Introduction
Now that we are over 10 years into the HAART era in resource-rich countries the major clinical impact of this therapy on reduced AIDS events and AIDS deaths has been well described (1) and in the past few years the focus has turned to describing and understanding what residual clinical disease remains in populations of HIV infected individuals. While death rates may be still falling in HIV-infected patients (2,3), at 0.5–1.0% per year, there is still a considerable excess risk compared with the general population. The breakdown of causes of death of patients has been described based on a study of deaths occurring in 2005 in France (4). AIDS diseases were responsible for only 36% of deaths, with a further 5% due to non-AIDS infections. Other leading causes of death were non-AIDS cancers (16%), liver disease (15%), cardiovascular disease (9%), suicide (5%). In a U.S. study, Lau and colleagues found that risk of death from non-AIDS conditions was greater than that for AIDS conditions for persons with CD4 count above 200 /mm3 (5).
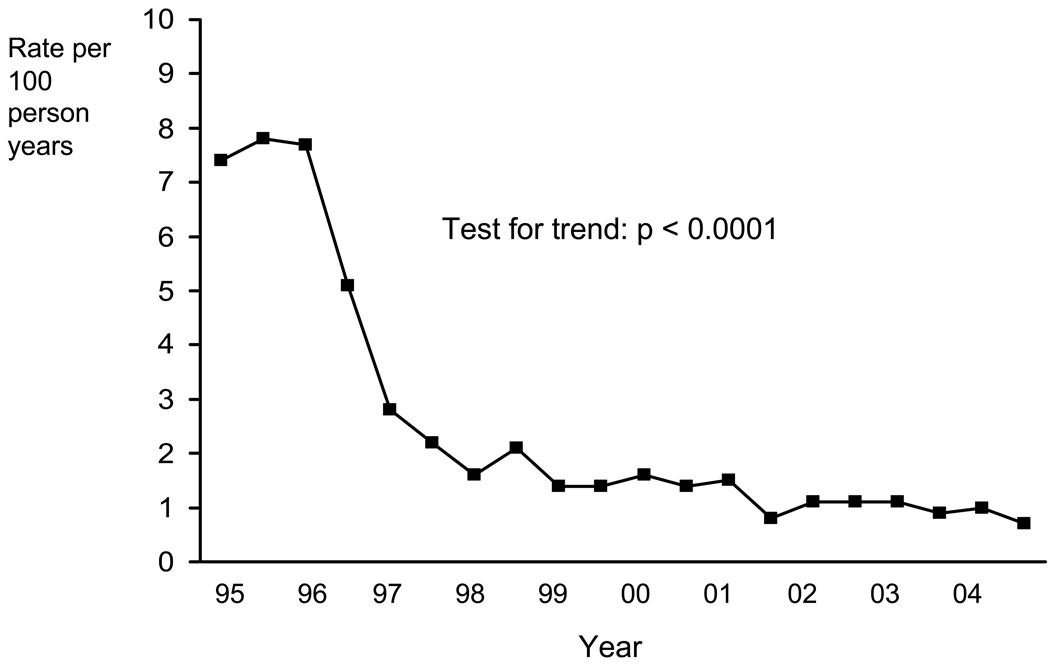
Now with fewer AIDS deaths we have of course observed an increase in the proportion of deaths due to non-AIDS causes, however, this does not mean to say that there has been an increase in the rate of non-AIDS – quite the reverse in fact. Unpublished data from EuroSIDA on trends over calendar time in rates of non-AIDS death (Figure 1), indicate that the start of the HAART era was actually accompanied by a decline in the rate of death from non-AIDS causes (6 and Personal communication), a finding which has been observed in other studies (7). This at first somewhat puzzling observation suggests that perhaps HIV may be playing a role in other diseases well beyond the 26 diseases which characterize AIDS. As we keep people with HIV alive for longer there will be an ever growing number of people with HIV. If HIV is influencing risk of these events, particularly if it does so in the higher CD4 count range where the majority of treated patients hopefully will be, then it is very important to be aware of this and to study it. In this article we briefly consider what rationale (i.e. potential mechanisms) there might be to hypothesize that HIV may play a role in causing diseases such as non-AIDS malignancy, liver cirrhosis, end stage renal disease and serious cardiovascular events (e.g., myocardial infarction, stroke and surgery for coronary artery disease). Collectively, we refer to these as serious non-AIDS events. We then go on to review studies which have data on incidence of serious non-AIDS disease and/or death from these diseases to build up a picture of the current state of knowledge. We finish by suggesting the implications for future research and current clinical care of patients. This review is not intended to consider adverse effects due to antiretroviral drugs (ART), although it is recognised that it is not always easy to separate the effects of HIV from the effects of ART.
Figure 1.
Rate of non-AIDS death by calendar time: EuroSIDA (ref 6 and Mocroft A, Lundgren JD et al, unpublished data).
Potential Mechanisms
There are a range of plausible mechanisms by which HIV might lead to raised risk of non-AIDS morbidity and mortality. As well as causing immunodeficiency, HIV also causes a generalized immune activation and leads to increased T cell turnover (8,9) which is perhaps linked to what has been referred to as microbial translocation, resulting early from depletion of CD4 lymphocytes in lymphoid tissue in the gut (10) which could have consequences for the speed of pathogenic processes leading to a variety of conditions of the cardiovascular and digestive system. Thinking specifically of CVD, whilst HIV infection seems to lead to a decrease in total cholesterol, it also causes decreases in HDL-cholesterol (e.g. 11–13) and results in increases in markers of inflammation (e.g. C-reactive protein), endothelial activation and damage (e.g. intercellular adhesion molecule, ICAM, and vascular cell adhesion molecule, VCAM), and in fact also of coagulation (e.g. D-dimer) - factors which have been linked to increased risk of cardiovascular disease in the general population (11, 14–21). Considering liver disease, there is fairly well established evidence that HIV infection results in faster progression of fibrosis and increased risk of hepatocellular cancer in people with hepatitis B and C virus although the mechanism is not entirely clear (22–25). Whether HIV can affect progression of fibrosis due to an effect on adaptive immunity to HCV, alteration of the dominant HCV subtype or increased hepatocyte apoptosis is not certain. Recent work suggests a possible role of decreased interferon gamma production by HCV-specific CD4 cells in co-infected patients (26).
With regard to renal disease, HIV associated nephropathy (HIVAN), a glomerulopathy demonstrating focal segmental glomerulosclerosis with collapsing features, is a condition well established as being related to HIV (27, 28), and possible mechanisms have been suggested (29–31). However, HIV has been linked with other kidney pathologies, such as immune complex glomerulonephritis (27,28,32). Further, there is a high prevalence of proteinuria in HIV-infected people, and the level of proteinuria is associated with the HIV RNA level and the CD4 count (33,34). HIV RNA and CD4 count also predict raised creatinine levels and both proteinurea & elevated creatinine are associated with all cause mortality in HIV patients, implying that HIV effects of the kidney may lead indirectly to raised risk of death from a wider range of causes other than those ascribed to kidney failure itself (35). Lastly, considering non-AIDS malignancies, the immunodeficiency induced by HIV provides a potential reason for HIV playing a role in some non-AIDS cancers, particularly those known to be caused by an infectious agent; infections may play a wider role in some other cancers than previously appreciated. Further, there has been a suggestion that infections such as Chlamydia pneumoniae is a cause of lung cancer (36), perhaps linked to the resulting inflammation (37), and that a similar mechanism could be linked to gastric cancer associated with H Pylori (38). Direct inflammatory effects of HIV could also raise cancer risk.
Types of evidence linking serious non-AIDS diseases with HIV
In the sections which follow we review data from three types of investigations: 1) studies comparing HIV-infected and uninfected individuals; 2) cohort studies in which the association between CD4 cell count and HIV RNA with serious non-AIDS diseases has been investigated; and 3) randomized trials of HIV-infected individuals that experimentally manipulated CD4 count and HIV RNA by use of ART. For the latter two types of studies some original data that help focus key points are presented.
