Abstract
Body size reduction in mammals is usually associated with only moderate brain size reduction as the brain and sensory organs complete their growth before the rest of the body during ontogeny1,2. On this basis “phyletic dwarfs” are predicted to have a higher relative brain size than “phyletic giants”1,3. This trend has been questioned, however, in the special case of dwarfism of mammals on islands4. Here we show that the endocranial capacities of extinct dwarf species of hippopotamus from Madagascar are up to 30% smaller than those of a mainland African ancestor scaled to equivalent body mass. These results show brain size reduction is much greater than predicted from an intraspecific ‘late ontogenetic’ model of dwarfism where brain size scales to body size with an exponent of 0.35. The nature of the proportional change or grade shift2,5 observed here indicates that selective pressures upon brain size are potentially independent from those on body size. This study demonstrates empirically that it is mechanistically possible for dwarf mammals on islands to evolve significantly smaller brains than would be predicted from a model of dwarfing based on the intraspecific scaling of the mainland ancestor. Our findings challenge our understanding of brain-body allometric relationships in mammals and suggest that the process of dwarfism could in principle explain small brain size, a factor relevant to the interpretation of the small-brained hominin found on the Island of Flores, Indonesia6.
Brain tissue is energetically expensive and it has been suggested that a reduction in brain volume may be advantageous to an animal’s survival if subjected to the environmental conditions associated with islands4,7,8. This phenomenon, if real, can be difficult to test because not only are most examples of dwarf island mammals extinct and known only from incomplete sub-fossil material, but knowledge of the founding ancestor can be difficult to ascertain. The strongest case previously documented is that of a fossil bovid Myotragus, isolated on the Mediterranean island of Mallorca for more than 5 million years4,9. The brain mass of Myotragus was reduced by up to 50% relative to values of extant bovids of equivalent body mass4. A link between the relatively small brain of Myotragus and the process of dwarfism has, however, been disputed10,11 in the light of the length of the bovid’s isolation and its uncertain ancestry9.
One aspect of the ongoing debate12,13,14 over whether the small-brained hominin discovered on the Island of Flores, Indonesia6, evolved via insular dwarfism centres around scaling exponents between brain and body size10,11. In terms of predicting the brain size of a mammal at a smaller body size these ‘intraspecific’ scaling models are the most appropriate to accommodate the likely correlated effects of body size adjustment on brain size in closely related species10,15,16 (see Supplementary Discussion). Here we demonstrate, however, that two species of extinct dwarf hippopotamus do not correspond to such dwarfing models because even though the same scaling exponents relating brain size to body size apply, large intercept (grade2,5) shifts distinguish these ‘phyletic dwarfs’ from their mainland ancestor (Fig. 1; Supplementary Table 1 and Supplementary Discussion).
Figure 1. The relationship between brain size and cranial size for an intraspecific ‘late ontogenetic’ model of dwarfing.
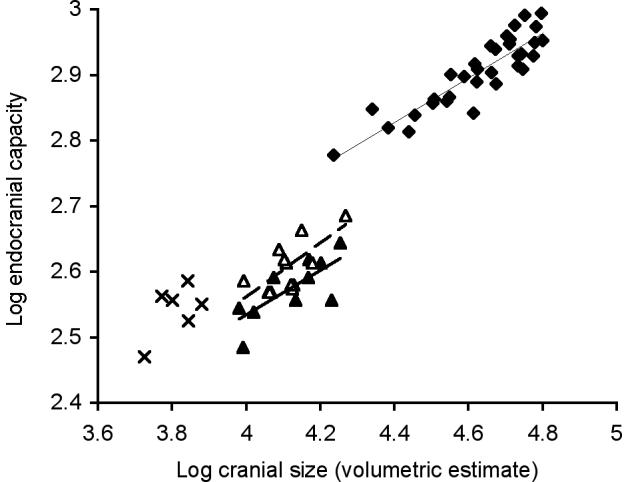
Major axis slopes and 95% confidence intervals: H. amphibius 0.3482 (0.28 - 0.41) thin line, H. lemerlei 0.369 (0.15 - 0.63) bold line, H. madagascariensis 0.4587 (0.20 - 0.79) dashed line. H. amphibius (filled diamonds; n = 33), H. lemerlei (filled triangles; n = 12), H. madagascariensis (open triangles; n = 12), C. liberiensis (crosses; n = 6). For statistical comparisons see Supplementary Table 1.
