Abstract
Carbohydrate and peptide-based antitumor vaccine constructs featuring clusters of both tumor associated carbohydrate antigens and mucin-like peptide epitopes have been designed, synthesized, and studied. The mucin-based epitopes are included to act, potentially, as T-cell epitopes in order to provoke a strong immune response. Hopefully the vaccine will simulate cell surface architecture, thereby provoking levels of immunity against cancer cell types displaying such characteristics. With this central idea in mind, we designed a new vaccine type against ovarian cancer. Following advances in glycohistology, our design is based on clusters of Gb3 antigen, and also incorporates a MUC5AC peptide epitope. The vaccine is among the most complex targeted constructs to be assembled by chemical synthesis to date. The strategy for the synthesis employed a Gb3-MUC5AC thioester cassette as a key building block. Syntheses of both non-conjugate and KLH-conjugated vaccines constructs have been accomplished.
Introduction
In the quest to develop effective vaccines to combat cancer, tumor immunologists seek to identify the characteristic phenotypes which differentiate tumor cells from normal cells. In this vein, it has been noted that malignantly transformed cells often display aberrant levels and patterns of cell surface glycosylation. 1 Presumably, it should be possible to exploit these distinguishing features by designing vaccine constructs which incorporate these tumor-associated carbohydrate domains. Such constructs, if properly presented to the immune system, could stimulate the formation of antibodies which would selectively bind and eradicate tumor cells overexpressing the carbohydrate epitopes at issue. Particularly impressive progress in this area of anticancer vaccines has been achieved by Boons,2 Kunz,3 Schmidt4 and their associates.
Over the past two decades, our laboratory has been engaged in the design and de novo synthesis of complex oligosaccharides and glycoconjugates, with an eye toward developing increasingly potent and versatile vaccines.5 Our emphasis has been on the development of immunostimulating strategies allowing for enhanced protection against tumor recurrence and metastasis following resection of tumor burden through surgery, radiation, or chemotherapeutic treatment.
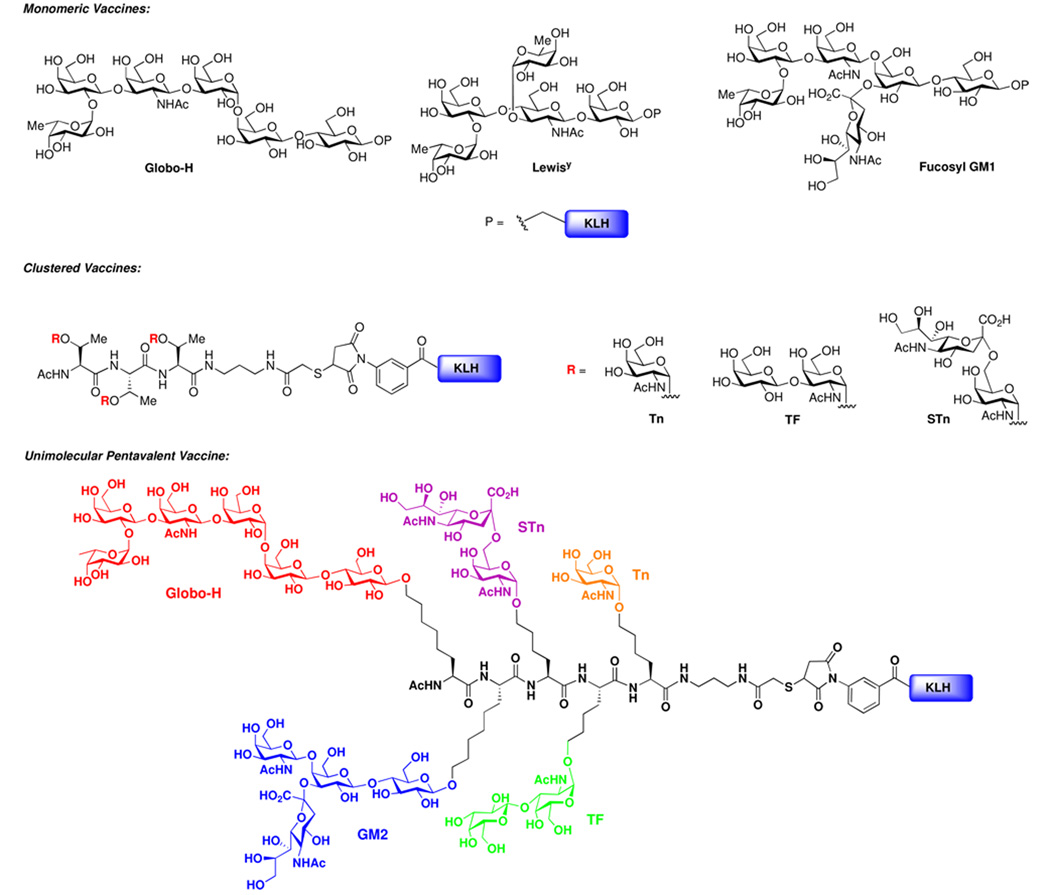
Our initial studies focused on the preparation of constructs, in which a single carbohydrate antigen is attached to an immunogenic carrier molecule, such as KLH (Keyhole Limpet Hemocyanin) (Figure 1).6 These monovalent vaccines – which include Globo-H, fucosyl GM1, and Lewisy (Ley) – have shown varying degrees of promise in early clinical settings. In our second generation studies, we are turning our attention to the preparation and evaluation of more elaborate constructs, in which multiple repeats, or “clusters,” of a carbohydrate epitope are presented on a peptide backbone. The design of these constructs was inspired by findings from the field of glycohistology which demonstrate that mucins – a family of glycoproteins overexpressed on tumor cell surfaces – often present clusters of two to five adjacent carbohydrates domains.7 The hope is that vaccines designed on the basis of these “clustered” antigens would better mimic the surfaces of targeted tumor cells. In this phase of our program, we prepared a number of clustered vaccine constructs, such as Tn(c), TF(c) and STn(c), each of which performed as hoped in preclinical studies. For instance, in a Phase I clinical trial against prostate cancer, the Tn(c)-KLH conjugate has produced positive serological results.8 These earlier vaccine constructs did not take full account of the multiplicity of carbohydrate epitopes overexpressed within a particular cancer type. Thus, even within the lifetime of a single tumor cell, there is a significant amount of heterogeneity of tumor cell surface carbohydrate expression.9 In order to achieve potency of a broader base, a carbohydrate-based antitumor vaccine should incorporate multiple antigenic components.
