin, placental growth factor
Introduction
Pre-eclampsia - the onset of maternal hypertension and proteinuria usually following the twentieth week of gestation - remains an important cause of maternal and fetal morbidity and mortality.(1). Placental secretion of excessive quantities of the antiangiogenic proteins soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin (sEng) into maternal blood, causing widespread maternal endothelial dysfunction, has been proposed to be the final common pathway leading to pre-eclampsia.(2, 3) Women with pre-eclampsia have increased serum concentrations of sFlt-1 and sEng and reduced concentrations of free vascular endothelial growth factor (VEGF) and free placental growth factor (PlGF), proangiogenic proteins which are bound and neutralized by sFlt-1.(3-8) Women with gestational hypertension, but not proteinuria, appear to have similar, but modest, alterations of circulating angiogenic proteins.(3)
Angiogenic factors play an important role in the development and maintenance of renal glomeruli. Mice lacking expression of one or more of the isoforms of VEGF are born with fewer glomeruli, develop glomerular injury with proteinuria, or die in the perinatal period. (9, 10) Injection of an antibody against transforming growth factor-β1, which may mimic the action of sEng, inhibits glomerular capillary lumen formation and endothelial cell fenestration in newborn rats.(11) Adult male mice injected with exogenous sFlt-1 or with anti-VEGF antibody develop proteinuria and glomerular endothelial damage.(12, 13) Pregnant and non-pregnant rats exposed to sFlt-1 and/or sEng manifest hypertension, proteinuria and glomerular endotheliosis, the renal lesion observed in pre-eclampsia.(2, 7) Administration of VEGF to rats improves glomerular health and kidney function in various renal disease models including pre-eclampsia.(14-18) Patients undergoing chemotherapy with a monoclonal antibody to VEGF frequently develop hypertension and proteinuria.(19)
The pathogenesis of gestational proteinuria in healthy women – the new onset of proteinuria following the twentieth week of gestation in the absence of hypertension – has not been studied in depth. Proteinuria which antedates conception, that is usually due to underlying renal disease and in which proteinuria is exacerbated early in pregnancy, has been associated with increased risk of adverse obstetrical outcomes.(20) Whether gestational proteinuria in healthy women is associated with adverse obstetrical or perinatal outcomes is not known. We hypothesized that proteinuria without hypertension in healthy nulliparous pregnancy might be similar to a mild form of pre-eclampsia and would be accompanied by similar alterations of circulating angiogenic factors. In order to determine the relationship of these factors to the development of gestational proteinuria, we conducted a nested case control study within the Calcium for Pre-eclampsia Prevention (CPEP) trial database and specimen repository. Circulating concentrations of sFlt-1, sEng and free PlGF in women with gestational proteinuria were compared to those in women who remained normotensive without proteinuria during pregnancy and in women who developed pre-eclampsia.
Methods
Participants and Specimens
CPEP was a randomized, double-blind clinical trial conducted from 1992 to 1995 to determine if calcium supplementation would prevent pre-eclampsia in healthy nulliparous women.(21) Women with singleton pregnancies were enrolled between 13 and 21 weeks of gestation at five participating medical centers and followed until 24 hours after delivery. Written consent was provided by all participants. Women were excluded from entry into the trial if they had: (1) a history of hypertension, renal disease, diabetes, or collagen vascular disease, (2) elevated blood pressure (≥ 135/85 mm Hg), (3) elevated serum creatinine (≥1.0 mg/dl), or (4) proteinuria (1+ [30 mg/dl] or greater on dipstick) at either of two screening clinic visits. Serum and urine specimens were requested prior to enrollment at 13 to 21 weeks of gestation, at 26 to 29 weeks, at 36 weeks if still pregnant, and when pre-eclampsia was suspected. Calcium supplementation did not reduce the incidence or severity of pre-eclampsia. (21)
Of a total of 4589 women enrolled in CPEP, 153 developed gestational proteinuria and 326 developed pre-eclampsia. Among women with gestational proteinuria, six were excluded owing to loss to follow-up, inadequate chart review, or lack of serum samples obtained at ten or more weeks of gestation before labor or delivery. Because proteinuria might have resulted from urinary tract infection, we excluded 39 women with positive urine cultures, leaving 108 women with gestational proteinuria for study. Seven women were excluded who developed pre-eclampsia, but lacked appropriate serum specimens, leaving 319 women with pre-eclampsia for comparison.
Controls were selected as follows: Among 4589 CPEP participants, 253 lost to follow-up, 26 with incomplete outcome data or smoking history, 20 whose pregnancy had terminated before 20 weeks, and 21 without repository serum or urine specimens were excluded. From the remaining 4269 women a random sample of 2200 was selected. After further excluding women without appropriate serum specimens as well as those who had developed pre-eclampsia, gestational hypertension, or gestational proteinuria, 1564 remained whose pregnancies had been normotensive and without proteinuria.
Gestational proteinuria was defined as the onset of proteinuria after the twentieth week of gestation in women who remained normotensive throughout pregnancy. Proteinuria was defined by either of the following: (1) 24-hour urine collection of ≥ 300 mg protein, (2) a single random urine specimen with protein / creatinine ratio ≥ 0.35, (3) ≥ 2+ (100 mg/dl) protein by dipstick in one random specimen, or (4) 1+ (30 mg/dl) protein in two random urine specimens obtained 4 to 168 hours apart. Severe proteinuria was urinary protein excretion ≥3.5 g per 24 hours or urine dipstick ≥3+ [300 mg/dl] in two random urine specimens obtained 4 to 168 hours apart. All dipsticks were Ames reagent strips (Miles Inc., Elkhart, IN). Gestational hypertension was the onset of hypertension after the twentieth week of gestation. Hypertension was an elevated diastolic blood pressure of at least 90 mm Hg on two occasions 4 to 168 hours apart. Pre-eclampsia was defined as the occurrence of hypertension and proteinuria within seven days of each other. Gestational age was determined using the earliest obstetrical ultrasound before enrollment in CPEP. The onset of gestational proteinuria was the time of the first urinary protein measurement leading to the diagnosis. Detailed definitions of pre-eclampsia and gestational (or pregnancy-associated) proteinuria have been published. (21,22)
The length of an episode of gestational proteinuria was computed from the day when it was first noted to the day it was no longer detected or delivery, whichever came first. An episode of gestational proteinuria was considered resolved when there was a 24-hour urine collection with less than 300 mg protein, a protein/creatinine ratio below 0.35, or at the first of two successive urine dipsticks at least four hours apart with trace or no protein.