To model the dwarfing process, we took the extant large H. amphibius, a generalised representative of the genus Hippopotamus, to be the probable ancestor of two recently extinct dwarfed hippos, H. lemerlei and H. madagascariensis, from the island of Madagascar17 (see Supplementary Discussion). These species can be unequivocally separated taxonomically17 but both share apomorphies with the modern genus Hippopotamus18. The modern pygmy hippopotamus Choeropsis liberiensis represents a lineage distinct from all other hippopotamids, diverging from its closest relative before 5 Myr18. Madagascar is a large island of diverse habitats that has prehistorically supported up to three species of hippopotamus19 whose times of dispersal to Madagascar are unknown, but whose remains persist to within the last 6000 years20.
Brain-body scaling trends in hippos can be inferred from the relationship of brain to cranial size, as the relationship between a volumetric estimate of cranial size and a postcranial estimate of body mass across the two living hippos and the Madagascan dwarf species is approximately isometric (Supplementary Figs 3-7, Supplementary Table 4 and Supplementary Discussion). Predictions of brain size reduction given in Table 1 are thus based on observed cranial size data and not on estimates of body mass (the latter are provided for contextual purposes only). In Table 1 we evaluate predictions of brain-body scaling in insular dwarf hippos and a dwarf elephant through consideration of two intraspecific dwarfing models: ‘late ontogenetic’ and ‘ontogenetic’. In mammals that commonly dwarfed on islands, such as hippos, elephants and deer (see Supplementary Discussion), all of which have precocial young, there is generally thought to be a transition from rapid to slow growth of the brain that closely coincides with birth2,15,16. In H. amphibius, however, the rapid early phase of brain growth continues after birth for up to 2 years (Supplementary Fig. 1). The ‘late ontogenetic’ exponent (0.35) in this context is calculated from the late, slower phase of postnatal brain growth (2 - 40 years) and not just from static adult data. This distinction is important, as the ‘growth’ as opposed to the ‘static adult’ exponent will characterise best the size adjustments (developmental allometry) associated with the late phase of brain maturation1. However, as all late postnatal brain development in mammals typically involves a low exponent value (0.2 - 0.4 for intraspecific brain-body scaling)5, ‘late ontogenetic’ exponents should be similar to, or only moderately higher than, those derived from static adult data (see Supplementary Discussion). The higher ‘ontogenetic’ exponent (0.47) is derived from a complete postnatal series from birth to adulthood where rapid and slow growth phases are combined.
Table 1. Estimates of brain size based on dwarfing models.
| Endocranial capacity prediction based on intraspecific dwarfing models using cranial size | ||||
|---|---|---|---|---|
| Species of extinct island dwarfs and the extant pygmy hippo (C. liberiensis) | Body mass (kg) (Malagasy hippo estimates based on cranial size) | Endocranial capacity (cm3) | Late ontogenetic scaling(k = 0.35: late phase of brain growth: 2 - 40 yrs) | Ontogenetic scaling(k = 0.47: early and late phases of brain growth: 0 - 40 yrs) |
| Hippopotamus lemerlei | 374 (25 %) | 380 (43 %) [70 %, 83 %] | 544 (62 %) | 456 (52 %) |
| Hippopotamus madagascariensis | 393 (26 %) | 421 (48 %) [76 %, 91%] | 553 (63 %) | 465 (53 %) |
| Choeropsis liberiensis | 22819 (15 %) | 350 (40 %) [83 %, 105 %] | 421 (48 %) | 334 (38 %) |
| Palaeoloxodon falconeri (a) | 20022 (2 %) | 1800 (20 %) [80 %, 125 %] | 2250 (25 %) | 1440 (16 %) |
| Palaeoloxodon falconeri (b) | 10023 (1 %) | 1800 (20 %) [100 %, 167 %] | 1800 (20 %) | 1080 (12 %) |
The values given in parentheses are expressed as a percentage of the original mean value of the mainland ancestor (H. amphibius for the Malagasy hippos and P. antiquus for P. falconeri) or in the case of C. liberiensis the larger sister taxon (H. amphibius) (see Table 2). For endocranial capacity, numbers in square brackets indicate the observed values as a percentage of those predicted based on the ‘late ontogenetic’ and ‘ontogenetic’ scaling models. For example the endocranial capacity of H. lemerlei is reduced by 30% of the value predicted by the late ontogenetic model (endocranial capacity = 70% of predicted value) whereas the endocranial capacity of H. madagascariensis is reduced by 24% of the value predicted by the late ontogenetic model (endocranial capacity = 76% of predicted value). The scaling exponents (k) are modelled from H. amphibius postnatal cranial data (Supplementary Table 1). The Malagasy hippo body masses are estimated assuming isometry to cranial volume of the ancestor using an adult body mass of 1495 ± 29.5 kg for H. amphibius (see Supplementary Discussion, and Table 2 for limb-bone estimates of body mass). P. falconeri brain size reduction is estimated from both (a) cranial size, a 50 fold difference22 and (b) an estimate of body mass, a 100 fold difference23 (P. antiquus has an approximate average mass of 10 tonnes estimated from limb-bone data in ref. 25 and P. Davies, unpublished data).