Figure 1.
Representative Previously Developed Vaccine Structures.
Toward this end, we have synthesized a number of unimolecular multiantigenic vaccine constructs, such as the one shown in Figure 1, which contains five different carbohydrate antigens: Globo-H, GM2, STn, TF and Tn.10 Preclinical biological studies have demonstrated that the unimolecular pentavalent vaccine–KLH conjugate is well tolerated and induces promising IgG and IgM responses against the target carbohydrate epitopes. Trials evaluating the clinical impact of these multiantigenic vaccines in the adjuvant setting are expected to commence in the near future.11
It will be noted that both the clustered and multiantigenic vaccine constructs consist of a number of carbohydrate domains presented along a peptide backbone. In the evolution of our vaccine design, we began by considering the possibility that the peptide backbone might also provide for additional antigenic markers, beyond its role as a linker to carrier protein. In this regard, we took specific note of the mucin family of O-linked glycoproteins.12 As noted above, the mucins, which carry highly clustered glycodomains on adjacent serine and threonine residues, are overexpressed on a variety of tumor cell surfaces. Numerous mucin types have been identified, and correlated with tumor types.13 For example, MUC1 expression for this section is most intense in cancers of breast, lung, ovarian, and endometrial origin; MUC2 is overexpressed in cancers of colon and prostate origin; MUC5AC is associated with breast and gastric cancers; MUC4 was found to be highly expressed in 50% of cancers of colon and pancreas origin; and MUC3, MUC5B, and MUC7 are overexpressed in a variety of epithelial cancers, though not intensely so. It has been theorized that these mucins may potentially serve as CD8+ cytotoxic T cell and CD4+ helper T cell epitopes.14 MUC1 has also previously been used as a B-cell epitope for generating anti-MUC1 antibodies.2a,3, 15
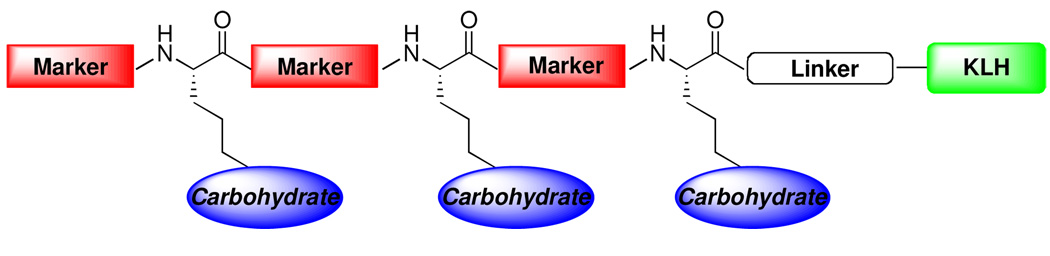
Based on these observations, we have designed a new type of antitumor vaccine structure featuring both a carbohydrate-based antigen and a mucin derived peptide-based marker in an alternating pattern (Figure 2). This design seeks to mimic the molecular architecture on tumor cell surfaces, thus provoking a more robust immune response. In these clustered carbohydrate–peptide antigenic constructs, either repeats of the same carbohydrate antigen or a combination of diverse carbohydrate antigens associated with a particular carcinoma can be incorporated. We envision that this type of vaccine structure has two potential advantages. First, a mucin derived peptide fragment is incorporated as both a linker and a marker, which may behave not only as a B-cell epitope for the production of antibodies against mucins, but also as a helper T-cell epitope to activate T-cells. Furthermore, the tandem repeats of both the carbohydrate-based antigen and the peptide-based epitope are anticipated to expose these B-cell and helper T-cell epitopes to the maximum extent on the surface of the carrier protein (KLH). Hopefully, this feature will prove to be quite important in stimulating a strong immune response, as our previous immunogenic studies in related design have demonstrated that clustered monomeric antigenic peptide did elicit substantial IgG and IgM antibody titers.8 Finally, vaccines composed of numerous carbohydrate antigens associated with a specific cancer type may provide a heightened and more varied responses, thereby increasing the efficiency of binding to the target cells. It is envisioned that success in this design and synthesis would pave the way for the preparation of more complex vaccine structures which mimic the natural cell surface.
Figure 2.
A Proposal for A Novel Carbohydrate–Peptide Based Vaccine.