Comparison of risk of events between HIV-infected and HIV-uninfected people
A limitation of these studies is that the HIV-uninfected comparison group will differ from the HIV-infected people in more ways than just the HIV infection, and the factors that differ are likely risk factors for the disease under study. In most cases, such potential confounding factors (e.g. smoking, blood pressure, lipids for CVD risk) are not measured in the infected and uninfected groups so adjustment cannot be made. Even where adjustments can be made, residual bias remains a strong possibility. A second limitation is that HIV infected subjects in these investigations include those on ART and ART-naïve, so it is not possible in all studies to distinguish the effect of HIV from effect of ART.
A meta-analysis from Grulich et al (39) looked at risk of different cancers in people with HIV (444,172 patients) compared to the general population. To control for confounding factors the authors performed a similar analysis for transplant patients (31,977 patients) - a group who also have immunosuppression, in their case induced by medicine to avoid transplant rejection. The rationale was that if there is raised risk of a cancer in HIV infected people compared with the general population and there is also raised risk of the same cancer in transplant patients compared with the general population then this was less likely to be explained by confounding because the same confounders are unlikely to operate in both groups. A raised risk of cancer was found in both immunosuppressed groups for 20 of the 28 cancers studied. Examples of standardized incidence ratios for various sites in the HIV-infected patients were 2.7 for lung, 3.2 for leukaemia, 1.5 for kidney, 1.6 for oesophagus and 1.9 for stomach. Further studies on transplant patients have shown an increased risk of cancers after kidney transplantation compared with before, strengthening the interpretation that the raised risk results from immunodeficiency and not confounding due to other features of transplant patients (40). In support of this, recent studies have focussed on lung cancer and found that there is an increased risk that is not explained by higher smoking rates in HIV-infected people (41,42). A recent large comparison of rates of non-AIDS malignancy in patients in the Adult and Adolescent Spectrum of Disease and the HIV Outpatient Study cohorts with the United States general population has also revealed increased incidence of many types of cancer (43).
Turning to end stage renal disease, a recent comparison within US Veterans showed that while among whites there was no indication of a raised risk of end stage renal disease for HIV-infected compared with uninfected, among blacks there was over double the risk after adjustment for several factors (44).
A number of studies have compared rates of cardiovascular events (variously defined) between HIV-infected and uninfected groups. Based on an administrative and clinical management database there was an increased risk of hospitalisation for coronary heart disease (CHD) or myocardial infarction (MI) (45). Comparing patients in an HIV cohort with the general population, Mary-Krause et al also identified a raised risk of MI in HIV-infected people but this was not present in those who had used protease inhibitors for < 18 months, suggesting it may have been an effect of drugs rather than HIV (46). In the largest such study, Currier et al made use of an administrative database for the California Medicaid population and found a significantly increased risk of CHD in the HIV-infected population but this was only present in the younger (age 18–33) age group (47). More recently, Triant et al used another patient data registry and estimated a raised risk of MI in the HIV-infected group. In these studies it was not possible to adjust for the full range of cardiovascular risk factors (blood pressure, smoking, lipids) (48).
A number of studies have compared risk of liver disease in patients infected with hepatitis B or C according to whether HIV is also present. The largest considered 4865 men and boys with haemophilia (and probable HCV infection) in the UK, of whom 1218 were HIV-infected. The estimated 25 year cumulative risk of liver death for those with severe haemophilia but not HIV was 1.4% (95% CI 0.7 – 3.0), compared with 1.2% (0.5 – 2.6) for those with moderate / mild haemophilia but not HIV and 6.5% (4.5 – 9.5) in HIV-infected patients (all haemophilia severities) (49). Co-infected patients were similarly found to have a raised risk of liver-related death compared with those infected with HCV alone among injection drug users in the Amsterdam cohort (50). Similarly, in a study of men with hepatitis B in the Multicenter AIDS Cohort Study (MACS), those with HIV infection were found to experience higher risk of liver death than those not HIV infection (24).
Studies of the association between CD4 count and HIV RNA level with risk of serious non-AIDS events
The second main source of evidence concerning the possible link between HIV and increased risk of serious non-AIDS disease or death comes from studies considering the association between CD4 count (and HIV RNA level) and risk of serious non-AIDS events. This involves comparing HIV infected people when they are in different states of immunodefiency so there is not such a clear problem with confounding than when comparing with HIV-uninfected groups, although as in any comparison not protected by randomization the possibility of residual bias is always present.
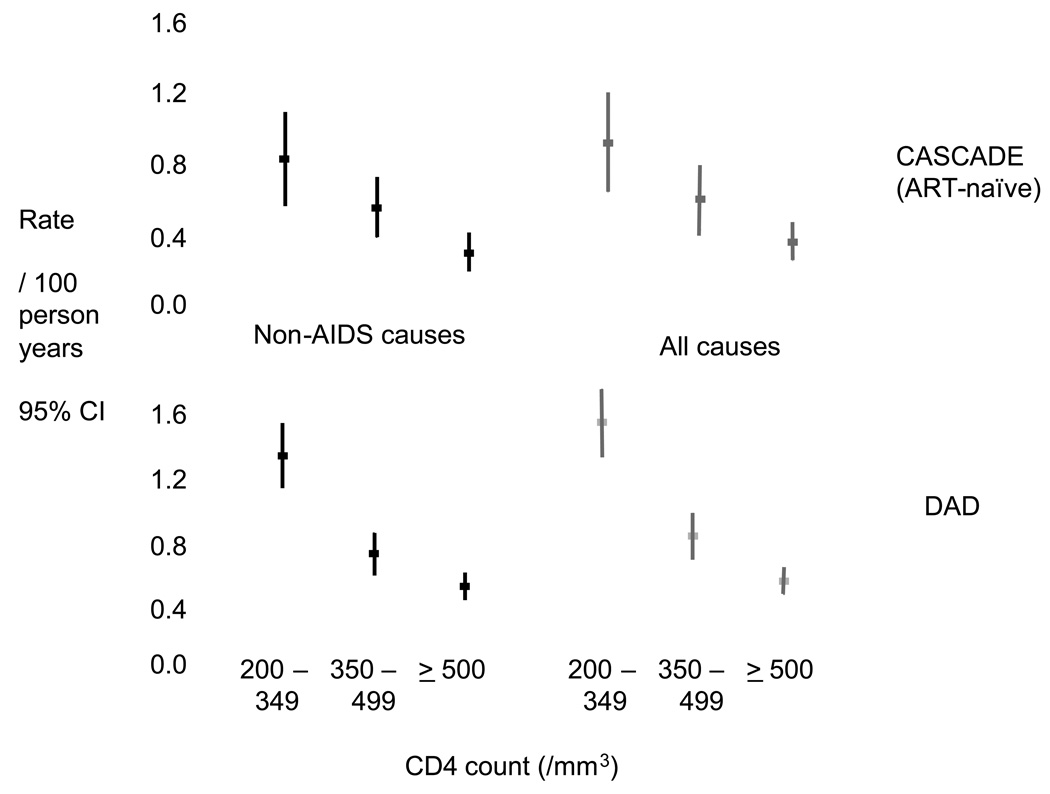
Figure 2 shows data from the DAD study of around 33,000 people with HIV followed in 10 cohorts and data from the large CASCADE collaboration of seroconverter cohorts, restricted to ART-naïve patients (51). On the left is the rate of death from causes other than AIDS, according to the most recent CD4 count at the time. There is a strong tendency for the rate of death from non-AIDS causes to be higher at lower CD4 count. Importantly, this trend seems to continue all the way through the CD4 count range, so it is not just the case that those with low CD4 count have raised risk of death from causes other than AIDS, those with counts of 350–500 even appear to be at increased risk compared with those with counts above 500. These trends are essentially unaffected by adjustment for other factors. On the right are the corresponding rates for all-cause mortality. At higher CD4 counts the rate of non-AIDS death is almost as high as those for all cause death, emphasising that in people in this higher CD4 count range, serious morbidity and mortality is dominated by non-AIDS conditions. These trends are highly consistent across the two studies. This trend in risk of non-AIDS death with CD4 count was also found in New York cases with a previous AIDS diagnosis, based on minimum CD4 count (52). Also, in collaborative analysis the rate of death in ART-naïve patients referred to above, Lodwick et al confirmed this trend for mortality (53).