If the endocranial capacity of H. lemerlei scaled as (cranial size)0.35 in accord with the ‘late ontogenetic’ model (Fig. 1; Supplementary Table 1), an endocranial capacity of 544 cm3 would be predicted, that is 62 % of the value of an ancestor four times its cranial size (Tables 1 and 2). The observed mean endocranial capacity of H. lemerlei is 380 ± 7.25 cm3 (Table 2), 30 % smaller than that predicted from the ‘late ontogenetic’ model (Table 1). The observed mean endocranial capacity of H. madagascariensis is 421 ± 11.9 cm3 (Table 2), 24 % smaller than that predicted from the ‘late ontogenetic’ model (Table 1). The ‘ontogenetic’ model, where the endocranial capacity of H. lemerlei would scale as (cranial size)0.47 (Fig. 2; Supplementary Table 1), predicts a endocranial capacity of 456 cm3, 52 % of the value of the ancestor, but the observed endocranial capacity is still 17% smaller than that predicted under this model and in H. madagascariensis it is 9 % smaller (Table 1).
Table 2. Values of endocranial capacity, cranial size (volumetric estimate) and body mass for species in Table 1.
| Species | Endocranial Capacity (cm3) | Adult cranial size(cm3) | Body mass range (kg) |
|---|---|---|---|
| H. amphibius | 882 ± 16 (n = 18) | 52533 ± 1657 (n = 20) | 1210 - 2001† |
| H. lemerlei | 380 ± 7.25 (n = 24) | 13298 ± 810 (n = 12) | 274 - 393 |
| H. madagascariensis | 421 ± 11.9 (n = 12) | 13948 ± 751 (n = 12) | 310 - 642 |
| C. liberiensis | 350 ± 12.7 (n = 6) | 6524 ± 338 (n = 6) | 180 - 27519 |
| P. antiquus | 900022 (n = 1) | 31100022 (n = 1) | 5055 - 1767525 |
| P. falconeri | 180022 (n = 1) | 612022 (n = 1) | 80 - 20022,23 |
The endocranial capacity and cranial size data for all species of hippopotamus were collected as part of this study (see Methods). The elephant cranial data are from ref. 22. Observed ranges of adult body mass are given for the extant species (see Supplementary Discussion), and estimates based on postcrania are given for the extinct species (see Supplementary Table 4 for Malagasy hippo data and Supplementary Discussion). Standard errors are given with mean values.
Figure 2. The relationship between brain size and cranial size for an intraspecific ‘ontogenetic’ model of dwarfing.
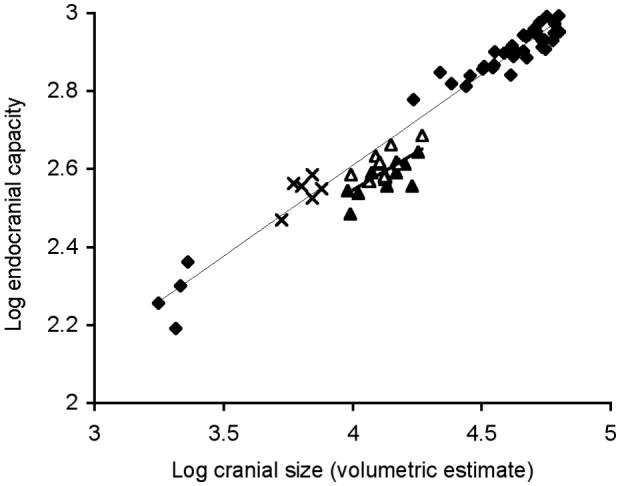
Major axis slopes and 95% confidence intervals: H. amphibius 0.468 (0.44 - 0.50) thin line, dwarf species pooled 0.454 (0.27 - 0.67) bold line. H. amphibius (filled diamonds; n = 37), H. lemerlei (filled triangles; n= 12), H. madagascariensis (open triangles; n = 12), C. liberiensis (crosses; n = 6). For statistical comparisons see Supplementary Table 1.