Because specimens could not be linked to identifiable women, the Office of Human Subjects Research of the National Institutes of Health granted the study an exemption from the requirement for review and approval by the institutional review board.
Procedures
Serum samples were frozen shortly after collection and sent to a central repository where they were stored at -70°C. Specimens were randomly ordered for analysis, and assays were performed by personnel who were unaware of pregnancy outcomes. Enzyme-linked immunosorbent assays (ELISA) for human sFlt-1, sEng, and free PlGF were conducted in duplicate by R&D Systems Analytical Testing Services (Minneapolis, MN) using their commercially available kits. These assays have been validated by recovery studies from serum of pregnant women. Intraassay and interassay coefficients of variation were 3.2 and 7.4 percent, 3.0 and 6.5 percent, and 5.4 and 11.2 percent for sFlt-1, sEng, and PlGF, respectively. Minimal detectable levels for sFlt-1, sEng, and PlGF were, respectively, 5 pg per milliliter, 7 pg per milliliter, and 7 pg per milliliter. Calcium supplementation did not affect angiogenic factor levels. Specimens from 192 of the 319 women with pre-eclampsia had been analyzed for angiogenic factors previously.(3, 6) Since the sFlt-1 assay had undergone recent changes, we analyzed fresh aliquots for all three angiogenic factors in all women.
Statistical Analysis
Chi-square tests were used for comparison of categorical variables; and t-tests, for comparison of continuous variables. Angiogenic factor levels were compared within pre-selected intervals using analysis of variance and covariance. Although arithmetic mean levels of angiogenic proteins are reported in the text and figures, statistical testing was conducted after logarithmic transformation. Within gestational age intervals, if more than one specimen was available from a woman, the earliest collection was used. For case-control comparisons made in the weeks prior to or after onset of proteinuria, serum specimens from women with gestational proteinuria were randomly matched by gestational age at collection. If more than one specimen from a particular case was available within an interval before or after onset of proteinuria, the specimen closest to the onset of proteinuria was selected. All P values are two-tailed.
Results
At enrollment in CPEP women with gestational proteinuria or pre-eclampsia had greater body-mass index, higher blood pressure, and they smoked less frequently than normotensive controls without proteinuria. Serum specimens from controls had been stored slightly longer at -70°C (Table 1). Black race was more common among women with pre-eclampsia. Whereas in women with gestational proteinuria obstetrical and perinatal outcomes were generally favorable, those for women with pre-eclampsia often were not (Table 2). Women with gestational proteinuria were more frequently delivered by cesarean section than controls (22% versus 13%, P=0.009). The incidence of renal dysfunction did not differ significantly from controls. Infants of women with gestational proteinuria were usually delivered at term and were larger than infants born to control mothers. The two-fold excess of large-for-gestational-age infants born to women with gestational proteinuria remained significantly different from controls and women with pre-eclampsia, even after adjustment for maternal weight or body-mass index.
Table 1. Characteristics of Women with Gestational Proteinuria, Pre-eclampsia and Controls at Enrollment and of their Serum Specimens*.
| Controls (n=1564) | Gestational Proteinuria (n=108) | P Value1 | Pre-eclampsia (n=319) | P Value2 | P Value3 | |
|---|---|---|---|---|---|---|
| Women | ||||||
| Age – yr | 20.8 ± 4.2 | 20.5 ± 4.3 | 21.0 ± 4.4 | |||
| Height – cm | 163 ± 7 | 163 ± 6 | 162 ± 7 | 0.03 | ||
| Weight – kg | 66.8 ± 15.5 | 73.8 ± 18.2 | <0.001 | 72.9 ± 19.7 | <0.001 | |
| Body-mass index† | 25.2 ± 5.4 | 27.9 ± 6.5 | <0.001 | 27.8 ± 6.8 | <0.001 | |
| Systolic blood pressure – mm Hg | 106 ± 9 | 108 ± 9 | 0.006 | 110 ± 8 | <0.001 | 0.03 |
| Diastolic blood pressure - mm Hg | 58.7 ± 7.4 | 60.1 ± 7.5 | 0.049 | 62.4 ± 7.7 | <0.001 | 0.009 |
| Serum creatinine – mg/dL | 0.72 ± 0.1 | 0.70 ± 0.1 | 0.74 ± 0.1 | 0.03 | ||
| Previous abortion (%) | 403 (25.8) | 24 (22.2) | 68 (21.3) | |||
| Gestational age at enrollment – wk | 17.6 ± 2.5 | 17.7 ± 2.5 | 17.4 ± 2.6 | |||
| Current smoker – no. (%) | 205 (13.1) | 10 (9.3) | 29 (9.1) | 0.048 | ||
| Calcium treatment – no. (%) | 826 (52.8) | 59 (54.6) | 154 (48.3) | |||
| Private health insurance – no. (%) | 173 (11.1) | 8 (7.4) | 25 (7.8) | |||
| Ever married - no. (%) | 344 (22.0) | 23 (21.3) | 65 (20.4) | |||
| Race – no. (%)‡ | 0.002 | 0.006 | ||||
| White, non-Hispanic | 553 (35.4) | 46 (42.6) | 85 (26.6) | |||
| White, Hispanic | 281 (18.0) | 20 (18.5) | 50 (15.7) | |||
| Black | 695 (44.4) | 39 (36.1) | 171 (53.6) | |||
| Other or unknown | 35 (2.2) | 3 (2.8) | 13 (4.1) | |||
| Specimens | ||||||
| Freezer storage – yr | 11.5 ± 0.8 | 11.3 ± 0.7 | <0.001 | 11.4 ± 0.8 | 0.02 | 0.01 |
Plus–minus values are means ± SD. P values are given only for significant differences
Comparing women with gestational proteinuria to controls,
women with pre-eclampsia to controls, and
women with pre-eclampsia to those with gestational proteinuria
Body-mass index is the weight in kilograms divided by the square of the height in meters.
Race or ethnic group was self-reported.
