Abstract
Available protocols for stripping antibodies from immunoblots involve the use of SDS or low pH buffers. SDS was shown to remove transferred proteins from membranes and low pH buffer shown to inefficiently strip off antibodies. A solution containing 6M guanidine hydrochloride, 0.2% non-denaturing detergent and a reducing agent can rapidly strip off tightly bound antibodies from aged PVDF immunoblots at room temperature, without removing significant amounts of transferred protein.
Article
Electroblotting of gel-resolved proteins to synthetic membranes coupled with immunoblotting of the transferred proteins with specific antibody has become one of the most commonly used procedures in protein analysis. In many instances, it is desirable to reprobe the membrane with antibodies specific for different proteins and/or antibodies specific for covalently modified regions. Various methods have been described for dissociating antibodies from nitrocellulose or polyvinylidene fluoride (PVDF) membranes after immunoblotting [1]. Currently, two treatments are commonly used for removing antibody from the membrane after immunoblotting in order to reprobe with new antibody. These are washing with a low pH glycine-HCl (Gly-HCl, 0.1 M, pH 2.5) buffer [2,3] or with a sodium dodecyl sulfate (SDS) solution (2% SDS, 0.1M mercaptoethanol, 50mM Tris-HCl, pH 7) [4,5]. Although the low pH Gly-HCl stripping solution will not remove any transferred proteins from the membrane, it does not always completely remove the antibody, especially from blots with a high signal that have been stored for an extended time. The SDS solution is effective at removing the antibody, but requires heating at an elevated temperature (50 to 70°C for 20 to 30 min), and can remove significant amounts of transferred protein from the membrane [6]. We have developed a guanidine hydrochloride-based (GnHCl) stripping solution (6M GnHCl, 0.2% Nonidet P-40 (NP-40), 0.1M β-mercaptoethanol, 20mM Tris-HCl, pH7.5) that can rapidly dissociate antibodies from immunoblots at room temperature without removing significant amounts of the transferred proteins.
Since an ideal solution for stripping antibodies from immunoblots for reprobing should not remove the transferred proteins from the membrane, we studied the effectiveness of the GnHCl, the SDS and the low pH Gly-HCl solutions for the removal of transferred proteins from PVDF membrane. Individual pieces of PVDF membrane with equivalent amounts of transferred proteins were subjected to three cycles of stripping with each stripping solution. Each cycle of stripping consisted of two 30 min washes at 60°C for the SDS solution, two 30 min washes at room temperature for the Gly-HCl solution, or two five min washes at room temperature for the GnHCl solution, each with gentle shaking. After these incubations with the stripping solution, membranes were washed 4 times with shaking (3 min each time) with 0.14M NaCl, 10mM Tris-HCl, pH7.2, (TBS) containing 0.05% NP-40 (TBSN) to remove the stripping solution. To quantitate the pre-stained proteins after stripping, these membrane pieces were placed side by side between two transparent plastic sheets and the intensity of the protein bands were recorded with a Fujifilm LAS-3000 imager. The protein bands were analyzed by the software Multi-Gauge (SL2005) from Fujifilm. Figure 1A shows images of the prestained proteins and Figure 1B a histogram representing the pixel density of protein bands after treatment with various stripping solutions from three independent experiments of the type depicted in Figure 1A. Stripping with SDS solution removed 39% of the 126 kDa and 17% of the 53 kDa proteins. There was no significant change in the amount of protein on the membrane after Gly-HCl or GnHCl stripping demonstrating that stripping with either of these agents did not remove the blotted proteins.
Figure 1.