Figure 2.
Rate of non-AIDS and all cause death according to most recent CD4 count in D:A:D and CASCADE (derived from refs 50, 56 and unpublished data).
The association between the CD4 count and incidence of serious but non-fatal non-AIDS events has also been considered in some studies, including the Strategies for Management of Antiretroviral Therapy (SMART) trial (54). The primary aim of SMART was to compare incidence of opportunistic disease (similar, but not identical, definition to AIDS) and death between use of either continuous ART or intermittent ART (Stop or defer ART when CD4 count > 350, restart or start ART when CD4 count < 250) (SMART) among 5472 patients with a CD4 count > 350 at enrolment. The intermittent arm was stopped in Jan 2006, and the protocol modified so that ART experienced patients recommended to restart ART, when there was found to be a raised rate of this primary endpoint but patient follow-up continued for another 18 months. Throughout the entire trial the incidence of serious non-AIDS diseases (non-AIDS cancer, CVD events, liver cirrhosis, renal failure, non-AIDS death) was assessed, as it was hypothesized that less use of ART would reduce the risk of contracting these diseases which were viewed as “complications” to ART. For serious non-AIDS events as a whole there were 346 events, compared with 164 AIDS events, emphasising the fact that risk of these types of events is higher than risk of AIDS events for patients in a relatively high CD4 count range (55). Considering both arms together, there was an overall hazard ratio for risk of 0.94 per 100 cell higher CD4 count (95% CI 0.90 – 0.98) after adjustment for age, gender, prior AIDS, hepatitis B or C infection and smoking status (SMART, unpublished data). Further, data from the Flexible Initial Retrovirus Suppressive Therapies (FIRST) trial that compared virological, immunological and clinical outcomes for patients randomly allocated to three different antiretroviral treatment strategies for initial ART were used to assess the association between latest CD4 count and risk of serious non-AIDS diseases (with a similar definition to SMART); the hazard ratio was 0.82 (0.72 – 0.93) after adjustment for age, gender, race, prior AIDS and hepatitis B or C infection (56).
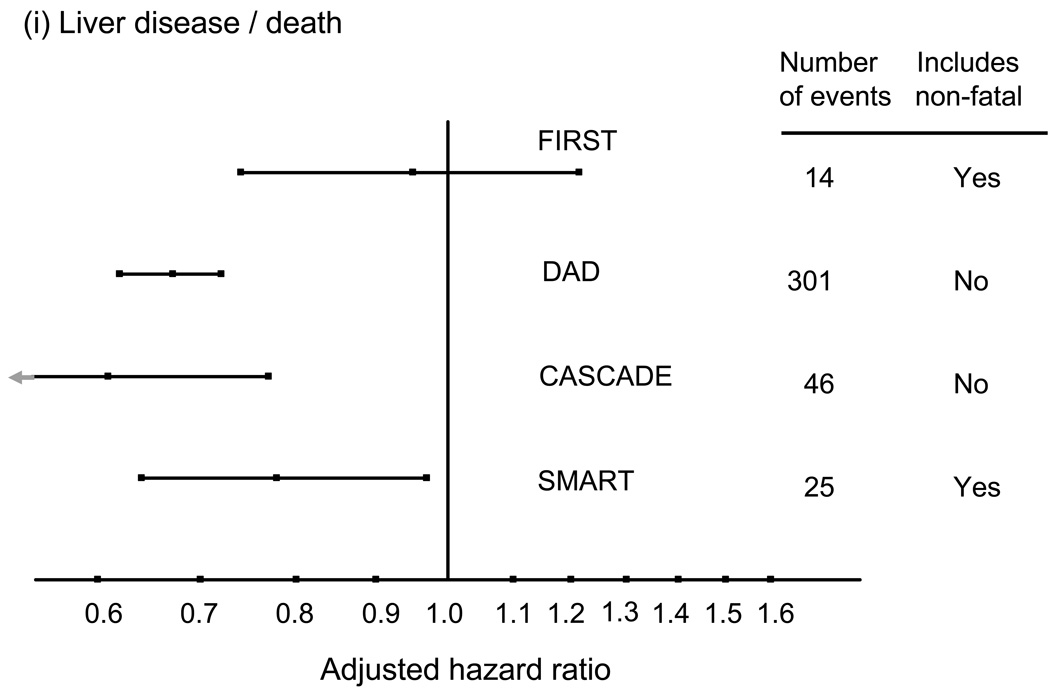
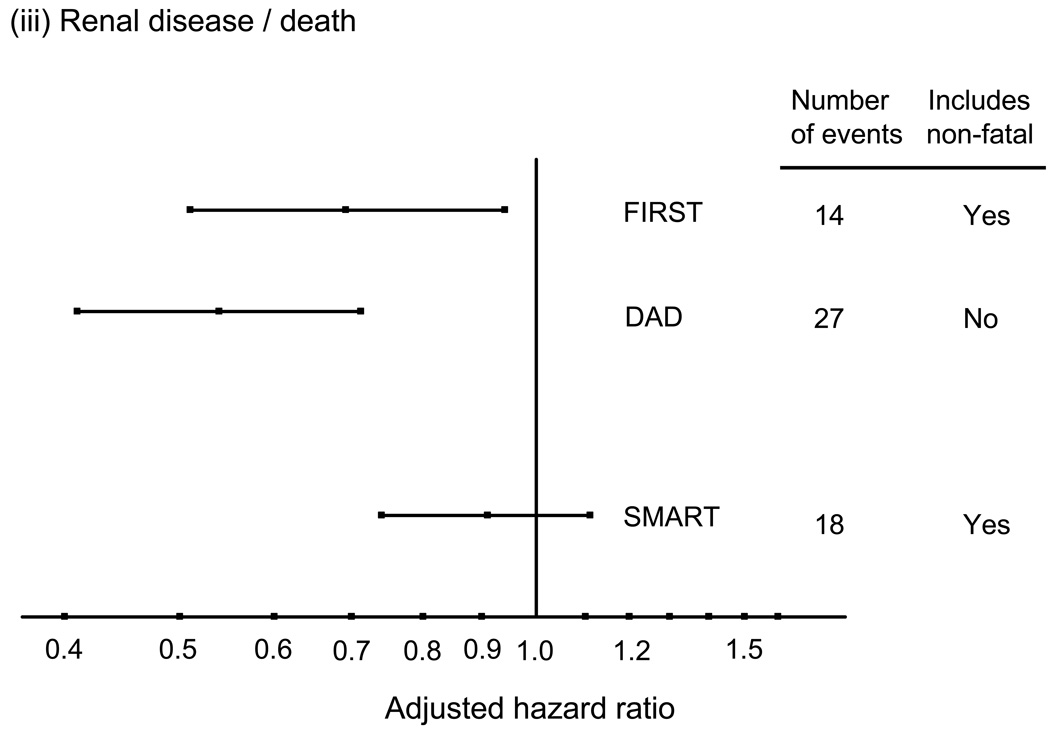
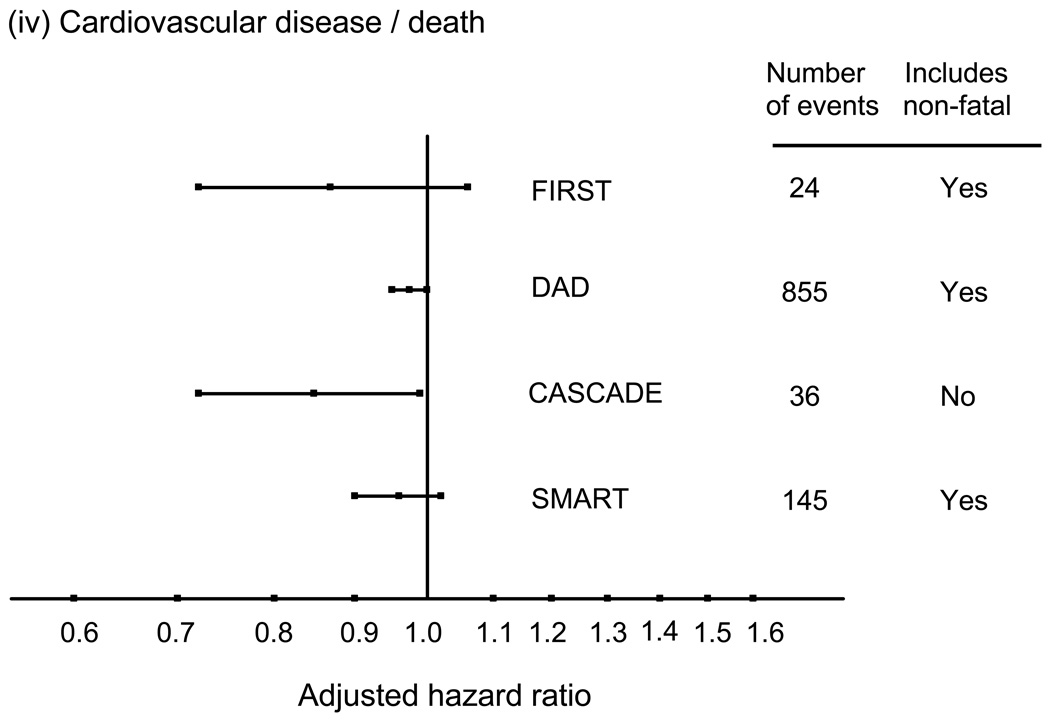
These associations between CD4 count and risk of non-AIDS diseases seem consistent across different types of events (57,58). Evidence of an association with CD4 count has been reported for liver death (51,59), non-AIDS malignancy (60), and kidney disease (34,61,62). The one exception is perhaps CVD where there is has not been strong evidence for a link with the CD4 count when considering risk of myocardial infarction that includes non-fatal as well as fatal cases (63), and in one study considering CVD mortality a suggestion of a stronger association with HIV RNA level than with CD4 count (57). Figure 3 shows adjusted relative hazards for the effect of CD4 cell count on risk of these four conditions for four studies, SMART, DAD, CASCADE and FIRST, generally illustrating the consistency of these effects. For cardiovascular disease, there is a suggestion of a stronger effect when considering fatal CVD.