Table 2. Obstetrical and Perinatal Outcomes in Women with Gestational Proteinuria, Pre-eclampsia and Controls*.
| Controls (n=1564) |
Gestational Proteinuria (n=108) |
P Value1 | Pre-eclampsia (n=319) |
P Value2 | P Value3 | |
|---|---|---|---|---|---|---|
| Obstetrical Outcomes | ||||||
| Preterm delivery < 37 wk - no. (%) | 160 (10.2) | 12 (11.1) | 67 (21.0) | <0.001 | 0.02 | |
| Preterm delivery < 34 wk - no. (%) | 55 (3.5) | 2 (1.9) | 24 (7.5) | 0.001 | 0.03 | |
| Post-term delivery (≥ 42 wk) – no. (%) | 50 (3.2) | 7 (6.5) | 10 (3.1) | |||
| Induction of labor – no. (%) | 189 (12.1) | 18 (16.7) | 151 (47.3) | <0.001 | <0.001 | |
| Cesarean delivery – no. (%) | 207 (13.2) | 24 (22.2) | 0.009 | 103 (32.3) | <0.001 | 0.048 |
| Placental abruption – no. (%) | 8 (0.5) | 2 (1.9) | 7 (2.2) | 0.007 | ||
| Disseminated intravascular coagulation - no. (%) | 0 (0.0) | 1 (0.9) | 3 (0.9) | 0.005 | ||
| Cerebral thrombosis/hemorrhage - no. (%) | 1 (0.1) | 0 (0.0) | 1 (0.3) | |||
| Platelet count < 100,000 – no. (%)$ | 9 (1.3) | 2 (3.2) | 23 (8.8) | <0.001 | 0.04 | |
| Elevated liver enzymes – no. (%)‡$ | 0 (0.0) | 2 (5.3) | 29 (10.2) | <0.001 | 0.01 | |
| Plasma glucose 1-hour after 50 g oral glucose challenge (mg/dL)$ | 107.2 ± 25.3 | 110.1 ± 28.0 | 112.1 ± 24.5 | 0.003 | ||
| Gestational diabetes mellitus‡$ - no. (%) | 28 (2.1) | 2 (2.1) | 8 (2.8) | |||
| Renal dysfunction‡$ - no. (%) | 5 (2.1) | 2 (4.7) | 25 (9.5) | <0.001 | 0.03 | |
| Perinatal Outcomes | ||||||
| Week of gestation at delivery† | 39.3 ± 2.7 | 39.4 ± 2.0 | 38.6 ± 2.9 | <0.001 | <0.001 | |
| Birth weight – g† | 3181 ± 607 | 3318 ± 590 | 0.02 | 3035 ± 766 | 0.002 | <0.001 |
| Small for gestational age infant (<10th percentile) – no. (%)† | 130 (8.4) | 11 (10.2) | 50 (15.8) | <0.001 | ||
| Large for gestational age infant (>90th percentile) – no (%)† | 100 (6.5) | 18 (16.7) | <0.001 | 20 (6.3) | 0.001 | |
| Apgar score at 1 minute† | 7.9 ± 1.5 | 7.7 ± 1.5 | 7.2 ± 1.9 | <0.001 | 0.002 | |
| Apgar score at 5 minutes† | 8.8 ± 0.7 | 8.8 ± 0.6 | 8.6 ± 1.0 | <0.001 | 0.002 | |
| Admission to the neonatal intensive care unit – no. (%) | 206 (13.3) | 14 (13.0) | 102 (32.3) | <0.001 | <0.001 | |
| Perinatal death - no. (%) | 21 (1.3) | 0 (0.0) | 4 (1.3) | |||
Plus–minus values are means ± SD. P values are given only for significant differences.
Comparing women with gestational proteinuria to controls,
women with pre-eclampsia to controls, and
women with pre-eclampsia to those with gestational proteinuria
Values are based on all live births.
Elevated liver enzymes were defined as an AST (SGOT) twice the upper limit of normal by local laboratory standards. Gestational diabetes mellitus was defined by a plasma glucose level ≥ 200 mg/dL one hour after a 50-g oral glucose screen in the absence of an oral glucose tolerance test or ≥ two abnormal plasma glucose values in a 3-hour 100-g oral glucose tolerance test (≥ 105 mg/dL fasting, ≥ 190 mg/dL at 1 hour, ≥165 mg/dL at 2 hours, or ≥ 145 mg/dL at 3 hours). Renal dysfunction was defined as an increase in serum creatinine of ≥ 0.5 mg/dL over baseline.
Indicates variables with more than 5 percent missing values. The number of subjects with complete information in controls, gestational proteinuria, and pre-eclampsia groups were as follows, respectively: Platelet count <100,000/mm3 (689, 63, 261); Plasma glucose 1 hr following 50 g oral glucose challenge (1378, 97, 294); Gestational diabetes mellitus (1345, 94, 283); Elevated liver enzymes (119, 38, 284); Renal dysfunction (235, 43, 263).
Only one woman with gestational proteinuria had severe proteinuria. The median gestational age at onset of proteinuria was 37.9 weeks. Proteinuria began within a week of delivery in about half the subjects (N=52, 48%). The mean of the estimated duration of proteinuria in women with a single episode was 9 days. In 43 of the 108 women (40 percent) with gestational proteinuria, proteinuria resolved prior to delivery; and in these women the mean of the estimated duration of proteinuria was 13 days. While almost all women had proteinuria documented only once, three had two episodes and one had three.
To evaluate gestational patterns of angiogenic factors before onset of proteinuria, we first compared concentrations in serum specimens obtained at 10-20, 21-32, or 33-42 weeks of gestation from women in whom proteinuria had been documented by a 24-hour urine collection or by a protein / creatinine ratio with those of specimens obtained from women with proteinuria determined by dipstick and from normotensive women without evidence of proteinuria (Table 3). After adjustment for body-mass index, race/ethnicity, and gestational age at specimen collection, in the women with gestational proteinuria PlGF appeared to be lower than controls throughout pregnancy (at least in the much larger group with dipstick proteinuria) while sFlt1 and sEng were higher at 33-42 weeks. There were no significant differences in concentrations of angiogenic factors between specimens from the 22 women whose proteinuria had been documented in a 24-hour urine or by protein / creatinine ratio and those from the 86 women with proteinuria by dipstick. Therefore, in subsequent analyses we combined both proteinuria subgroups.