The Malagasy dwarf hippos do not correspond to either ‘intraspecific’ scaling model (Figs 1 and 2; Table 1), suggesting that ‘insular dwarfing’ does not necessarily comply with predictions based on the ontogenetic scaling of the mainland ancestor. The Malagasy dwarf hippos are approximately equivalent in body mass to a 1 - 2 year old H. amphibius (353 - 544 kg), a stage in development that marks the completion of the first rapid phase of brain growth. In contrast, the extant pygmy hippopotamus (C. liberiensis), with a body mass 6 - 8 times less than that of H. amphibius (ref. 19 and Table 2), has a mean endocranial capacity (350 cm3 ± 12.7), approximately equal to the values predicted from the ‘ontogenetic’ scaling model (Table 1) but reduced by 17 % relative to the values predicted from the ‘late ontogenetic’ model (Table 1). The extant pygmy hippopotamus is not an insular dwarf21 and is phylogenetically more basal than H. amphibius, its only extant sister taxon18 (see Supplementary Discussion). Adult C. liberiensis has a body mass equivalent to a 6-month-old H. amphibius (see Supplementary Discussion), a stage of development where rapid early brain growth persists in the hippopotamus. In primates, too, a marked increase in relative brain size has been attributed to the prolongation of the rapid ‘prenatal’ phase of brain development3. In ‘phyletic dwarfs’ the opposite developmental adjustment, involving a reduction in the duration of rapid early brain growth, could potentially explain a “grade shift” to a lower brain-body ratio as demonstrated by the change in ratio of brain to cranial size in Fig. 1 (see Supplementary Discussion).
A further example is provided by Palaeoloxodon falconeri22, 23, the smallest insular dwarf elephant from Sicily and Malta, derived from the mainland P. antiquus22, 23. The brain of a modern elephant grows relatively little after birth (an elephant neonate brain is 50% of its adult brain weight whereas that of a common hippopotamus is 22% and that of a human is 25%)24 suggesting that rapid early brain growth is completed by the end of gestation. Adult P. falconeri is estimated to be approximately the size of a neonate modern elephant23 and hence brain mass is expected to scale to body mass with a ‘late ontogenetic’ exponent typical of a precocial mammal. With brain size scaled on (body mass)0.35, brain size matches the prediction of the ‘late ontogenetic’ model using a 100-fold estimated reduction of body mass (Table 1b and see refs 10, 11). However, estimates of body mass reduction in these elephants are imprecise, especially given the magnitude of the dwarfing (Table 2). Cranial volume, determined by direct measurement22, is reduced by only 50-fold, and when scaled as (cranial volume)0.35, P. falconeri would have an endocranial capacity (2250 cm3) 25% of the original value of its ancestor (P. antiquus) 50 times its size (Tables 1 and 2). The actual endocranial capacity of P. falconeri is 1800 cm3 (ref. 22), 20 % smaller than the value predicted from this ‘late ontogenetic’ model (Table 1a), in line with our results on the dwarf hippos. This discrepancy between the predictions based on cranial size22 and those based on estimates of body mass23,25 in these elephants urges caution in the use of the latter for the interpretation of brain-size scaling (see refs 7, 10, 11).