Figure 3.
Adjusted hazard ratios (per 100 cell/mm3 higher CD4 count; with 95% confidence intervals) for the association between latest CD4 count and risk of various serious non-AIDS diseases, or deaths from these diseases. (derived from refs 50, 55, 56 and unpublished data, also see 79).
Two further recent studies looking at the association between the CD4 count and non-fatal as well as fatal non-AIDS have come from France. The Aquitaine cohort looked at all hospitalisations in HIV-infected people in that study between 2000 and 2004 (64). There was the same trend in CD4 count with the percent hospitalised present for non-AIDS cancers and digestive events, again not much so for CVD events. Further, the distribution of the burden of morbidity according to cause was found to be heavily weighted towards non-AIDS-related events, with less than 1% of hospitalizations in those with CD4 count above 500 were ascribed to AIDS. This association with latest CD4 count was also found in APROCO COPILOTE (65).
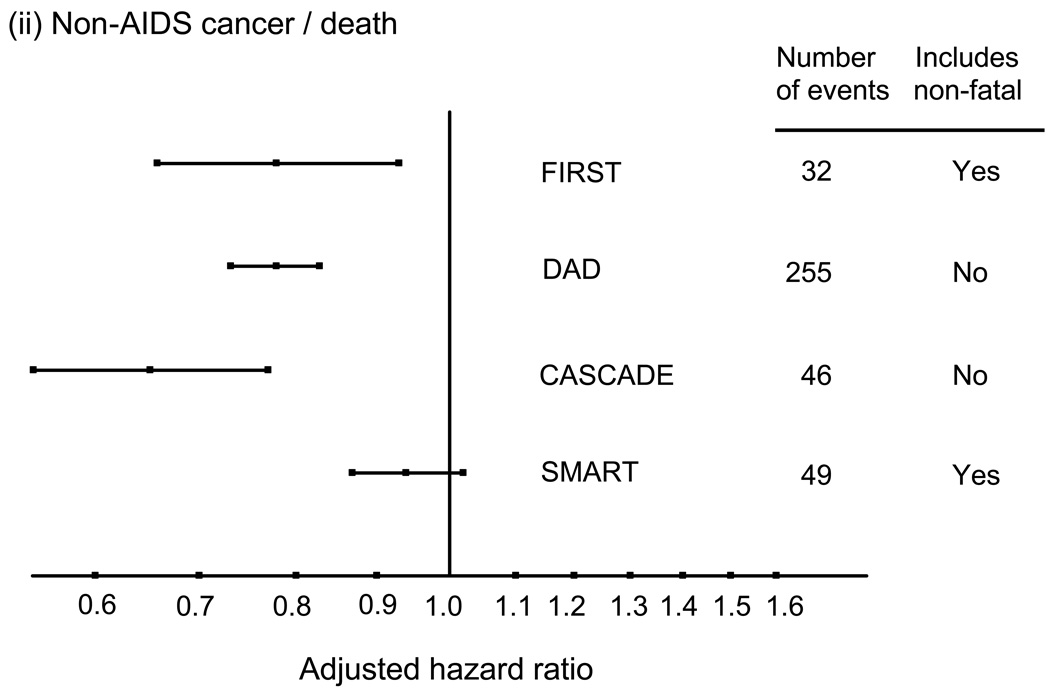
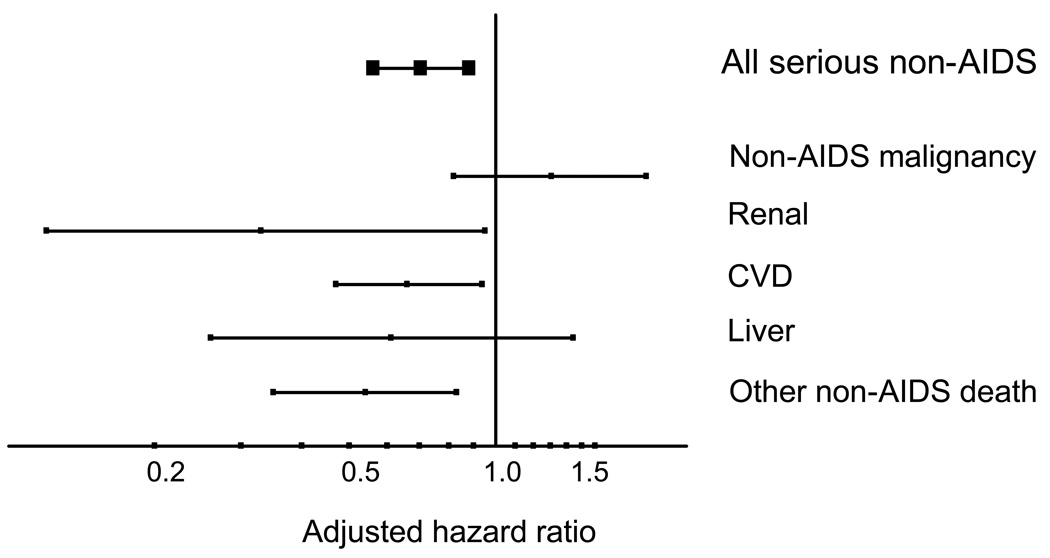
It is also worth considering whether there is an effect of latest HIV RNA as well as of latest CD4 count. If we are thinking about whether ART can have a benefit in terms of risk of these diseases then the reduction in viral load induced by ART could be an additional benefit on top of that gained from the resulting rise in CD4 count. In most studies HIV RNA is high either in those who are ART naïve, ART-experienced but off ART, or virologically failing so there is confounding between HIV RNA level and the factors that lead people to be in these situations. This complicates interpretation of the association. In the SMART trial however - this was not the case because patients were randomly allocated to be on ART or not. The adjusted (including for the most recent CD4 count) hazard ratio for serious non-AIDS events according to the most recent HIV RNA level – above or below 400 copies/mL – was 0.70 (95% 0.56 – 0.88) (Figure 4). Considering separate conditions, this was strongest for renal, CVD and deaths due to other causes, but there is no such trend when looking at non-AIDS cancer. When additionally adjusted for latest HIV RNA level the hazard ratio for the latest CD4 count became 0.96 (0.92 – 1.01). Of note, impairment of immune function exists for both HIV and the above mentioned transplant patients, whereas HIV-RNA obviously only affects HIV patients; hence, the CD4 gradient data does suggest that immunodeficiency per se increases risk of these diseases, but that HIV-RNA additionally may also contribute.