Table 3. Angiogenic Factor Concentrations at 10-20, 21-32, and 33-42 Weeks of Gestation in Normotensive Controls and in Women Who Subsequently Developed Proteinuria Documented either by Dipstick or by 24-hour Urine or Protein/Creatinine Ratio.
| 10-20 wks | Control | GP by Dipstick | GP by 24 Hr Urine or P/C Ratio |
|---|---|---|---|
| N | 1353 | 79 | 19 |
| GA at collection (days) | 114 ± 19 | 113 ± 19 | 116 ± 21 |
| sFlt-1 (pg/ml) | 3786 ± 1989 | 3368 ± 1814 | 3636 ± 2074 |
| PlGF (pg/ml) | 146 ± 126 | 115 ± 72* | 163 ± 151 |
| sEng (ng/ml) | 5.6 ± 5.4 | 5.0 ± 1.0 | 5.30 ± 1.13 |
| 21-32 wks | |||
| N | 1363 | 65 | 15 |
| GA at collection (days) | 192 ± 11 | 194 ± 11 | 194 ± 11 |
| sFlt-1 (pg/ml) | 4005 ± 2356 | 3769 ± 2078 | 4391 ± 2901 |
| PlGF (pg/ml) | 733 ± 621 | 591 ± 455 | 496 ± 198 |
| sEng (ng/ml) | 5.5 ± 7.6 | 5.3 ± 2.1 | 5.5 ± 1.5 |
| 33-42 wks | |||
| N | 1217 | 43 | 6 |
| GA at collection (days) | 255 ± 6 | 257 ± 9 | 256 ± 7 |
| sFlt-1 (pg/ml) | 7823 ± 4725 | 9450 ± 6086* | 10108 ± 3524* |
| PlGF (pg/ml) | 583 ± 697 | 384 ± 436* | 170 ± 66* |
| sEng (ng/ml) | 10.2 ± 7.9 | 11.8 ± 8.7 | 15.2 ± 6.6** |
Data is presented as N for number of specimens or arithmetic mean ± s.d.
GA = gestational age GP = gestational proteinuria
sFlt-1 = soluble fms-like tyrosine kinase 1 PlGF = placental growth factor sEng = soluble endoglin
Comparisons of angiogenic factor concentrations used logarithmically transformed values.
P <0.05,
P <0.01
P < 0.001 for comparisons with controls after adjusting for BMI, race/ethnicity, and GA at collection
There were no significant differences in angiogenic factor concentrations between GP by dipstick and GP by 24-hour urine or P/C ratio
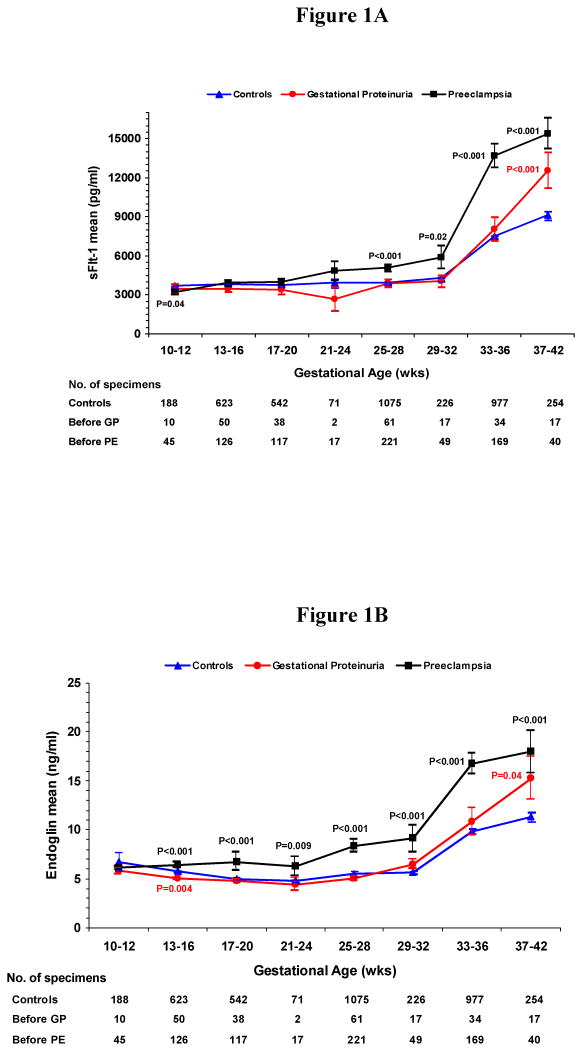
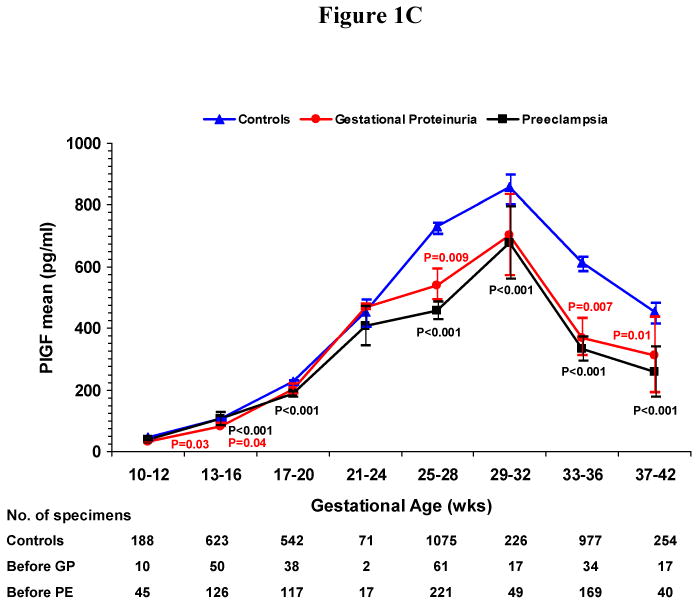
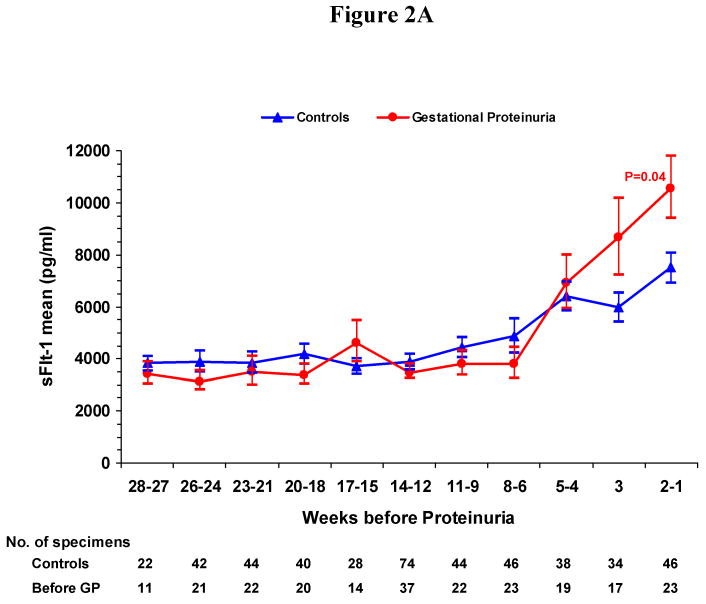
We conducted a cross-sectional analysis of serum concentrations before onset of gestational proteinuria or pre-eclampsia usually within four week gestational-age intervals. In women who developed gestational proteinuria and in controls, serum levels of sFlt-1 (Figure 1A) and sEng (Figure 1B) remained stable until rising at 33-36 weeks of gestation. In women who later developed pre-eclampsia, levels of sFlt-1 rose compared to controls beginning at 25-28 weeks and sEng, beginning at 13-16 weeks. Peak concentrations attained at 37-42 weeks were greatest in women who subsequently developed pre-eclampsia (15,390 pg/ml sFlt-1, 18.0 ng/ml sEng), intermediate in women who developed gestational proteinuria (12,540 pg/ml sFlt-1, 15.3 ng/ml sEng), and lowest in normotensive non-proteinuric controls (9160 pg/ml sFlt-1, 11.3 ng/ml sEng). While at term concentrations in women who later developed pre-eclampsia or gestational proteinuria did not differ significantly, both were significantly greater than controls. PlGF increased with gestation, reaching maximal values at 29-32 weeks of gestation, then declining (Figure 1C). In women who later developed gestational proteinuria, PlGF concentrations were lower throughout pregnancy, beginning at 10-12 weeks. Concentrations in women with subsequent pre-eclampsia were similar to those in women who later developed gestational proteinuria, but were usually somewhat lower.