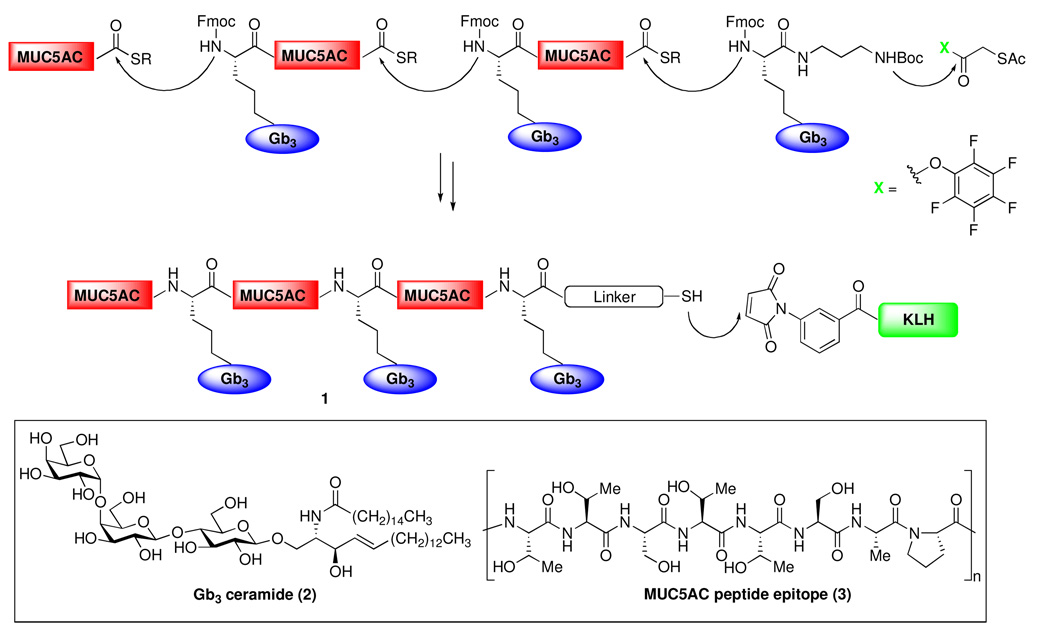
Ovarian cancer is the fifth leading cause of cancer deaths in women and the leading cause of death from gynecological malignancies. 16 A number of carbohydrates have been found to be overexpressed on ovarian tumor cell surfaces, including Ley, 17 STn, 18 Globo-H 19 and Gb3 (globotriaosyl ceramide, cf. 2, Figure 3). 20 Also found on ovarian cancer cell surfaces are the mucin antigens, MUC1 (vide supra), MUC5AC (cf. 3)21 and MUC16 (CA125 antigen). 22 Structurally, MUC1 and MUC5AC consist of tandem repeats of a 20-amino acid sequence (VTSAPDTRPAPGSTAPPAHG) and an 8-amino acid sequence (TTSTTSAP), which are potentially responsible for the activation of T cells. In the hope of exploring the promise of a chimeric vaccine construct, composed of alternating immunogenic carbohydrate and peptide domains, we have designed a vaccine which incorporates alternating repeats of the Gb3 antigen and the MUC5AC-based peptide marker (1, Figure 3). Due to the fact that Gb3 exists as a ceramide form, we decided to prepare Gb3 glycosylamino acid using nonnatural extended hydroxynorleucine linker in the hopes of mimicking the ceramide chain. In addition, our previous experience demonstrated that these nonnatural linkages avoid problems associated with the instability of the O-glycosyl serine23 and are able to simulate the activity of their native counterparts.10 Glycosylamino acids bearing nonnatural linkers may be more immunogenic because they are potentially more recognizable as “nonself” by the immune system.24
Figure 3.
Design and Synthetic Strategy for a Vaccine Candidate Targeting Ovarian Cancer (1).
Our initial program for the total synthesis of construct 1 required the assembly of three repeats of both the protected Gb3 glycosylamino acid and the MUC5AC peptide C-terminal thioester, which would then be iteratively coupled to form the fully glycosylated polypeptide backbone, in analogy to our synthesis of unimolecular polyantigenic vaccine constructs.10 We have further refined our synthetic approach by preparing a Gb3–MUC5AC thioester cassette, to be employed as a key building block (Figure 3). We elected to block the N-termini of the cassettes with fluorenylmethyl carbonate (Fmoc) protecting groups, so that the coupling sequence would consist of iterative peptide couplings following deprotection of the N-termini. The Gb3 glycosylamino acid would ultimately be linked to the carrier protein (KLH) via a Boc-protected diaminopropyl unit.10
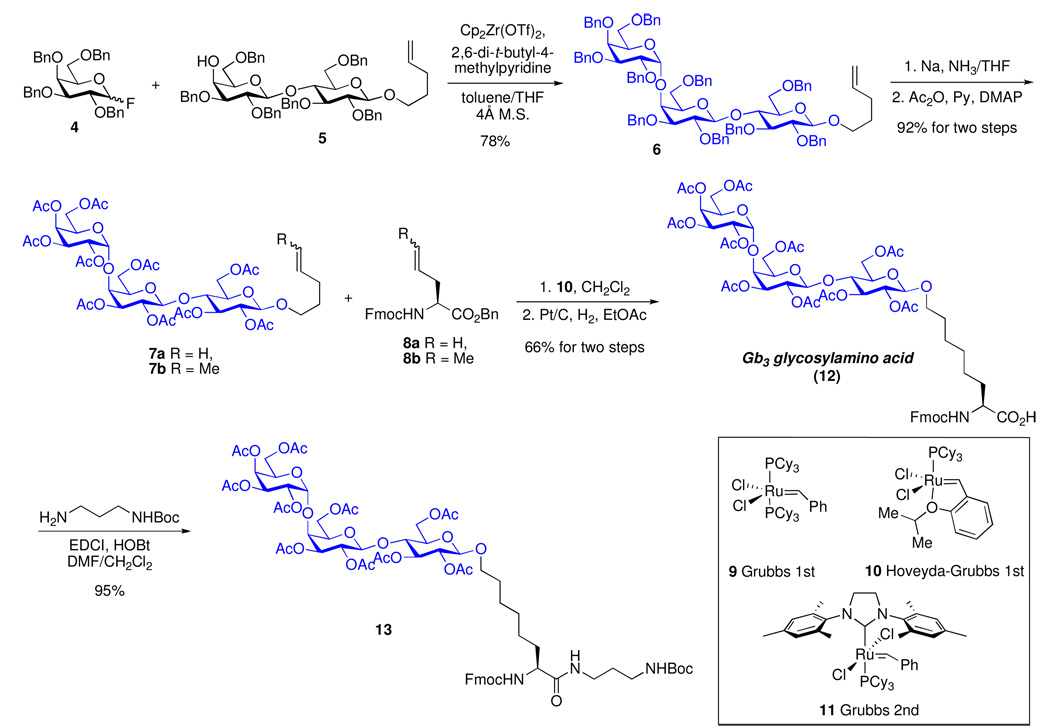