One argument put forward to refute the idea that the small brain of H. floresiensis was linked to the process of insular dwarfism is that its small brain size could not be accommodated within predictions made from mammalian intraspecific brain-body scaling models10,11. Here we demonstrate empirically that it is mechanistically possible for dwarf mammals on islands to evolve significantly smaller brains in relation to their cranial size than would be predicted from models of dwarfing based on intraspecific scaling of the mainland ancestor. If the hippo model is applied to a typical H. erectus ancestor with a body mass of 60 kg10,11,26 and average endocranial capacity of 991 (cm3)10,11,27 that reduced its body mass by 62 % to 23 kg (the median of the 16 - 29 kg estimated body mass of H. floresiensis)6, an endocranial capacity of 704 cm3 (71 % of the original value) would be predicted from scaling (body mass)0.35. If the brain were reduced by a further 30 % of that value, as in the case of the Malagasy hippo H. lemerlei, an endocranial capacity of 493 cm3 would result. This is still larger than the actual value of H. floresiensis (380 - 430 cm3)6,28, but if the ancestor had an endocranial capacity of 804 cm3, as in the African H. erectus KNM-ER 388327, scaling the body mass of the ancestor down to 23 kg and then reducing the brain size by a further 30 % of the scaled value gives an endocranial capacity of 405 cm3, comparable to that of H. floresiensis (see Supplementary Table 5). By the same analogy if the Dmanisi adult Homo remains (skull D3444 cf. H. erectus)29, with an endocranial capacity 650 cm3 (ref. 29) and body mass of 40 kg (ref. 30) are considered, an endocranial capacity of 378 cm3 would result, a value close to that of H. floresiensis (see Supplementary Table 5). If cranial variables are used instead of body mass for scaling these hominins’ endocranial capacity, as in the hippo example, a similar result is obtained (see Supplementary Table 5).
Similar intercept shifts to those illustrated in Figure 1 have been reported from domesticated mammals that on average have smaller brains than their wild relatives16. Domesticated mammals provide proof of the developmental malleability of the brain among closely related species or within species, but there are some noteworthy distinctions between the phenomena of domestication and island dwarfism. The brain size reduction in domestic mammals is not necessarily associated with body size reduction, as it is in phyletic dwarfism, and domestication is usually associated with a reduction in the size of the sense organs16. H. floresiensis, unlike the bovid Myotragus and domesticated mammals, does not have reduced orbital dimensions11, although such skeletal variation does not necessarily correlate with the actual size of the sense organ. This is also evident in the Malagasy hippos where orbit area is actually larger in the dwarf hippos relative to that of H. amphibius (Supplementary Fig. 2). A pathological explanation for this condition in the Malagasy species can be ruled out through the existence of over 40 individual dwarf hippo specimens with intact braincases (Supplementary Table 2). Whatever the explanation for the tiny brain of H. floresiensis relative to its body size, the evidence presented here suggests that the phenomenon of insular dwarfism could have played a part in its evolution.
METHODS SUMMARY
A cross-sectional postnatal series of crania of H. lemerlei, H. madagascariensis H. amphibius and C. liberiensis (see Supplementary Tables 2 and 3) were digitised with a MicroScribe G2 and inter-landmark distances calculated to compute a volumetric measure of the entire cranium. Endocranial capacity was used as a surrogate for brain size. Exponents derived from brain-body allometric scaling relationships in mammals are widely applied in analyses of relative brain size5 but here brain size has been regressed against cranial volume. Supplementary data are provided, demonstrating that results generated using estimates of body mass, and those reported using cranial size, are similar (see Supplementary Figs 3-7 and Supplementary Discussion). The data and procedure used to determine body mass estimates from Malagasy dwarf postcrania are given in Supplementary Table 4 and in the Supplementary Discussion.
Supplementary Material
Acknowledgements
We thank A. Currant, C. Lefèvre, C. Sagne, E. Gilissen, F. Renoult, H. Chatterjee, J. Ashby, M. Nowak-Kemp, M. Harman, P. Jenkins, P. Tassy, R. Sabin, R. Symonds, and S. Stuenes for facilitating access to museum collections. We thank A. Rasoamiaramanana, G. Ravololonarivo, H. Andriamialison, T. Rakotondrazafy, M. Ramarolahy, S. Bourlat, for permission and/or assistance with study of the subfossil material held in the University of Antananarivo and the L’ Academie, Malagache and B. Ramanivosoa, D. Gommery, C. Guérin, M. Faure for facilitating the study of material at the Akiba Museum, Mahajanga, Madagascar. We thank R. Portela Miguez for assistance with recording endocranial capacity measures from H. amphibius specimens in the NHM, London, A. Friday for assistance with data collection in the UMZC, Cambridge, UK and C. Anderung, J-R. Boisserie, S. Walsh and V. Herridge for discussion and helpful comments. We thank J. Kappelman, J. Niven, D. Lieberman and A. Gordon for comments on earlier versions of this manuscript. This research was supported by the Biotechnology and Biological Sciences Research Council, United Kingdom.