Figure 1. Figure 1A. Mean Serum Concentration of Soluble Fms-like Tyrosine Kinase 1 (sFlt-1) According to Gestational Age, Figure 1B. Mean Serum Concentration of Soluble Endoglin (sEng) According to Gestational Age, Figure 1C. Mean Serum Concentration of Placental Growth Factor (PlGF) According to Gestational Age.
Figures 1A-C show the mean serum concentrations of sFlt-1, sEng and PlGF within gestational age intervals in women who later had gestational proteinuria (GP), those who later had pre-eclampsia (PE) and normotensive controls. I bars represent standard errors. Specimens from women with gestational proteinuria and pre-eclampsia were collected before onset and analyzed within pre-selected intervals, using the earliest serum specimen when more than one serum specimen was available. The number of specimens within each gestational age interval is noted below the figures. Statistical testing was performed on logarithmically transformed values. Only significant P values are reported and are for the comparisons with controls. The difference between mean serum concentrations of sFlt-1 (Figure 1A) in women who developed gestational proteinuria and women who later had pre-eclampsia was significant at 25 to 28 (P=0.01) and 33 to 36 weeks (P<0.001). For sEng (Figure 1B), the difference between women who developed gestational proteinuria and pre-eclampsia was significant at 13 to 16 weeks (P<0.001), 17 to 20 weeks (P=0.001), 25 to 28 weeks (P<0.001), and 33 to 36 weeks (P<0.001). For PlGF (Figure 1C), the difference was significant only at 25 to 28 weeks (P=0.008), the interval with the greatest number of serum samples from all three groups of women.
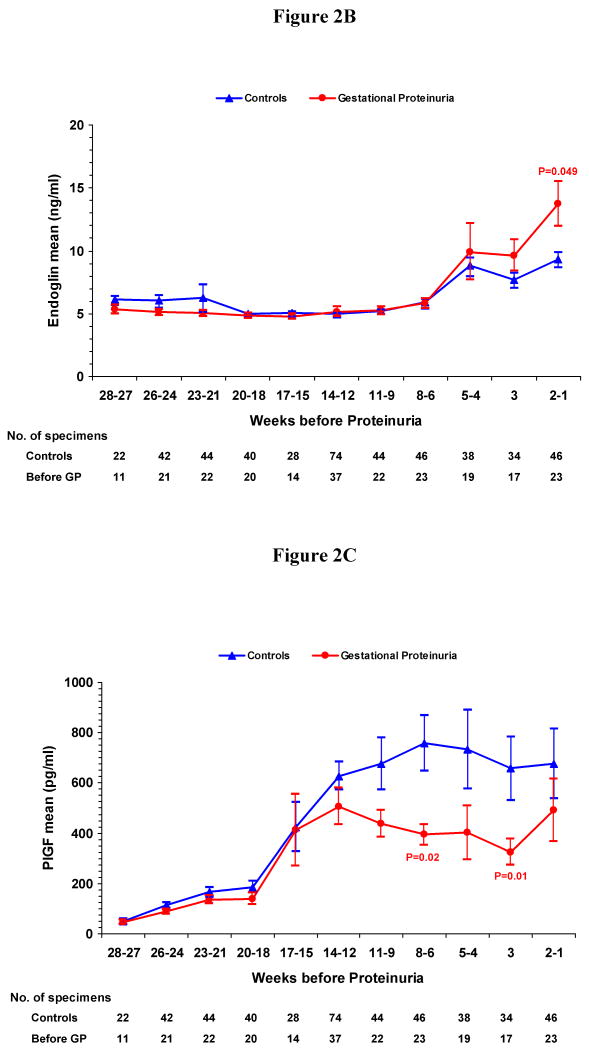
In order to determine the progression of serum angiogenic factor concentrations with proximity to the onset of gestational proteinuria, we compared serum samples from women who would later develop gestational proteinuria to gestational age–matched samples from normotensive, non-proteinuric controls (Figure 2A-C). During the two weeks before onset of proteinuria, the mean serum sFlt-1 and sEng levels significantly increased (sFlt-1: 10,550 pg per milliliter versus 7510 pg per milliliter, P=0.04; sEng: 13.8 ng per milliliter versus 9.4 ng per milliliter, P=0.049) in the women who developed gestational proteinuria. The PlGF concentration was reduced significantly at 6-8 weeks and at 3 weeks before proteinuria.
Figure 2. Figure 2A. Mean Serum Concentration of Soluble Fms-like Tyrosine Kinase 1 (sFlt-1) According to Weeks before the Onset of Gestational Proteinuria and in Gestational-Age Matched Controls. Figure 2B. Mean Serum Concentration of Soluble Endoglin (sEng) According to Weeks before the Onset of Gestational Proteinuria and in Gestational-Age Matched Controls. Figure 2C. Mean Serum Concentration of Placental Growth Factor (PlGF) According to Weeks before the Onset of Gestational Proteinuria and in Gestational-Age Matched Controls.