The synthesis of the Gb3 glycosylamino acid 12 commenced with glycosylation of fluoro-donor 425 with disaccharide acceptor 5, under conditions previously developed in our group,26 to afford the desired perbenzylated trisaccharide 6 in 78% isolated yield (Scheme 1). Dissolving metal reduction of 6 followed by peracetylation afforded 7a (92%, two steps).
Scheme 1.
An Improved Synthesis of Gb3 Glycosylamino Acid
In an earlier disclosure in a related context,27 we had noted that, in the presence of Grubbs 2nd generation catalyst (11), the direct cross-metathesis of the terminal olefins of 7a and 8a had been plagued by the formation of significant quantities of a truncated side product. To circumvent this complication, we had prepared compounds 7b and 8b through cross-metathesis of 7a and 8a with trans-2-butene, in the presence of catalyst 9. These modified substrates underwent cross-metathesis in the presence of catalyst 11.27 It was found that direct olefin cross metathesis of 7a and 8a can in fact be effectively accomplished through the use of the Grubbs-Hoveyda 1st generation catalyst (10)28, to provide the desired adduct, accompanied by only trace amounts of the truncated side product. 29 Hydrogenolysis, using Pt/C under a hydrogen atmosphere, provided the Gb3 glycosylamino acid 12 in 66% yield over two steps. The latter was further coupled with tert-butyl N-(3-aminopropyl)carbamate to provide 13, incorporating the C-terminal partial linker for eventual conjugation to the carrier protein (Scheme 1).
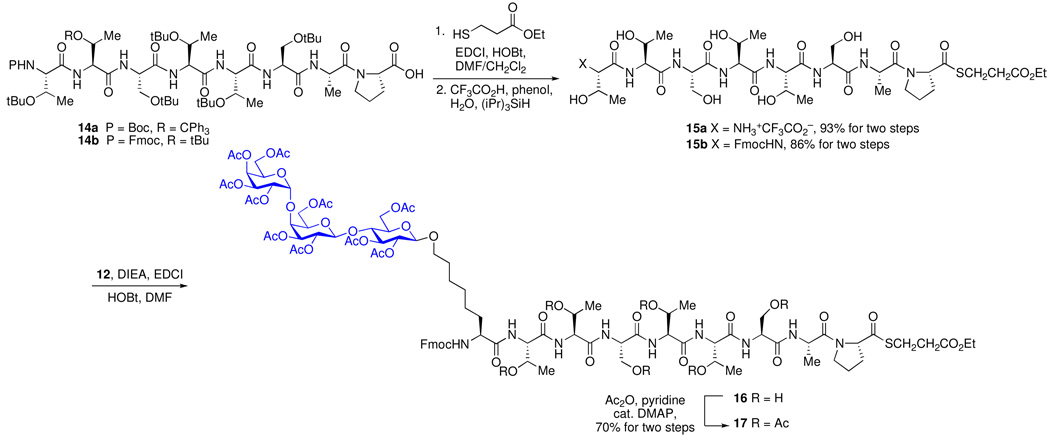
Next, peptides 14a and 14b were prepared through Fmoc solid-phase synthesis using Novabiochem proline-TGT resin. Installation of a C-terminal thioester on both 14a and 14b, followed by standard side-chain deprotection afforded 15a and 15b in 93% and 86% yield over two steps, respectively. Compound 15b was to be a key intermediate for later stage fragment assembly, because the N-terminal Fmoc can be selectively removed in the presence of the N-Boc functionality. Our initial attempts at Fmoc deprotection of 15b afforded the desired free amine, together with significant amounts of the corresponding diketopiperizine. This side reaction presented difficulties in attempts at subsequent separation. We thus prepared compound 15a for coupling with Gb3 glycosylamino acid 12. Standard coupling of 15a with Gb3 glycosylamino acid 12 using EDCI/HOBt afforded compound 16, which was subsequently subjected to peracetylation to furnish the Gb3-MUC5AC cassette 17 (70%, two steps). The acetate protection step facilitated isolation of the product. It will be noted that, in our peptide design, we chose to incorporate an activated L-proline thioester at the C-terminus of the peptide fragment, due to the rather non-racemizable nature of its α-stereocenter. This feature could prove crucial in the subsequent cassette assembly stage.