Appendix
METHODS
Sample size
The number of specimens included in different analyses is not constant due to missing data (MD); all relevant landmarks were not preserved on every specimen studied and endocranial capacity could not be measured accurately on some specimens due to braincase damage or poor state of preservation (see Supplementary Tables 2 and 3). Sample sizes are: H. lemerlei (n = 29; 17 with missing data ‘MD’), H. madagascariensis (n = 19; 7 with MD), H. amphibius (n = 50; 13 with MD) and C. liberiensis (n = 7; 1 with MD)
Estimation of cranial volume
A volumetric measure of the entire cranium was computed using the product of three variables: cranial length (the most posterior point of the nuchal crest to the most mesial point of the first incisor socket), cranial width (Zygion - Zygion) and cranial height (Akrokranion [median dorsal point of occipital region] - Basion). The static adult samples used to derive values given in Table 2 include specimens from dental ‘Age Group XI’ and above (see Supplementary Tables 2 and 3 and Supplementary Discussion).
Estimation of endocranial capacity
Endocranial capacity, the volume of the endocranial cavity, was measured by pouring precision plastic (polypropylene) balls of 5.5 mm diameter into the braincase cavity (large foramina plugged with plastazote foam), and then decanting the balls into a measuring cylinder.
Analysis of relative brain size
Brain size in mammals scales allometrically with body size and is described by the bivariate power function:
or after logarithmic transformation the linear equation:
where y and x are variables and k (exponent expressing slope) and b (y-intercept) are constants. Slopes were determined using Major Axis and least-squares regression (see Supplementary Table 1) but Major Axis values define k in Table 1. In light of the absence of associated body mass data for a growth series of modern H. amphibius skeletons, an analysis of cranial size versus global skeletal size (an estimate of body mass determined from postcranial evidence) has been provided in the Supplementary Material (see Supplementary Discussion). For the subfossil taxa, body mass values were estimated from crania or from unassociated skeletal elements (see Supplementary Table 4 and Supplementary Discussion). The endocranial cavity (used to represent brain size), forms part of the cranium and cranial size is therefore a conservative estimator of change that can be measured directly from both fossil and modern material, and in hippos is a good proxy for body size (see Supplementary Discussion).
References
- 1.Shea BT. Phyletic size change and brian/body allometry: a consideration based on the African pongids and other primates. Int. J. Primatol. 1983;4:33–62. [Google Scholar]
- 2.Martin RD. Human brain evolution in an ecological context: 52nd James Arthur Lecture on the evolution of the human brain. American Museum of Natural History; New York: 1983. [Google Scholar]
- 3.Gould SJ. Allometry in primates with emphasis on scaling and the evolution of the brain. Contrib. Primatol. 1975;5:244–292. [PubMed] [Google Scholar]
- 4.Köhler M, Moyà-Solà S. Reduction of brain and sense organs in the fossil insular bovid Myotragus. Brain. Behav. Evol. 2004;63:125–140. doi: 10.1159/000076239. [DOI] [PubMed] [Google Scholar]
- 5.Martin RD, Harvey PH. In: Size and Scaling in Primate Biology. Jungers WL, editor. Plenum Press; New York: 1985. pp. 147–173. [Google Scholar]
- 6.Brown P, et al. A new small-bodied hominin from the Late Pleistocene of Flores, Indonesia. Nature. 2004;431:1055–1061. doi: 10.1038/nature02999. [DOI] [PubMed] [Google Scholar]
- 7.Niven JE. Brains, islands and evolution: breaking all the rules. Trends Ecol. Evol. 2006;22:57–59. doi: 10.1016/j.tree.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Richards GD. Genetic, physiologic and ecogeographic factors contributing to variation in Homo sapiens: Homo floresiensis reconsidered. J. Evol. Biol. 2006;19:1744–1767. doi: 10.1111/j.1420-9101.2006.01179.x. [DOI] [PubMed] [Google Scholar]