2A-C show the mean serum concentrations of sFlt-1, sEng and PlGF by weeks before the onset of gestational proteinuria (GP) in women who later developed gestational proteinuria and in gestational-age matched normotensive controls. I bars represent standard errors. Specimens from women with gestational proteinuria were collected before onset of proteinuria and analyzed within pre-selected intervals, using the serum specimen closest to onset when more than one serum specimen was available. The number of specimens within each interval is noted below the figures. Statistical testing was performed on logarithmically transformed values. Only significant P values are reported and are for the comparisons with controls.
We also examined angiogenic factor concentrations after the onset of proteinuria, comparing them within pre-selected intervals to those of gestational-age matched controls. Whereas within 2 weeks before onset of proteinuria sFlt-1 and sEng were significantly elevated in the women who were to develop proteinuria, after onset of proteinuria the only significant difference was a reduction in PlGF one week later (268 pg per milliliter versus 624 pg per milliliter, P=0.01). Two or more weeks following the onset of gestational proteinuria, differences in serum angiogenic factor levels became unremarkable (Table 4).
Table 4. Angiogenic Factor Concentrations by Weeks Before or After the Onset of Gestational Proteinuria (GP) Compared to Gestational-Age Matched Controls.
| 1-2 Weeks Before |
<1 Week After |
1 Week After |
2-8 Weeks After |
||
|---|---|---|---|---|---|
| No. of specimens | GP | 23 | 20 | 11 | 16 |
| Controls | 46 | 40 | 22 | 32 | |
| Gestational Age (days) | GP | 251 ± 24 | 230 ± 39 | 245 ± 30 | 228 ± 34 |
| Controls | 251 ± 23 | 229 ± 38 | 245 ± 27 | 227 ± 33 | |
| sFlt-1 (pg/mL) | GP | 10550a ± 5740 | 10160 ± 7760 | 7380 ± 4380 | 6550 ± 4860 |
| Controls | 7510 ± 3930 | 7030 ± 5880 | 8330 ± 6190 | 6180 ± 3100 | |
| sEng (ng/mL) | GP | 13.8b ± 8.6 | 10.4 ± 7.6 | 9.4 ± 4.9 | 9.7 ± 8.6 |
| Controls | 9.4 ± 4.3 | 8.5 ± 6.3 | 10.8 ± 4.8 | 8.1 ± 4.5 | |
| PlGF (pg/mL) | GP | 492 ± 601 | 538 ± 652 | 268c ± 145 | 643 ± 535 |
| Controls | 676 ± 950 | 539 ± 431 | 624 ± 759 | 805 ± 899 | |
Removal of the five women with gestational proteinuria who developed elevated liver enzymes (N=2), low platelet counts (N=2), or disseminated intravascular coagulation (N=1) had only minor effects on mean angiogenic factor levels. Their inclusion did not account for the characteristic patterns noted within the women with gestational proteinuria.
Discussion
We have demonstrated that healthy pregnant nulliparous women who develop proteinuria, but not hypertension, manifest a modest and transient imbalance of circulating angiogenic factors, resulting in an antiangiogenic state in the blood. The imbalance of angiogenic factors in women who develop gestational proteinuria occurs before onset of clinical signs, as in pre-eclampsia; and it typically occurs within two weeks before onset of proteinuria. Serum concentrations of PlGF appear to be lower than those of controls as early as 10-12 weeks of gestation. However, levels of sFlt-1 and sEng are elevated only at term, when peak concentrations are attained. At term levels of sFlt-1, sEng, and PlGF before onset of gestational proteinuria are not altered as much as in women who will develop pre-eclampsia. Nevertheless, the similarities suggest that gestational proteinuria may be a mild variant of pre-eclampsia.
Women with gestational proteinuria had greater body-mass index, small increases in blood pressure at enrollment early in the second trimester, and a lower prevalence of smoking, characteristics which are also observed in women who develop pre-eclampsia.(24-26) Healthy women with gestational proteinuria have favorable pregnancy outcomes and a two-fold excess of large-for-gestational-age infants. Contrary to previous reports (23), an excess of large-for-gestational-age infants in women with pre-eclampsia was not observed here.
We can only speculate why gestational proteinuria did not progress to pre-eclampsia. Almost half the women had onset within a week of delivery, suggesting that delivery may have terminated the progression, but in 40 percent of the women proteinuria resolved prior to delivery. It is also likely that the complete syndrome of pre-eclampsia did not develop in this group of women since the abnormalities in circulating angiogenic factors were transient and modest. In serum specimens collected after the onset of proteinuria, but before labor or delivery, the antiangiogenic state appeared to dissipate within two weeks after proteinuria began. This contrasts with pre-eclampsia, where the greatest alterations of angiogenic factor concentrations occur after the onset of clinical disease.(3, 6) Resolution of angiogenic factor imbalance could explain why gestational proteinuria usually lasts less than 2 weeks and may not progress to pre-eclampsia. Finally, similar levels of PlGF, but not sFlt-1, in women with gestational proteinuria and pre-eclampsia suggest that while low levels of PlGF may be sufficient to develop proteinuria following modest brief increases in sFlt-1 and sEng, higher levels of sFlt-1 and sEng over a period exceeding one month may be required to develop hypertension.
The results are consistent with limited previous observations. During normal pregnancy serum concentrations of sFlt-1 were found to be positively correlated and free VEGF concentrations negatively correlated with urinary albumin excretion, suggesting that circulating levels of sFlt-1 may affect the health of glomerular endothelial cells even in normal pregnancy.(27) Indeed, a previous report involving renal biopsies has demonstrated that mild glomerular endotheliosis can be found in normal term pregnancy.(28) A small study which compared 10 women after onset of gestational proteinuria to 20 age- and gestational week-matched controls with normal pregnancies reported increased circulating sFlt-1 and decreased PlGF in the women with gestational proteinuria.(29) In the present study of a larger number of women with gestational proteinuria significant differences in serum angiogenic factor levels were observed before, but usually not after the onset of proteinuria. Some investigators have reported lower levels of PlGF in women who did not develop pre-eclampsia, but who delivered small-for-gestational-age infants. (30,31) We have observed lower levels of PlGF in women with pre-eclampsia or gestational proteinuria, but not in normotensive women who delivered small-for-gestational-age infants.(3) The different findings may result from differences in race/ethnicity, sample collection or storage, or accuracy of the clinical diagnoses. It is also possible that low levels of PlGF early in pregnancy may not be specific for pre-eclampsia and gestational proteinuria.