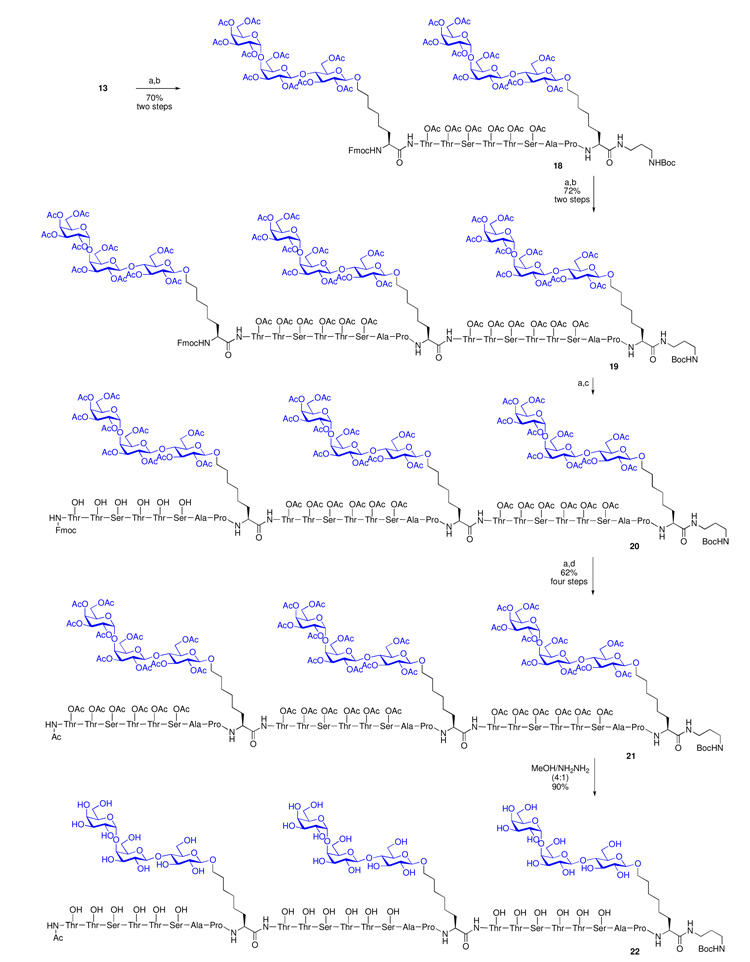
We were then able to devise a slightly modified procedure for Fmoc deprotection, using the relatively volatile diethylamine as a solvent, in lieu of piperidine in DMF (Scheme 3). With this modification, we needed only to remove the volatile reagents and solvents following Fmoc cleavage. The crude free amine thus exposed would be used in the next coupling step without further purification. In the event, Fmoc deprotection of the N-terminus of compound 13 afforded the desired free amine, which was subjected to peptide coupling with Gb3-MUC5AC thioester cassette 17 under the AgCl/HOOBt protocol.30 There was obtained the desired bis-Gb3-MUC5AC intermediate 18 (70% over two steps). This bis-Gb3-MUC5AC 18 was subsequently elongated to produce compound 19, via a two-step sequence involving Fmoc deprotection and subsequent coupling with the Gb3-MUC5AC thioester cassette 17 (72% over two steps). The next task would be that of installing the third MUC5AC peptidyl fragment. In an effort to facilitate a polarity–based separation of the target tris-Gb3-tris-MUC5AC glycopeptide (cf. 20) from other potential side products, we elected to install the final MUC5AC fragment in its deprotected, free hydroxyl form.
Scheme 3.
Synthesis of Construct 22.
(a) 5% Et2NH in DMF; (b) 17, AgCl, HOOBt, iPr2NEt, DMSO; (c) 15b, AgCl, HOOBt, iPr2NEt, DMSO; (d) Ac2O, cat. DMAP, pyridine.
Thus, as outlined in Scheme 3, Fmoc cleavage of tris-Gb3-bis-MUC5AC compound 19, followed by coupling with the deprotected MUC5AC thioester, 15b, afforded the desired tris-Gb3-tris-MUC5AC adduct, 20. As expected, glycopeptide 20 was readily separated from other side products. Next, N-terminal Fmoc cleavage followed by peracetylation furnished the desired clustered Gb3-MUC5AC construct 21 (62% over four steps). Thus, through the use of the Gb3-MUC5AC thioester cassette 17, we were indeed able to assemble, in a convergent manner, ample quantities of the clustered vaccine construct 21. Global deprotection of 21 using NH2NH2/MeOH (1:4, v/v) afforded the target fully synthetic clustered Gb3-MUC5AC construct 22 (90%). Biological evaluations of conjugate 22 are expected in the near future.