Figure 4.
HIV RNA and risk of serious non-AIDS events: SMART. Adjusted hazard ratio for latest value < 400 vs. > 400 copies/mL. Adjusted for age, gender, prior AIDS, hep B/C, smoking, latest CD4 count. (SMART unpublished data)
Randomized trials of HIV-infected individuals that experimentally manipulated CD4 count and HIV RNA by use of ART
There are limited data from randomized trials of interventions that induce changes in CD4 count and HIV RNA levels and examine subsequent risk of non AIDS events. The best type of study to tell us whether risk of non-AIDS diseases is related to HIV is one in which patients with HIV are randomized to either take ART, with the aim of having their HIV replication suppressed, or to not take ART. If HIV itself influences the risk of diseases other than AIDS we would expect to see more such diseases in those not on ART. Such a trial has to be large in order to observe incidence of serious clinical events. We do not have any large trials in which naïve patients are randomized to take ART or not but we do have one in which patients - whether on or off ART - were randomized to take ART or not. This was the SMART trial, which was mentioned above when considering the association with CD4 count and HIV RNA, but not in terms of the main randomized comparison (54,55).
At baseline in SMART, 84% of patients were on ART and 16% were not. The randomization to continuous or intermittent ART means that initially one group of patients was all to be off ART and the other group all to be on ART. At the time that the intermittent arm was stopped (January 2006) the proportion of time spent on ART in the two arms was 94% for the continuous arm and 33% for the intermittent arm. This was a trial generally in people with relatively high CD4 count - the proportion of person time In which the CD4 count was above 350 was 93% in the continuous arm and 68% in the intermittent arm.
Rates of serious non-AIDS defining events were also looked at (13,54,55,66) – originally because it was thought that there might well be a raised risk of some such conditions in those on continuous ART – because they were thought to be negatively affected as a side effect of antiretroviral therapy. Table 1 shows how many of these events: major cardiovascular events, kidney failure, liver cirrhosis, non-AIDS malignancy and non-AIDS deaths there were in the two trial arms (55,66 and unpublished data). These are given separately before the study protocol modification in January 2006 and after (when ART-experienced patients in the DC arm were recommended to re-commence ART). Pre-modification is the period during which there is by far the greatest difference in ART use between the arms. For serious non-AIDS as a whole, the hazard ratio was 1.6, with a highly significant p-value. It is important to remember that most patients in the trial had CD4 counts above 250 for the whole time, in fact over 95% of the patient time in the two arms pre-modification was spent with CD4 count > 250. So this difference in risk of serious events has occurred amongst a group of patients with what we would think of as fairly adequate CD4 counts. Further emphasising the importance of non-AIDS diseases in the CD4 count range only 8% of deaths in SMART were from AIDS diseases. The trends are generally consistent across the major end organ pathologies. After study modification when there was much greater use of ART in the DC arm the effects are markedly reduced.
Table 1.
Serious AIDS and Serious Non-AIDS Outcomes in SMART according to randomization to episodic ART (Drug Conservation, DC) arm or continuous ART (Viral suppression, VS) arm in the SMART study, before and after study modification on January 11, 2006.
| Number of events |
Rate per 100 person yrs | Hazard Ratio (95% CI) | P-value | ||
|---|---|---|---|---|---|
| DC | VS | ||||
| Pre-modification | |||||
| All Serious Non-AIDS Events (fatal or non-fatal) | 186 | 3.2 | 2.0 | 1.6 (1.2, 2.1) | 0.003 |
| CVD+ | 79 | 1.3 | 0.8 | 1.6 (1.0, 2.5) | 0.05 |
| Hepatic++ | 17 | 0.3 | 0.2 | 1.4 (0.6, 3.8) | 0.46 |
| Renal+++ | 11 | 0.2 | 0.1 | 4.5 (1.0, 20.9) | 0.05 |
| NADM++++ | 51 | 0.7 | 0.6 | 1.1 (0.7, 2.0) | 0.67 |
| Other Non-AIDS Death | 45 | 0.8 | 0.4 | 1.8 (1.0, 3.4) | 0.05 |
| Post-modification | |||||
| All Serious Non-AIDS Events (fatal or non-fatal) | 161 | 2.2 | 2.1 | 1.1 (0.8, 1.5) | 0.67 |
| CVD+ | 67 | 0.9 | 0.8 | 1.1 (0.7, 1.8) | 0.64 |
| Hepatic++ | 8 | 0.2 | 0.0 | 7.2 (0.9, 58.2) | 0.07 |
| Renal+++ | 7 | 0.0 | 0.2 | 0.2 (0.0, 1.4) | 0.10 |
| NADM++++ | 50 | 0.6 | 0.7 | 0.7 (0.4, 1.3) | 0.29 |
| Other Non-AIDS Death | 45 | 0.7 | 0.5 | 1.4 (0.8, 2.5) | 0.27 |
Fatal or non-fatal myocardial infarction (silent or clinical), stroke, procedure or surgery for coronary artery disease.
Fatal or non-fatal cirrhosis
Fatal or non-fatal end stage renal disease
Fatal or non-fatal non-AIDS-defining malignancies (NADM) except non-melanoma skin
A proportion of the patients in SMART were ART naïve at baseline. When the analysis is restricted to these patients, plus ART experienced patients for whom there was evidence of having been off ART for more than 6 months (477 patients in total), the comparison effectively becomes one of whether to start ART or to defer it (67). When this is done the difference in risk of serious non-AIDS for this subgroup is even more striking (HR 7.02 (95% CI 1.57 – 31.4) p=0.01), albeit that the numbers are very small. Unfortunately, SMART represents the only randomized study of ART vs no ART currently available to study the question of whether HIV increases the risk of serious diseases other than AIDS.
As a follow-up to these findings from SMART, a recent presentation looked at the possible role of several biomarkers in the excess risk of mortality and CVD observed in the intermittent arm of SMART. Two studies using stored plasma were undertaken – one to see the effect of the randomization arm on marker levels and the other to see the effect of marker levels on risk of death and major CVD events (21). Results for interleukin-6, a cytokine linked to the acute phase response, and D-dimer, a coagulation marker known to predict CVD and all-cause mortality in the general population (68), were particularly interesting. There was some evidence that the effect of the interruption strategy on risk of death and, to a lesser extent risk of CVD, may be partially mediated by these markers.
Implications
To sum up, in the HAART era, morbidity and mortality in all but those in the lowest CD4 count categories are dominated by non-AIDS rather AIDS events. As the HIV-infected population ages, these conditions will assume even more significance because older age is a risk factor for these conditions. Data from studies looking at incidence of serious non-AIDS diseases and death provide appreciable evidence for HIV playing a detrimental role in some of these serious non-AIDS diseases. However, the picture is not entirely consistent and the question requires further study in cohorts and, better if possible, large randomized trials – future studies should include these events as endpoints, along with AIDS. Studies storing plasma and other samples are particularly useful for developing nested case-control studies for exploring biomarkers that might mediate the raised risk. Work is needed to standardize data collection methods, to standardize diagnostic criteria, and to develop electronic methods for collecting information from multiple sources. A standardized means of coding causes of deaths exists (CoDe, 69), but the same would be useful for non-fatal conditions (case definitions to be used in the new START trial are now available on www.insight-trials.org and www.cphiv.dk). Furthermore, studies should attempt to collect data on risk factors for these serious diseases. This will mean that it will be possible to better evaluate whether standard approaches to predicting these serious non-AIDS events on the basis of these risk factors also hold in the HIV-infected population.