Our study had several advantages. The prospective collection of serum samples within the CPEP trial cohort of healthy nulliparas allowed us to examine changes in levels of angiogenic factors in women who developed gestational proteinuria without concern for selection bias that might otherwise be present in women presenting after the onset of the condition. Care was taken to exclude women who had renal disease or proteinuria prior to enrollment in CPEP. Exclusion criteria included women with a history of renal disease or diseases which could affect kidney function such as chronic hypertension, diabetes mellitus, or systemic lupus erythematosus. All women were screened twice for proteinuria prior to enrollment and were required to have a serum creatinine < 1.0 mg/dl at enrollment. The likelihood that proteinuria resulted from contamination was minimized by requirements for confirmation of positive dipsticks in a clean catch specimen, for catheterization after rupture of membranes or during vaginitis, and exclusion of specimens containing visible blood. Moreover, the characteristic patterns of angiogenic factors, greater body-mass index, small elevations in midtrimester blood pressure, and lower prevalence of smoking could not be explained if contamination or concentration of urine specimens had been an important cause of gestational proteinuria.
Besides the use of urine dipsticks to diagnose the majority of cases of gestational proteinuria, shortcomings include the cross-sectional nature of the data with the consequence that trends in angiogenic factor levels need not necessarily reflect changes which occur within individual women. Furthermore, the exact duration of proteinuria could not be ascertained as daily assessments for proteinuria were not performed. Women with gestational proteinuria in our study were healthy nulliparas who with one exception did not have severe proteinuria. Conclusions about outcomes can only be applied to this population and not to all women with proteinuria during pregnancy.
Since routine data collection concluded 24 hours following delivery, we were unable to evaluate the clinical course of women with gestational proteinuria. It is possible that some women who developed proteinuria during pregnancy were signaling the new onset of chronic renal disease. A recent review of chronic renal disease in pregnancy indicates that the prevalence ranges from 2 to 12 per 10,000.(32) This would extrapolate to 1 to 6 women in the CPEP cohort or even fewer since the above prevalence rates appear to include women diagnosed during pregnancy, but whose disease may have antedated pregnancy. CPEP participants were screened prior to entry and were unlikely to have had renal disease before pregnancy. While proteinuria which antedates pregnancy has been associated with later development of chronic and even end-stage renal disease,(20) it is not known whether long-term follow-up of healthy women with gestational proteinuria might reveal renal consequences. For this reason it is important for women with gestational proteinuria who manifest renal insufficiency or in whom proteinuria persists beyond pregnancy to be evaluated for kidney disease.
In conclusion, gestational proteinuria in healthy nulliparous women appears to be a benign condition that may not warrant intervention during pregnancy in the absence of hypertension or severe proteinuria. Modest angiogenic factor imbalances favoring an antiangiogenic state, greater body-mass index, small elevations in midtrimester blood pressure, and a lower prevalence of smoking suggest that gestational proteinuria without hypertension may represent a mild variant of pre-eclampsia.
Acknowledgments
We are indebted to the CPEP Study Group (Appendix), who assembled the database and specimen repository used here and to the patients who took part in the study. We thank Maren May for helpful discussions and Patricia Moyer for assistance with figures. This study was supported by funds and salary from the intramural research program of the National Institutes of Health and by salary from the U.S. Navy. CPEP was supported by the National Institute of Child Health and Human Development under contracts N01-HD-1-3121 through 3126, N01-HD-3154, and N01-HD-5-3246, with co-funding from the National Heart, Lung, and Blood Institute. S.A.K was supported by NIH grants (DK065997 and HL079594). S.A.K. is an investigator of the Howard Hughes Medical Institute.
Appendix
The following were members of the CPEP Study Group: University of Alabama at Birmingham: JC Hauth, R Goldenberg, BS Stofan; University of New Mexico at Albuquerque: LB Curet, GM Joffe, V Dorato; University of Tennessee at Memphis: BM Sibai, SA Friedman, BM Mercer, T Carr; Case Western Reserve University at MetroHealth Medical Center, Cleveland: PM Catalano, AS Petrulis, L Barabach; Oregon Health Sciences University, Portland: C Morris, S-L Jacobson, K McCracken; The EMMES Corporation, Rockville: JR Esterlitz, MG Ewell, DM Brown; NICHD: RJ Levine, R DerSimonian, JD Clemens, MA Klebanoff, EG Raymond, JG Rigau-Perez, H Shifrin; NHLBI: JA Cutler, DE Bild. Data Safety and Monitoring Board: M Lindheimer, C Begg, T Chalmers, M Druzin, R Sokol.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The views expressed are those of the authors and do not reflect the official policy or position of the Department of Defense or the U.S. Government.
Disclosures: S.A.K. reports having served as a consultant to Abbott, Beckman Coulter, Roche, and Johnson & Johnson and having been named coinventor on multiple provisional patents filed by Beth Israel Deaconess Medical Center for the use of angiogenesis-related proteins for the diagnosis and treatment of pre-eclampsia. These patents have been nonexclusively licensed to several companies.