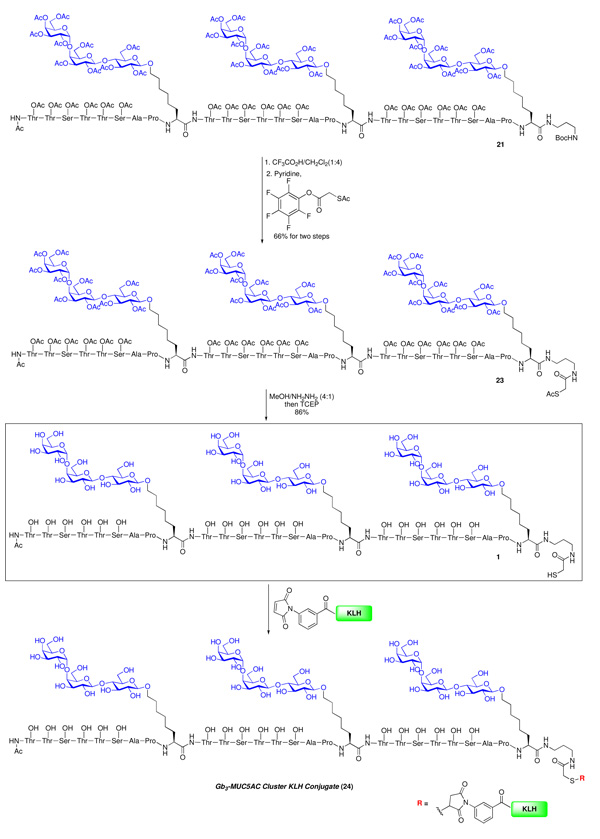
The final objective would be that of installing an appropriate handle for conjugation to the KLH carrier protein. Toward this end, 21 was treated with trifluoroacetic acid in dichloromethane to cleave the Boc carbamate functionality. Next, direct amidation with activated S-acetylthioglycolic acid pentafluorophenyl ester (SAMA-OPfp), provided 23 in 66% yield for two steps.10 Final global deprotection of 23 using NH2NH2/MeOH (1:4, v/v)31 afforded the desired construct 1 together with some of the corresponding dimmer, presumably arising from disulfide formation. This mixture was then subjected to reduction with tris(2-carboxyethyl)phosphine (TCEP) to provide vaccine construct 1 (86% yield)‥
The corresponding KLH conjugate 24 was prepared via 1 in two steps. The first involved activation of the carrier protein KLH with sulfo-MBS (m-maleimidobenzoyl-N-hydroxysuccinimide). This was followed by subsequent addition of the terminating thiol on the glycopeptide 1 (in a presumed Michael fashion) to the maleimide olefin center of the activated carrier protein (Scheme 4). 32 The ratio of glycopeptide-to-protein for KLH conjugate 24, as determined by hydrolytic carbohydrate analysis 33 and standard protein analysis (Bio-Rad dye-binding method) was ca. 698:1. This gratifyingly high ratio of construct incorporation into the carrier presumably reflects the steric accessibility of the linking thiol function in 1, as well as improved conjugation techniques. This phase of the synthesis is summarized in Scheme 3 and Scheme 4.
Scheme 4.
Synthesis of Vaccine Construct 1 and KLH conjugate 24.
In conclusion, we have designed and synthesized a vaccine construct targeting ovarian carcinoma, which consists of clusters of Gb3 carbohydrate antigen and MUC5AC peptide marker. The efficient synthesis was enabled by the preparation of a Gb3-MUC5AC thioester cassette as a key building block for constructing three alternating repeats of Gb3 and MUC5AC. Both non-conjugate and KLH-conjugate vaccine candidates have been prepared and the results of immunological evaluations will be forthcoming.
We note in passing that the capacity to build homogeneous structures such as 24 and 1 in a laboratory is convincing testimony of the awesome power of chemical synthesis. Clearly no such specified structures are available through strictly biological means. Total chemical synthesis can now be employed to enhance the performance of molecules (such as vaccines!) which had hitherto been seen as “biologics”. 34 The accessibility of “biologics” to the systematics of SAR-based medicinal chemistry is indeed an exciting prospect.
Supplementary Material
Experimental procedures, NMR spectra, and characterization for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Scheme 2.
Synthesis of Gb3-MUC5AC Cassette (17).
Acknowledgments
Support for this work was provided by the National Institutes of Health (CA28824 to SJD and PO1CA052477 to POL). A postdoctoral fellowship is gratefully acknowledged by Q.W. (Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research, and the Experimental Therapeutics Center, SKI). Special thanks go to Rebecca Wilson for editorial consultation, and to Dana Ryan and Monica Gonzalez for their assistance with the preparation of the manuscript. We thank Dr. Jin Chen for helpful discussions. We thank Dr. George Sukenick, Ms. Hui Fang, and Ms. Sylvi Rusli of the Sloan-Kettering Institute’s NMR core facility for mass spectral and NMR spectroscopic analysis.