Importantly, given the evidence presented above it is plausible that use of ART - with its ability to reduce HIV RNA levels and increase CD4 counts - may reduce risk of some serious non-AIDS events, as well reducing risk of AIDS events. This provides a new rationale to re-raise the question of whether we need to be considering starting ART earlier in ART-naive patients (54,70). Currently, most guidelines do not encourage ART initiation until the CD4 count is below 350 /mm3 (71–73) but the possible impact of HIV on risk of serious non-AIDS diseases in people in the high CD4 count range, together with the reduction in concern over long term risk of resistance (74,75) and steady improvements in virologic suppression (76–78), suggests that we need to evaluate whether to start ART in people with much higher CD4 counts (e.g. > 500 /mm3). In response to the data reviewed above and other considerations a trial (called START) has been designed in patients with CD4 count > 500 /mm3 in order to compare the effects of immediate initiation of ART with deferral until the CD4 count has reached 350 /mm3. Analyses of observational data indicate that the median time for a person with CD4 count > 500 /mm3 to reach a CD4 count < 350/mm3 is around 2.5 years (79), with this depending heavily on viral load. The trial will begin recruitment shortly in sites across all continents. As well as telling us whether to start ART earlier it will provide a rich source of nested biomarker studies to understand the role of HIV (and the various ART drugs) in the various serious non-AIDS diseases.
The evidence we have presented also suggests that clinicians caring for patients with HIV need to be sensitive to the possible raised risk of a variety of conditions and to become aware of the best means to try to prevent and to monitor for early signs of these outcomes, in much the same way as clinicians of other specialties need to be aware of the signs that may indicate presence of HIV infection in those undiagnosed.
Conclusion
There is a growing body of evidence to suggest that HIV impacts the risk of a far wider range of conditions than AIDS diseases alone. Since risk of these serious conditions is much higher than risk of AIDS diseases within the higher CD4 count range - the range within which increasingly large numbers of people will be - this has serious consequences for our management of patients over the coming decades. We need to adapt our research priorities to better understand the full role of HIV in causing a wide range of clinical diseases. Incidence of these diseases should be included as endpoints in epidemiological studies and trials using clinical endpoints. Partially as a result of these new data we have reviewed and presented the possibility that ART should be initiated much earlier than is currently generally the case is to be investigated in a randomized trial.
Acknowledgements
We would like to acknowledge the investigators of the following studies for allowing presentation of some unpublished data: DAD study (consisting of Aquitaine, Nice, CPCRA, EuroSIDA, ICONA,SHCS, Brussels, BASS, AHOD, ATHENA, HivBivus cohorts), EuroSIDA, SMART (INSIGHT - International Network for Strategic Initiatives in Global HIV Trials), FIRST, CASCADE. We are grateful to Jacquie Neuhaus (SMART), Grace Peng, Jason Baker (FIRST) (supported in part by NIH U01 AI068641), Benoit Marin, Genevieve Chene, Abdel Babiker (CASCADE), Colette Smith, Caroline Sabin (DAD), Amanda Mocroft (EuroSIDA) for help with producing these extra analyses. AP drafted the manuscript which was based on a presentation at 15th Conference on Retroviruses and Opportunistic Infections (CROI 2008), February 3–6, 2008 in Boston, MA, USA, a presentation which resulted to a large extent from discussions of results from the SMART trial between AP, JDL and JN along with the entire INSIGHT Executive Committee. AP drafted the manuscript, JDL and JN provided additional input and critical revisions.
Footnotes
Based on a talk prepared for the 15th Conference on Retroviruses and Opportunistic Infections (CROI 2008), February 3–6, 2008 in Boston, MA, USA.
REFERENCES
- 1.Mocroft A, Vella S, Benfield TL, Chiesi A, Miller V, Gargalianos P, d'Arminio Monforte A, Yust I, Bruun JN, Phillips AN, Lundgren JD. Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet. 1998 Nov 28;352(9142):1725–1730. doi: 10.1016/s0140-6736(98)03201-2. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 3.Crum NF, Riffenburgh RH, Wegner S, et al. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr. 2006;41:194–200. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- 4.Salmon D, Lewden C, Bonnet F, et al. Causes of liver related death in HIV-infected patients in France: mortality 2005 Survey. Int J Antimicrob Agents. 2007;29:S151–S152. [Google Scholar]
- 5.Lau B, Gange S, Moore RD. Risk of non-AIDS related mortality may exceed risk of AIDS-related mortality among individuals enrolling into care with CD4+ counts greater than 200 cells/mm3. J Acquir Immune Defic Syndr. 2006 doi: 10.1097/01.qai.0000247229.68246.c5. (epublication) [DOI] [PubMed] [Google Scholar]
- 6.Mocroft A, Brettle R, Kirk O, et al. Changes in the cause of death among HIV positive subjects across Europe: results from the EuroSIDA study. AIDS. 2002;16:1663–1671. doi: 10.1097/00002030-200208160-00012. [DOI] [PubMed] [Google Scholar]
- 7.Grabar S, Lanoy E, Allavena C, Mary_krause M, Bentata M, Fischer P, et al. Causes of the first AIDS-defining illness and subsequent survival before and after the advent of combined antiretroviral therapy. HIV Medicine. 2008;9:246–256. doi: 10.1111/j.1468-1293.2008.00554.x. [DOI] [PubMed] [Google Scholar]
- 8.Liu ZY, Cumberland WG, Hultin LE, et al. Elevated CD38 antigen expression on CD8(+) T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the multicenter AIDS cohort study than CD4(+) cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. JAIDS. 1997;16:83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 9.Douek DC, Betts MR, Hill BJ, Little SJ, Lempicki R, Metcalf RA, et al. Evidence for increased T cell turnover and decreased thymic output in HIV infection. J. Immunol. 2001;167:6663–6668. doi: 10.4049/jimmunol.167.11.6663. [DOI] [PubMed] [Google Scholar]
- 10.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 11.Riddler SA, Smit E, Cole SR, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289:2978–2982. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 12.Rose H, Woolley I, Hoy J, et al. HIV infection and high-density lipoprotein: the effect of the disease versus the effect of treatment. Metabolism Clinical and Experimental. 2006;55:90–95. doi: 10.1016/j.metabol.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Phillips AN, Carr A, Neuhaus J, Visnegarwala F, Prineas R, Burman WJ, et al. Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV-1 infection: exploratory analyses from the SMART trial. Antiviral Therapy. 2008;13:177–187. doi: 10.1177/135965350801300215. [DOI] [PubMed] [Google Scholar]
- 14.De Larrangas GF, Petroni A, Deluchi G, Alonso BS, Benetucci JA. Viral load and disease progression as responsible for endothelial activation and/or injury in human immunodeficiency virus-1-infected patients. Blood Caogul Fibrinolysis. 2003;14:15–18. doi: 10.1097/00001721-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Solages A, Vita JA, Thornton DJ, et al. Endothelial function in HIV-infected persons. Clin Infect Dis. 2006;42:1325–1332. doi: 10.1086/503261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Gaetano, Donati K, Rabagliati R, Iacoviello L, Cauda R. HIV infection, HAART, and endothelial adhesion molecules: current perspectives. Lancet Infect Dis. 2004;4:213–222. doi: 10.1016/S1473-3099(04)00971-5. [DOI] [PubMed] [Google Scholar]