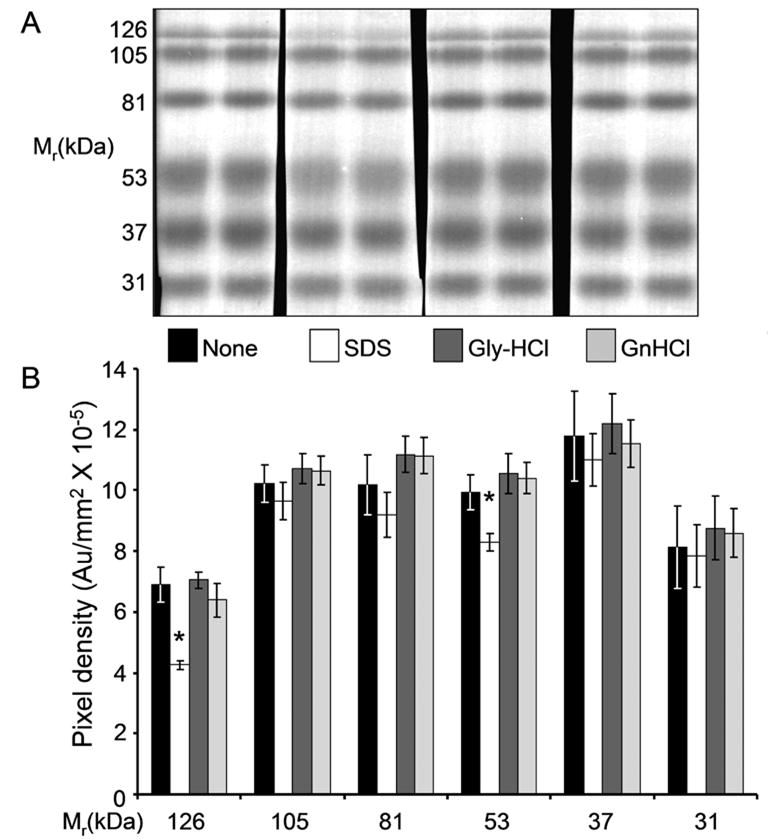
Effects of SDS, Gly-HCl and GnHCl stripping on PVDF membrane bound proteins. 12μl of pre-stained protein standards (covalently prestained molecular markers, Sigma-Aldrich, catalogue no. P8748) were applied to each lane of a 15 well 1mm thick 10% mini gel. SDS-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out in the Laemmli buffer system (7) and the resolved proteins were transferred from the gel onto PVDF membrane (0.45μ, Immobilon, Millipore) at 10°C overnight in the buffer system of Towbin et al (8) with 5% methanol. The membrane was cut into individual pieces containing two lanes of pre-stained proteins each, and each piece was subjected to three cycles of stripping with the indicated solution as described in the text. The membrane pieces were then washed with TBS, and the intensity of the protein bands was quantitated as described in the text. A. Image of the protein strips after treatment. None, no treatment; SDS, stripped with SDS; Gly-HCl, stripped with pH2.3 glycine-HCl; GnHCl, stripped with 6M GnHCl solution. B. Quantitative analysis of protein bands after stripping. Each bar in the diagram represents the mean pixel density (with background subtracted) of six protein bands from three separate experiments. Errors bars: ±1 standard deviation (n=6). *, significant difference between the pixel densities of protein bands on the SDS-stripped membranes compared to the densities of the same Mr bands on the untreated, Gly-HCl or GnHCl-stripped membranes (Student’s t test, P<0.001).
To demonstrate the effectiveness of the GnHCl stripping solution over the GlyHCl solution, a strongly stained immunoblot (Immunoblot 1, Fig. 2A and B) that had been stored for 4 weeks was stripped with the Gly-HCl solution then reprobed with the HRP-secondary antibody, followed by ECL reagent development (Fig. 2C). Following Gly-HCl stripping, the residual intensity at 5 min exposure (Fig. 2C) approximated the intensity of the original immunoblot observed with a 15 second exposure (Fig. 2A). Thus while reasonably efficient, Gly-HCl stripping was incomplete. In contrast, when the same Gly-HCl-stripped immunoblot was stripped with the GnHCl solution and the membrane reprobed with the HRP-secondary antibody, no signal was detected even after 30 min exposure (Fig. 2D), indicating complete removal of the primary antibody. Reprobing of this blot with a 15 sec exposure time yielded band intensities (Fig. 2E) approximating those observed in the 15 sec exposure prior to stripping (Fig. 2A), indicating minimal loss of PVDF-bound protein. In another experiment (Fig. 2F & G), the GnHCl solution was shown to completely remove antibodies from immunoblots without prior stripping with the Gly-HCl solution.
Figure 2.
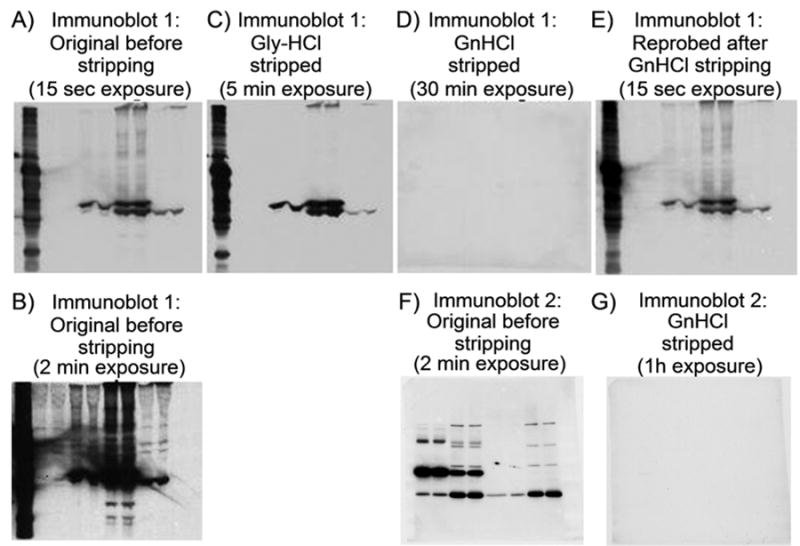
Effectiveness of GnHCl stripping of an aged immunoblot. SDS-PAGE and transfer of protein to PVDF membrane was performed as described in Figure 1. Immunoblotting was performed as described previously (9) with minor modifications. Briefly, the PVDF membrane was blocked by incubation with 5% non-fat dry milk in TBSN at 37°C for 1h. The primary antibody, diluted in the blocking solution, was incubated with the blocked membrane for 2h at room temperature, washed thoroughly with TBSN, and then incubated (1h, room temperature) with a secondary antibody coupled to horseradish peroxidase (HRP) diluted in the same blocking solution. Finally, the thoroughly washed blot was developed with ECL reagent (Amershampharmacia Biotech) and exposed to film (X-Omat, Kodak). Immunoblot 1: (A) 15sec exposure, (B) 2min exposure. After storage for 4 weeks in TBS (containing 0.02% NaN3) the blot was stripped with the Gly-HCl solution and reprobed with the HRP-coupled secondary antibody (C). Then the blot was stripped with the GnHCl solution and reprobed with HRP-coupled secondary antibody (D). Finally, the completely stripped blot (D) was reprobed again with the same primary antibody used in (A) and HRP-secondary antibodies (E). Immunoblot 2: (F) Prior to stripping and (G) after stripping with GnHCl solution (without prior Gly-HCl solution treatment) and reprobing with HRP-coupled secondary antibody.