References
- 1.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005 Jun 10;308(5728):1592–4. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 2.Venkatesha S, Toporsian M, Lam C, Hanai JI, Mammoto T, Kim YM, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006 Jul;12(6):642–9. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 3.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006 Sep 7;355(10):992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 4.Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005 Jan;17(1):3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 5.Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, et al. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab. 2003 May;88(5):2348–51. doi: 10.1210/jc.2002-021942. [DOI] [PubMed] [Google Scholar]
- 6.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004 Feb 12;350(7):672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 7.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polliotti BM, Fry AG, Saller DN, Mooney RA, Cox C, Miller RK. Second-trimester maternal serum placental growth factor and vascular endothelial growth factor for predicting severe, early-onset preeclampsia. Obstet Gynecol. 2003 Jun;101(6):1266–74. doi: 10.1016/s0029-7844(03)00338-7. [DOI] [PubMed] [Google Scholar]
- 9.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111(5):707–16. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattot V, Moons L, Lupu F, Chernavvsky D, Gomez RA, Collen D, et al. Loss of the VEGF(164) and VEGF(188) isoforms impairs postnatal glomerular angiogenesis and renal arteriogenesis in mice. J Am Soc Nephrol. 2002 Jun;13(6):1548–60. doi: 10.1097/01.asn.0000013925.19218.7b. [DOI] [PubMed] [Google Scholar]
- 11.Liu A, Dardik A, Ballermann BJ. Neutralizing TGF-beta1 antibody infusion in neonatal rat delays in vivo glomerular capillary formation 1. Kidney Int. 1999 Oct;56(4):1334–48. doi: 10.1046/j.1523-1755.1999.00661.x. [DOI] [PubMed] [Google Scholar]
- 12.Kitamoto Y, Takeya M, Tokunaga H, Tomita K. Glomerular endothelial cells are maintained by vascular endothelial growth factor in the adult kidney. Tohoku J Exp Med. 2001 Sep;195(1):43–54. doi: 10.1620/tjem.195.43. [DOI] [PubMed] [Google Scholar]
- 13.Sugimoto H, Hamano Y, Charytan D, Cosgrove D, Kieran M, Sudhakar A, et al. Neutralization of Circulating Vascular Endothelial Growth Factor (VEGF) by Anti-VEGF Antibodies and Soluble VEGF Receptor 1 (sFlt-1) Induces Proteinuria. J Biol Chem. 2003;278(15):12605–8. doi: 10.1074/jbc.C300012200. [DOI] [PubMed] [Google Scholar]
- 14.Kang DH, Joly AH, Oh SW, Hugo C, Kerjaschki D, Gordon KL, et al. Impaired angiogenesis in the remnant kidney model: I. Potential role of vascular endothelial growth factor and thrombospondin-1. J Am Soc Nephrol. 2001 Jul;12(7):1434–47. doi: 10.1681/ASN.V1271434. [DOI] [PubMed] [Google Scholar]
- 15.Li Z, Zhang Y, Ying Ma J, Kapoun AM, Shao Q, Kerr I, et al. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension. 2007 Oct;50(4):686–92. doi: 10.1161/HYPERTENSIONAHA.107.092098. [DOI] [PubMed] [Google Scholar]
- 16.Miyamoto K, Kitamoto Y, Tokunaga H, Takeya M, Ezaki T, Imamura T, et al. Protective effect of vascular endothelial growth factor/vascular permeability factor 165 and 121 on glomerular endothelial cell injury in the rat. Lab Invest. 2004 Sep;84(9):1126–36. doi: 10.1038/labinvest.3700134. [DOI] [PubMed] [Google Scholar]
- 17.Ostendorf T, Kunter U, Eitner F, Loos A, Regele H, Kerjaschki D, et al. VEGF(165) mediates glomerular endothelial repair. J Clin Invest. 1999;104(7):913–23. doi: 10.1172/JCI6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimizu A, Masuda Y, Mori T, Kitamura H, Ishizaki M, Sugisaki Y, et al. Vascular endothelial growth factor165 resolves glomerular inflammation and accelerates glomerular capillary repair in rat anti-glomerular basement membrane glomerulonephritis. J Am Soc Nephrol. 2004 Oct;15(10):2655–65. doi: 10.1097/01.ASN.0000141038.28733.F2. [DOI] [PubMed] [Google Scholar]
- 19.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003 Jul 31;349(5):427–34. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stettler RW, Cunningham FG. Natural history of chronic proteinuria complicating pregnancy. Am J Obstet Gynecol. 1992 Nov;167(5):1219–24. doi: 10.1016/s0002-9378(11)91692-5. [DOI] [PubMed] [Google Scholar]
- 21.Levine RJ, Hauth JC, Curet LB, Sibai BM, Catalano PM, Morris CD, et al. Trial of calcium to prevent preeclampsia. N Engl J Med. 1997;337(2):69–76. doi: 10.1056/NEJM199707103370201. [DOI] [PubMed] [Google Scholar]
- 22.Levine RJ, Esterlitz JR, Raymond EG, et al. Trial of Calcium for Preeclampsia Prevention (CPEP): rationale, design, and methods. Control Clin Trials. 1996;17(5):442–469. doi: 10.1016/s0197-2456(96)00106-7. [DOI] [PubMed] [Google Scholar]
- 23.Vatten LJ, Skjaerven R. Is pre-eclampsia more than one disease? Bjog. 2004 Apr;111(4):298–302. doi: 10.1111/j.1471-0528.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 24.Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. (Clinical research) 2005 Mar 12;330(7491):565. doi: 10.1136/bmj.38380.674340.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine RJ, Qian C, Maynard SE, Yu KF, Epstein FH, Karumanchi SA. Serum sFlt1 concentration during preeclampsia and mid trimester blood pressure in healthy nulliparous women. Am J Obstet Gynecol. 2006 Apr;194(4):1034–41. doi: 10.1016/j.ajog.2005.10.192. [DOI] [PubMed] [Google Scholar]
- 26.Sibai BM, Gordon T, Thom E, Caritis SN, Klebanoff M, McNellis D, et al. Risk factors for preeclampsia in healthy nulliparous women: a prospective multicenter study. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 1995 Feb;172(2 Pt 1):642–8. doi: 10.1016/0002-9378(95)90586-3. [DOI] [PubMed] [Google Scholar]
- 27.Yoshimatsu J, Matsumoto H, Goto K, Shimano M, Narahara H, Miyakawa I. Relationship between urinary albumin and serum soluble fms-like tyrosine kinase 1 (sFlt-1) in normal pregnancy. Eur J Obstet Gynecol Reprod Biol. 2006 Sep-Oct;128(12):204–8. doi: 10.1016/j.ejogrb.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 28.Strevens H, Wide-Swensson D, Hansen A, Horn T, Ingemarsson I, Larsen S, et al. Glomerular endotheliosis in normal pregnancy and pre-eclampsia. BJOG. 2003 Sep;110(9):831–6. [PubMed] [Google Scholar]
- 29.Masuyama H, Suwaki N, Nakatsukasa H, Masumoto A, Tateishi Y, Hiramatrsu Y. Circulating angiogenic factors in preeclampsia, gestational proteinuria, and preeclampsia superimposed on chronic glomerulonephritis. Am J Obstet Gynecol. 2006 Feb;194(2):551–6. doi: 10.1016/j.ajog.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 30.Taylor RN, Grimwood J, Taylor RS, McMaster MT, Fisher SJ, North RA. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am J Obstet Gynecol. 2003;188:177–82. doi: 10.1067/mob.2003.111. [DOI] [PubMed] [Google Scholar]
- 31.Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramin SM, Vidaeff AC, Yeomans ER, Gilstrap LC., 3rd Chronic renal disease in pregnancy. Obstet Gynecol. 2006 Dec;108(6):1531–9. doi: 10.1097/01.AOG.0000246790.84218.44. [DOI] [PubMed] [Google Scholar]