- 17.Birdsall HH, Siwak EB, Trial J, et al. Transendothelial migration of leukocytes carrying infectious HIV-1: an indicator of adverse prognosis. AIDS. 2002;16:5–12. doi: 10.1097/00002030-200201040-00002. [DOI] [PubMed] [Google Scholar]
- 18.Blum A, Hadas V, Burke M, Yust I, Kessler A. Viral load of human immunodeficiency virus could be an independent risk factor for endothelial dysfunction. Clin Cardiol. 2005;28:149–153. doi: 10.1002/clc.4960280311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lau B, Sharrett R, Kingsley LA, et al. C-reactive protein is a marker for human immunodeficiency virus disease progression. Arch Intern Med. 2006;166:64–70. doi: 10.1001/archinte.166.1.64. [DOI] [PubMed] [Google Scholar]
- 20.Wolf K, Tsakiris DA, Weber R, et al. Antiretroviral therapy reduces markers of endothelial and coagulation activation in patients with human immunodeficiency virus type 1. JID. 2002;185:456–462. doi: 10.1086/338572. [DOI] [PubMed] [Google Scholar]
- 21.Kuller L the SMART Study Group. Cardiovascular risk, mortality and TB complicating HIV infections. 15th Conference on Retroviruses and Opportunistic Infections; Feb 3–6 2008; Boston, USA. Abstract 139. [Google Scholar]
- 22.Eyster ME, Diamondstone LS, Lien JM, et al. Natural-history of hepatitis-C virus infection in multitransfused hemophiliacs – effect of co-infection with HIV. JAIDS. 1993;6:602–610. [PubMed] [Google Scholar]
- 23.Tan YJ, Lim SG, Hong WJ. Understanding human immunodeficiency virus type 1 and hepatitis C virus coinfection. Cur HIV Res. 2006:21–30. doi: 10.2174/157016206775197600. [DOI] [PubMed] [Google Scholar]
- 24.Thio CL, Seaberg EC, Skolasky R, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360:1921–1926. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 25.Soto B, SanchezQuijano A, Rodrigo L, et al. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol. 1997;26:1–5. doi: 10.1016/s0168-8278(97)80001-3. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez SA, Zhang C, Fiel MI, Chung S, Zhang L, Jacobson IM, Talal AH. Hepatic inflammatory cytokine mRNA expression in hepatitis C virus–human immunodeficiency virus co-infection. J Viral Hepatitis. 2008;15:331–338. doi: 10.1111/j.1365-2893.2007.00949.x. [DOI] [PubMed] [Google Scholar]
- 27.Szczech LA, Gupta SK, Habash R, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int’l. 2004;66:1145–1152. doi: 10.1111/j.1523-1755.2004.00865.x. [DOI] [PubMed] [Google Scholar]
- 28.Kimmel PL, Barisoni L, Koop JB. Pathogenesis and treatment of HIV-associated renal disease: lesson from clinical and animal studies, molecular pathologic correlations, and genetic investigations. Annal Intern Med. 2003;139:214–226. [PubMed] [Google Scholar]
- 29.Doublier S, Zennarob C, Spatola T, Lupiad E, Bottelli A, Deregibusa MC, Carrarob M, Conaldie PG, Camussia G. HIV-1 Tat reduces nephrin in human podocytes: a potential mechanism for enhanced glomerular permeability in HIV-associated nephropathy. AIDS. 2007;21:423–432. doi: 10.1097/QAD.0b013e328012c522. [DOI] [PubMed] [Google Scholar]
- 30.Bruggeman LA, Dikman S, Meng C, Quaggin SE, Coffman TM, Klotman PE. Nephropathy in human immunodeficiency virus-1 transgenic mice is due to renal transgene expression. J Clin Invest. 1997;100:84–92. doi: 10.1172/JCI119525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto T, Noble NA, Miller DE, Gold LI, Hishida A, Nagase M, et al. Increased levels of transforming growth factor-beta in HIV-associated nephropathy. Kidney Int. 1999;55:579–592. doi: 10.1046/j.1523-1755.1999.00296.x. [DOI] [PubMed] [Google Scholar]
- 32.Jones R, Scott C, Nelson M, Levy J. Renal complications in HIV. Int J Clin Practice. 2007;61:991–998. doi: 10.1111/j.1742-1241.2007.01376.x. [DOI] [PubMed] [Google Scholar]
- 33.Gupta S, Franceschini N, Szczech LA, Smurzynski M, Kalayjian R. The effects of HIV-1 viral suppression and non-viral factors on clinically significant proteinurea in the HAART era. 15th Conference on Retroviruses and Opportunistic Infections; Feb 3–6 2008; Boston, USA. Abstract 974. [Google Scholar]
- 34.Szczech LA, Gange S, van der Horst C, et al. Predictors of proteinuria and renal failure among women with HIV infection. Kidney International. 2002;61:195–202. doi: 10.1046/j.1523-1755.2002.00094.x. [DOI] [PubMed] [Google Scholar]
- 35.Lucas GM, Eustace JA, Sozio S, et al. Highly active antiretroviral therapy and the incidence of HIV-1-associated nephropathy: a 12-year cohort study. AIDS. 2004;18:541–546. doi: 10.1097/00002030-200402200-00022. [DOI] [PubMed] [Google Scholar]
- 36.Littman AJ, Jackson LA, Vaughan TL. Chlamydia pneumoniae and Lung Cancer: Epidemiologic Evidence. 2005;14:773–778. doi: 10.1158/1055-9965.EPI-04-0599. [DOI] [PubMed] [Google Scholar]
- 37.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Omar, Rabkin CR, Gammon MD, Vaughan T, Risch HA, Schoenberg JB, et al. Increased Risk of Noncardia Gastric Cancer Associated With Proinflammatory Cytokine Gene Polymorphisms. Gasttroenterology. 2003;124:1193–1201. doi: 10.1016/s0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 39.Grulich AE, van Leeuwen MT, Falster MO, Vajdic C. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 40.Vajdic CM, McDonald SP, McCredie MRE, van Leeuwen MT, Stewart JH, Law M, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823–2831. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 41.Kirk GD, Merlo C, O’Driscoll P, et al. HIV infection is associated with an increased risk of lung cancer, independent of smoking. CID. 2007;45:103–110. doi: 10.1086/518606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaturvedi A, Pfeiffer RM, Chang L, et al. Elevated risk of lung cancer among people with HIV. AIDS. 2007;21:207–213. doi: 10.1097/QAD.0b013e3280118fca. [DOI] [PubMed] [Google Scholar]
- 43.Patel P, Hanson DL, Sullivan PS, Novak RM, Moorman AC, Tong TC, et al. for the Adult and Adolescent Spectrum of Disease Project and HIV Outpatient Study Investigators. Incidence of Types of Cancer among HIV-Infected Persons Compared with the General Population in the United States, 1992–2003. Ann Intern Med. 2008;148:728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 44.Choi AI, Rodriguez RA, Bacchetti P, et al. Racial differences in end-stage renal disease rates in HIV infection versus diabetes. J Am Soc Nephrol. 2007;18:2968–2974. doi: 10.1681/ASN.2007040402. [DOI] [PubMed] [Google Scholar]
- 45.Klein D, Hurley LB, Quesenberry CP, Jr, Sidney S. Do Protease Inhibitors Increase the Risk for Coronary Heart Disease in Patients With HIV-1 Infection? JAIDS. 2002;30:471–477. doi: 10.1097/00126334-200208150-00002. [DOI] [PubMed] [Google Scholar]
- 46.Mary-Krause M, Cotte L, Simon A, Partisani M, Costagliola D. Increased risk of myocardial infarction with duration of protease inhibitor therapy in HIV-infected men. AIDS. 2003;17:2479–2486. doi: 10.1097/00002030-200311210-00010. [DOI] [PubMed] [Google Scholar]
- 47.Currier JS, Taylor A, Boyd F, et al. Coronary heart disease in HIV-infected individuals. JAIDS. 2003;33:506–512. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- 48.Triant VS, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with HIV disease. J Clin Endocrin Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Darby SC, Ewart DW, Giangrande PLF, Spooner RJD, Rizza CR, Dusheiko GM, et al. Mortality from liver cancer and liver disease in haemophilic men and boys in UK given blood products contaminated with hepatitis C. Lancet. 1997;350:1425–1431. doi: 10.1016/s0140-6736(97)05413-5. [DOI] [PubMed] [Google Scholar]
- 50.Smit C, van den Berg C, Geskus R, Berkhout B, Coutinho R, Prins M. Risk of hepatitis-related mortality increased among hepatitis C virus/HIV-coinfected drug users compared with drug users infected only with hepatitis C virus - A 20-year prospective study. JAIDS. 2008;47:221–225. doi: 10.1097/QAI.0b013e31815d2f59. [DOI] [PubMed] [Google Scholar]