These experiments demonstrate that the GnHCl solution strips antibody from PVDF immunoblots completely, without significant removal of the transferred proteins and that stripping is effective with a very short incubation. Furthermore, no loss of transferred proteins was observed with the GnHCl solution even on prolonged incubation (more than 30 min), or repeated stripping (Fig. 1B). We have stripped and reused PVDF blots more than 20 times without noticeable loss of protein. Hence the PVDF blot can be re-used almost infinitely with GnHCl stripping, provided the blot is stored wet in TBS or phosphate-buffered saline containing an antimicrobial agent like sodium azide (0.02%). Drying the blot at any time will severely decrease the immuno-reactivity of the protein on the blot. However, GnHCl stripping is not the method of choice for nitrocellulose membrane protein blots. Approximately 50% of the prestained proteins mixture spotted onto nitrocellulose membrane was washed off with the GnHCl solution (unpublished observation).
In this GnHCl stripping solution, high purity of GnHCl is not necessary and the GnHCl we routinely used is 95% pure. The pH of the solution can vary from 7.5 to 8.5. NP-40 can be substituted with triton X-100, or dodecyltrimethyl ammonium bromide and the detergent concentration should be between 0.2 to 0.4%. Antibody will not be completely stripped in old blots with less than 0.1% detergent. Detectable loss of protein begins to occur at detergent concentration higher than 0.5%. Any reducing agent, which cleaves disulfide bonds (e.g., dithiothreitol, tris[2-carboxyethyl] phosphine, etc.), can be used instead of mercaptoethanol. Without mercaptoethanol, the solution is stable at room temperature. The mercaptoethanol should be added immediately prior to use.
Acknowledgments
This work was supported by National Institutes of Health Grants CA26504 (E.R.S.) and the Albert Einstein College of Medicine Cancer Center Grant 5P30-CA13330.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaufmann SH. Reutilization of western blots after chemiluminescent detection or autoradiography. In: Walker JM, editor. The protein protocol handbook. 2. Humana Press Inc.; NJ: 2002. pp. 439–416. [Google Scholar]
- 2.Lioubin MN, Myles GM, Carlberg K, Bowtell D, Rohrschneider LR. Shc, Grb2, Sos1, and a 150-kilodalton tyrosine-phosphorylated protein form complexes with Fms in hematopoietic cells. Mol Cell Biol. 1994;14:5682–5691. doi: 10.1128/mcb.14.9.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Legocki RP, Verma DPS. Multiple immunoreplica technique: screening for specific proteins with a series of different antibodies using one polyacrylamide gel. Anal Biochem. 1981;111:385–392. doi: 10.1016/0003-2697(81)90577-7. [DOI] [PubMed] [Google Scholar]
- 4.Harlow E, Lane D. Using antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press; NY: 1999. [Google Scholar]
- 5.Kaufmann SH, Ewing CM, Shaper JH. The erasable western blot. Anal Biochem. 1987;161:89–95. doi: 10.1016/0003-2697(87)90656-7. [DOI] [PubMed] [Google Scholar]
- 6.Szewyk B, Summer D. Preparative elution of proteins blotted to immobilon membranes. Anal Biochem. 1988;168:48–53. doi: 10.1016/0003-2697(88)90008-5. [DOI] [PubMed] [Google Scholar]
- 7.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 8.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeung YG, Berg KL, Pixley FJ, Angeletti RH, Stanley ER. Protein tyrosine phosphatase-1C is rapidly phosphorylated in tyrosine in macrophages in response to colony stimulating factor-1. J Biol Chem. 1992;267:23447–23450. [PubMed] [Google Scholar]