References
- 1.Le Poole IC, Gerberi MAT, Kast WM. Curr. Opin. Oncol. 2002;14:641–648. doi: 10.1097/00001622-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 2.(a) Ingale S, Wolfert MA, Gaekwad J, Buskas T, Boons G-J. Nat. Chem. Biol. 2007;3:663–667. doi: 10.1038/nchembio.2007.25. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Buskas T, Ingale S, Boons G-J. Angew. Chem., Int. Ed. 2005;44:5985–5988. doi: 10.1002/anie.200501818. [DOI] [PubMed] [Google Scholar]
- 3.(a) Kunz H, Dziadek S, Wittrock S, Becker T. ACS Symposium Series. 2008;989:293–310. (Carbohydrate-Based Vaccines) [Google Scholar]; (b) Westerlind U, Hobel A, Gaidzik N, Schmitt E, Kunz H. Angew. Chem., Int. Ed. 2008;47:7551–7556. doi: 10.1002/anie.200802102. [DOI] [PubMed] [Google Scholar]; (c) Wittrock S, Becker T, Kunz H. Angew. Chem., Int. Ed. 2007;46:5226–5230. doi: 10.1002/anie.200700964. [DOI] [PubMed] [Google Scholar]; (d) Dziadek S, Brocke C, Kunz H. Chem. Eur. J. 2004;10:4150–4162. doi: 10.1002/chem.200400228. [DOI] [PubMed] [Google Scholar]
- 4.(a) Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, Schmidt R, Harris Adrian L, Old L, Cerundolo V. J. Immunol. 2003;171:5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]; (b) Schmidt RR, Castro-Palomino JC, Retz O. Pure Appl. Chem. 1999;71:729–744. [Google Scholar]
- 5.(a) Danishefsky SJ, Allen JR. Angew. Chem., Int. Ed. 2000;39:836–863. doi: 10.1002/(sici)1521-3773(20000303)39:5<836::aid-anie836>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]; (b) Keding SJ, Danishefsky SJ. Carbohydrate-Based Drug Discovery. 2003;1:381–406. [Google Scholar]; (c) Ouerfelli O, Warren JD, Wilson RM, Danishefsky SJ. Expert Rev. Vaccines. 2005;4:677–685. doi: 10.1586/14760584.4.5.677. [DOI] [PubMed] [Google Scholar]; (d) Warren JD, Geng X, Danishefsky SJ. Top Curr. Chem. 2007;267:109–141. [Google Scholar]; (e) Wilson RM, Warren JD, Ouerfelli O, Danishefsky SJ. ACS Symposium Series. 2008;989:258–292. (Carbohydrate-Based Vaccines) [Google Scholar]
- 6.(a) Ragupathi G, Park TK, Zhang SL, Kim IJ, Graber L, Adluri S, Lloyd KO, Danishefsky SJ, Livingston PO. Angew. Chem., Int. Ed. Engl. 1997;36:125–128. [Google Scholar]; (b) Slovin SF, Ragupathi G, Adluri S, Ungers G, Terry K, Kim S, Spassova M, Bornmann WG, Fazzari M, Dantis L, Olkiewicz K, Lloyd KO, Livingston PO, Danishefsky SJ, Scher HI. Proc. Natl. Acad. Sci. U.S.A. 1999;96:5710–5715. doi: 10.1073/pnas.96.10.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gilewski T, Ragupathi G, Bhuta S, Williams LJ, Musselli C, Zhang X-F, Bencsath KP, Panageas KS, Chin J, Hudis CA, Norton L, Houghton AN, Livingston PO, Danishefsky SJ. Proc. Natl. Acad. Sci. U.S.A. 2001;98:3270–3275. doi: 10.1073/pnas.051626298. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Krug LM, Ragupathi G, Hood C, Kris MG, Miller VA, Allen JR, Keding SJ, Danishefsky SJ, Gomez J, Tyson L, Pizzo B, Baez V, Livingston PO. Clin. Cancer Res. 2004;10:6094–6100. doi: 10.1158/1078-0432.CCR-04-0482. [DOI] [PubMed] [Google Scholar]; (e) Sabbatini PJ, Kudryashov V, Ragupathi G, Danishefsky SJ, Livingston PO, Bornmann W, Spassova M, Zatorski A, Spriggs D, Aghajanian C, Soignet S, Peyton M, O'Flaherty C, Curtin J, Lloyd KO. Int. J. Cancer. 2000;87:79–85. [PubMed] [Google Scholar]
- 7.Carlstedt I, Davies JR. Biochem. Soc. Trans. 1997;25:214. doi: 10.1042/bst0250214. [DOI] [PubMed] [Google Scholar]
- 8.Kuduk SD, Schwarz JB, Chen X-T, Glunz PW, Sames D, Ragupathi G, Livingston PO, Danishefsky SJ. J. Am. Chem. Soc. 1998;120:12474–12485. [Google Scholar]
- 9.(a) Zhang S, Cordon-Cardo C, Zhang HS, Reuter VE, Adluri S, Hamilton WB, Lloyd KO, Livingston PO. Int. J. Cancer. 1997;73:42–49. doi: 10.1002/(sici)1097-0215(19970926)73:1<42::aid-ijc8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]; (b) Zhang S, Zhang HS, Cordon-Cardo C, Reuter VE, Singhal AK, Lloyd KO, Livingston PO. Int. J. Cancer. 1997;73:50–56. doi: 10.1002/(sici)1097-0215(19970926)73:1<50::aid-ijc9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 10.For the first unimolecular pentavalent vaccine, see: Keding SJ, Danishefsky SJ. Proc. Natl. Acad. Sci. USA. 2004;101:11937–11942. doi: 10.1073/pnas.0401894101. For the synthesis of the unimolecular pentavalent vaccine shown in Figure 1, see: Ragupathi G, Koide F, Livingston PO, Cho YS, Atsushi E, Wan Q, Spassova MK, Keding SJ, Allen J, Ouerfelli O, Wilson RM, Danishefsky SJ. J. Am. Chem. Soc. 2006;128:2715–2725. doi: 10.1021/ja057244+.