- 9.Lalueza-Fox C, Shapiro B, Bover P, Alcover JA, Bertranpetit J. Molecular phylogeny and evolution of the extinct bovid Myotragus balearicus. Mol. Phylogenet. Evol. 2002;25:501–510. doi: 10.1016/s1055-7903(02)00290-7. [DOI] [PubMed] [Google Scholar]
- 10.Martin RD, et al. Comment on “the brain of LB1, Homo floresiensis”. Science. 2006;312:999b. doi: 10.1126/science.1121144. [DOI] [PubMed] [Google Scholar]
- 11.Martin RD, Maclarnon AM, Phillips JL, Dobyns WB. Flores hominid: new species or microcephalic dwarf? Anat. Rec. 2006;288A:1123–1145. doi: 10.1002/ar.a.20389. [DOI] [PubMed] [Google Scholar]
- 12.Köhler M, Moyà-Solà S, Wrangham RW. Island rules cannot be broken. Trends Ecol. Evol. 2008;23:6–7. doi: 10.1016/j.tree.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Argue D, Donlon D, Groves C, Wright R. Homo floresiensis: microcephalic, pygmoid, Australopithecus, or Homo? J. Hum. Evol. 2006;51:360–374. doi: 10.1016/j.jhevol.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Niven JE. Impossible arguments about possible species? Trends Ecol. Evol. 2008;23:8–9. Response to Köhler et al. [Google Scholar]
- 15.Lande R. Quantitative genetic analysis of multivariate evolution, applied to brain:body size allometry. Evolution. 1979;33:402–416. doi: 10.1111/j.1558-5646.1979.tb04694.x. [DOI] [PubMed] [Google Scholar]
- 16.Kruska DCT. On the evolutionary significance of encephalization in some eutherian mammals: effects of adaptive radiation, domestication and feralization. Brain Behav. Evol. 2005;65:73–108. doi: 10.1159/000082979. [DOI] [PubMed] [Google Scholar]
- 17.Stuenes S. Taxonomy, habits, and relationships of the subfossil Madagascan hippopoptami Hippopotamus lemerlei and H. madagascariensis. J. Vert. Paleontol. 1989;9:241–268. [Google Scholar]
- 18.Boisserie J-R. The Phylogeny and taxonomy of Hippopotamidae (Mammalia: Artiodactyla): a review based on morphology and cladistic analysis. Zool. J. Linn. Soc. 2005;143:1–26. [Google Scholar]
- 19.Eltringham SK. The Hippos: Natural History and Conservation. Academic Press; London: 1999. [Google Scholar]
- 20.Burney DA, et al. A chronology for late prehistoric Madagascar. J. Hum. Evol. 2004;47:25–63. doi: 10.1016/j.jhevol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Weston EM. Evolution of ontogeny in the hippopotamus skull: using allometry to dissect developmental change. Biol. J. Linn. Soc. 2003;80:625–638. [Google Scholar]
- 22.Accordi FS, Palombo MR. Morfologia endocranica degli elefanti nani pleistocenici di Spinagallo (Siracusa) e comparazione con l’ endocranio di Elephas antiquus. Atti. Accad. Naz. Lincei, Rc. 1971;51:111–124. [Google Scholar]
- 23.Roth VL. Inferences from allometry and fossils: dwarfing of elephants on islands. Oxford Surveys in Evolutionary Biology. 1992;8:259–288. [Google Scholar]
- 24.Shoshani J, Kupsky WJ, Marchant GH. Elephant brain Part I: Gross morphology, functions, comparative anatomy, and evolution. Brain Res. Bull. 2006;70:124–157. doi: 10.1016/j.brainresbull.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 25.Christiansen P. Body size in proboscideans, with notes on elephant metabolism. Zool. J. Linn. Soc. 2004;140:524–549. [Google Scholar]
- 26.Kappelman J. The evolution of body mass and relative brain size in fossil hominids. J. Hum. Evol. 1996;30:243–276. [Google Scholar]
- 27.Stanyon R, Consigliere S, Morescalchi MA. Cranial capacity in hominid evolution. Hum. Evol. 1993;8:205–216. [Google Scholar]
- 28.Jacob T, et al. Pygmoid Australomelanesian Homo sapiens skeletal remains from Liang Bua, Flores: population affinities and pathological abnormalities. Proc. Natl. Acad. Sci. U.S.A. 2006;103:13421–13426. doi: 10.1073/pnas.0605563103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lordkipanidze D, et al. A Fourth Hominin Skull From Dmanisi, Georgia. Anat. Rec. 2006;288A:1146–1157. doi: 10.1002/ar.a.20379. [DOI] [PubMed] [Google Scholar]
- 30.Lordkipanidze D, et al. Postcranial evidence from early Homo from Dmanisi, Georgia. Nature. 2007;449:305–310. doi: 10.1038/nature06134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