- 51.The DAD Study Group. Liver-related deaths in persons infected with the human immunodeficiency virus - The D:A:D study. Arch Intern Med. 2006;166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 52.Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145:397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 53.Lodwick R, Porter K, Sabin CA, Ledergerber B, Cozzi Lepri A, Khaykin P, et al. Age-and sex- specific death rates in ART-naïve patients with CD4 count above 350 /mm3 compared with the general population. 15th Conference on Retroviruses and Opportunistic Infections; Feb 3–6 2008; Boston, USA. Abstract 141. [Google Scholar]
- 54.The Strategies for Management of Antiretroviral Therapy (SMART) Study Group. CD4+ count - guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 55.Neaton JD, Grund B. Earlier Initiation of Antiretroviral Therapy in Treatment-Naïve Patients: Implications of Results of Treatment Interruption Trials. Cur Opin HIV/AIDS. 2008;3:112–117. doi: 10.1097/COH.0b013e3282f3808b. [DOI] [PubMed] [Google Scholar]
- 56.Bakerc JV, Peng G, Rapkin J, et al. CD4+ cell count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS. 2008;22:841–848. doi: 10.1097/QAD.0b013e3282f7cb76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marin B, Thiebaut R, Rondeau V, Costagliola D, Dorrucci M, Bucher H, et al. on behalf of the CASCADE Collaboration. Association between CD4 and HIV RNA with non AIDS-related causes of death in the era of combination Antiretroviral Therapy (cART). 4th IAS Conference on HIV Pathogenesis and Treatment; July2007; Sydney. [Google Scholar]
- 58.Smit C, Geskus R, Walker S, et al. Effective therapy has altered the spectrum of cause-specific mortality following HIV seroconversion. AIDS. 2006;20:741–749. doi: 10.1097/01.aids.0000216375.99560.a2. [DOI] [PubMed] [Google Scholar]
- 59.Mocroft A, Soriano V, Rockstroh J, et al. Is there evidence for an increase in the death rate from liver-related disease in patients with HIV? AIDS. 2005;19:2117–2125. doi: 10.1097/01.aids.0000194799.43799.ea. [DOI] [PubMed] [Google Scholar]
- 60.d’Arminio Monforte A, et al. The incidence of fatal AIDS-defining (ADM) and non-AIDS-defining (nADM) malignancies in the D:A:D study, and their relationship with immunodeficiency. 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles. 2007. [Google Scholar]
- 61.Krawczyka CS, Holmberg SD, Moorman AC, Gardner LI, McGwin G. Factors associated with chronic renal failure in HIV-infected ambulatory patients. AIDS. 2004;18:2171–2178. doi: 10.1097/00002030-200411050-00009. [DOI] [PubMed] [Google Scholar]
- 62.Kirk O, Mocroft A, d’Arminio Monforte A, Hansen AB, Gatell J, Caplinskas S, et al. Deterioration in renal function associated with current level of immunodeficiency. 15th Conference on Retroviruses and Opportunistic Infections; Feb 3–6 2008; Boston, USA. Abstract 971. [Google Scholar]
- 63.The D:A:D Study Group. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 64.Bonnet F, Chene G, Thiebaut R, Dupon M, Lawson-Ayayi S, Pellegrin JL, et al. Trends and determinants of severe morbidity in HIV-infected patients: the ANRS CO3 Aquitaine Cohort, 2000–2004. HIV Medicine. 2007;8:547–554. doi: 10.1111/j.1468-1293.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- 65.Ferry T, Raffi F, Collin F, Garre M, Duval X, Morlat P, et al. Incidence And Risk Factors For The Occurrence Of Non-AIDS-defining Non-HAART-related Severe Clinical Events In HIV-infected Adults With Long-term Follow-up, Aproco-copilote Cohort (ANRS CO8). 47th ICAAC; Sept 17–20; Chicago, IL, USA. Abstract H-1722. [Google Scholar]
- 66.The SMART Study Group. Risk for Opportunistic Disease and Death after Reinitiating Continuous Antiretroviral Therapy in Patients with HIV Previously Receiving Episodic Therapy. A Randomized Trial. Ann Intern Med. doi: 10.7326/0003-4819-149-5-200809020-00003. (in press) [DOI] [PubMed] [Google Scholar]
- 67.SMART Study Group. Major Clinical Outcomes in Antiretroviral Therapy (ART)–Naive Participants and in Those Not Receiving ART at Baseline in the SMART Study. J Infect Dis. 2008;197:1133–1144. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- 68.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C. Multiple Biomarkers for the Prediction of First Major Cardiovascular Events and Death. N Engl J Med. 2006;355:2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 69.CoDe. http://www.cphiv.dk/CoDe/Documents/tabid/101/Default.aspx.
- 70.Phillips AN, Gazzard B, Clumeck N, Losso M, Lundgren JD. When should antiretroviral therapy for HIV be started? BMJ. 2007;334:76–78. doi: 10.1136/bmj.39064.406389.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Panel on Clinical Practices for Treatment of HIV infection convened by the Department of Health and Human Services (DHHS) [accessed 3 May 2008];Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. 2008 Jan 29; http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf.
- 72.Hammer SM, Saag MS, Schechter M, et al. Treatment for adult HIV infection - 2006 recommendations of the International AIDS Society-USA panel. JAMA. 2006;296:827–843. doi: 10.1001/jama.296.7.827. [DOI] [PubMed] [Google Scholar]
- 73.BHIVA Treatment Guidelines Writing Committee. Treatment of HIV infected adults with antiretoroviral therapy. http://www.bhiva.org/cms1191540.asp.
- 74.Phillips AN UK HIV Drug Resistance Database. Long term probability of detection of HIV-1 drug resistance after starting antiretroviral therapy in routine clinical practice. AIDS. 2005;19(5):487–494. doi: 10.1097/01.aids.0000162337.58557.3d. [DOI] [PubMed] [Google Scholar]
- 75.Phillips AN, Leen C, Wilson A, Anderson J, Dunn D, Schwenk A, Orkin C, Hill T, Fisher M, Walsh J, Pillay D, Bansi L, Gazzard B, Easterbrook P, Gilson R, Johnson M, Sabin CA for the UK Collaborative HIV Cohort (CHIC) Study. Risk of extensive virologic failure to the three original antiretroviral drug classes over long term follow-up from the start of therapy among patients with HIV infection. Lancet. 2007;370:1923–1928. doi: 10.1016/S0140-6736(07)61815-7. [DOI] [PubMed] [Google Scholar]
- 76.Lampe FC, Smith CJ, Madge S, Kinloch-de Loes S, Tyrer M, Sabin CA, et al. Success of HIV clinical care according to demographic group among sexually-infected patients in a routine clinic population, 1999 to 2004. Arch Intern Med. 2007;167:692–700. doi: 10.1001/archinte.167.7.692. [DOI] [PubMed] [Google Scholar]
- 77.Lampe FC, Gatell J, Staszewski S, et al. Changes over time in risk of initial virological failure of combination antiretroviral therapy. A multi-cohort analysis, 1996 to 2002. Arch Intern Med. 2006 Mar 13;166(5):521–528. doi: 10.1001/archinte.166.5.521. [DOI] [PubMed] [Google Scholar]
- 78.Bannister WP, Kirk O, Gatell JM, Knysz B, Viard J-P, Mens H, D’Arminio Monforte A, Phillips AN, Mocroft A, Lundgren JD the EuroSIDA Study Group. Regional Changes over Time in Initial Virologic Response Rates to Combination Antiretroviral Therapy Across Europe. JAIDS. 2006;42:229–237. doi: 10.1097/01.qai.0000214815.95786.31. [DOI] [PubMed] [Google Scholar]
- 79.UK CHIC Steering Committee. HIV diagnosis at CD4 count above 500 Cells/mm(3) and progression to below 350 Cells/mm(3) without Antiretroviral therapy. JAIDS. 2007;46:275–278. doi: 10.1097/qai.0b013e3181514441. [DOI] [PubMed] [Google Scholar]
- 80.Lifson AR, Belloso WH, Carey C, Davey RT, Duprez D, El-Sadr WM, et al. INSIGHT Cause of Death Writing Group. Determination of the underlying cause of death in three multicenter international trials. HIV Clin Trials. 2008;9:177–185. doi: 10.1310/hct0903-177. [DOI] [PMC free article] [PubMed] [Google Scholar]