- 11.Livingston PO, Ragupathi G. Human Vaccines. 2006;2:137–143. doi: 10.4161/hv.2941. [DOI] [PubMed] [Google Scholar]
- 12.(a) Van den Steen P, Rudd PM, Dwek RA, Opdenakker G. Crit. Rev. Biochem. Mol. Biol. 1998;33:151–208. doi: 10.1080/10409239891204198. [DOI] [PubMed] [Google Scholar]; (b) Brockhausen I. In: Glycoproteins. Montreuil J, Vliegenthart JFG, Schachter H, editors. New York: Elsevier Science; 1995. pp. 201–259. [Google Scholar]
- 13.Zhang S, Zhang HS, Cordon-Cardo C, Ragupathi G, Livingston PO. Clin. Cancer Res. 1998;4:2669–2676. [PubMed] [Google Scholar]
- 14.(a) Barratt-Boyes SM, Vlad A, Finn OJ. Clin. Cancer Res. 1999;5:1918–1924. [PubMed] [Google Scholar]; (b) Hiltbold EM, Ciborowski P, Finn OJ. Cancer Res. 1998;58:5066–5070. [PubMed] [Google Scholar]; (c) Kocer B, McKolanis J, Soran A. BMC gastroenterology. 2006;6:4. doi: 10.1186/1471-230X-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Bondurant KL, Crew MD, Santin AD, O'Brien TJ, Cannon MJ. Clin. Cancer Res. 2005;11:3446–3454. doi: 10.1158/1078-0432.CCR-04-2043. [DOI] [PubMed] [Google Scholar]; (e) Cannon MJ, O'Brien TJ, Underwood LJ, Crew MD, Bondurant KL, Santin AD. Expert Rev. Anticancer Ther. 2002;2:97–105. doi: 10.1586/14737140.2.1.97. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, Graeber LA, Helling F, Ragupathi G, Adluri S, Lloyd KO, Livingston PO. Cancer Res. 1996;56:3315–3319. [PubMed] [Google Scholar]
- 16.Merck Manual of Diagnosis and Therapy Section 18. Gynecology And Obstetrics Chapter 241. Gynecologic Neoplasms.
- 17.Yin BW, Finstad CL, Kitamura K, Federici MG, Welshinger M, Kudryashov V, Hoskins WJ, Welt S, Lloyd KO. Int. J. Cancer. 1996;65:406. doi: 10.1002/(SICI)1097-0215(19960208)65:4<406::AID-IJC2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S, Zhang HS, Cordon-Cardo C, Reuter VE, Singhal AlK, Lloyd KO, Livingston PO. Int. J. Cancer. 1997;73:50–56. doi: 10.1002/(sici)1097-0215(19970926)73:1<50::aid-ijc9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 19.Livingston PO. Semin. Cancer Biol. 1995;6:357–366. doi: 10.1016/1044-579x(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 20.(a) Kiguchi K, Iwamori Y, Suzuki N, Kobayashi Y, Ishizuka B, Ishiwata I, Kita T, Kikuchi Y, Iwamori M. Cancer Science. 2006;97:1321–1326. doi: 10.1111/j.1349-7006.2006.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lingwood CA, Khine AA, Arab S. Acta Biochimica Polonica. 1998;45:351–359. [PubMed] [Google Scholar]; (c) Arab S, Russel E, Chapman WB, Rosen B, Lingwood CA. Oncology Research. 1997;9:553–563. [PubMed] [Google Scholar]
- 21.Giuntoli RL, II, Rodriguez GC, Whitaker RS, Dodge R, Voynow JA. Cancer Res. 1998;58:5546–5550. [PubMed] [Google Scholar]
- 22.Yin BW, Lloyd KO. J. Biol. Chem. 2001;276:27371–27375. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- 23.Kilberg J, Elofsson M. Curr. Med. Chem. 1997;4:85. [Google Scholar]
- 24.Glunz PW, Hintermann S, Williams LJ, Schwarz JB, Kuduk SD, Kudryashov V, Lloyd KO, Danishefsky SJ. J. Am. Chem. Soc. 2000;122:7273. [Google Scholar]
- 25.Fluoro donor 4 was prepared in 81% yield (α:β = 1:1.4) by treatment of commercial available 2,3,4,6-tetra-O-benzyl-D-galactopyranose with DAST (diethylaminosulfur trifluoride) in THF. For a representative example of synthesis of fluoro donor 4, see: Nicolaou KC, Caulfield T, Kataoka H, Kumazawa T. J. Am. Chem. Soc. 1988;110:7910–7912.
- 26.Allen JR, Allen JG, Zhang X-F, Williams LJ, Zatorski A, Ragupathi G, Livingston PO, Danishefsky SJ. Chem. Eur. J. 2000;6:1366–1375. doi: 10.1002/(sici)1521-3765(20000417)6:8<1366::aid-chem1366>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 27.Wan Q, Cho YS, Lambert TH, Danishefsky SJ. J. Carbohydr. Chem. 2005;24:425–440. [Google Scholar]
- 28.Kingsbury JS, Harrity JPA, Bonitatebus PJ, Jr, Hoveyda AH. J. Am. Chem. Soc. 1999;121:791. [Google Scholar]
- 29.Zhu J, Wan Q, Yang G, Ouerfelli O, Danishefsky SJ. Heterocycles. 2009;79 doi: 10.3987/COM-08-S(D)82. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawakami T, Yoshimura S, Aimoto S. Tetrahedron Lett. 1998;39:7901–7904. [Google Scholar]
- 31.Allen JR, Harris CR, Danishefsky SJ. J. Am. Chem. Soc. 2001;123:1890–1897. doi: 10.1021/ja002779i. [DOI] [PubMed] [Google Scholar]
- 32.Zhang S, Graeber LA, Helling F, Ragupathi G, Adluri S, Lloyd KO, Livingston PO. Cancer Res. 1996;56:3315–3319. [PubMed] [Google Scholar]
- 33.(a) Lloyd KO, Savage A. Glycoconjugate J. 1991;8:439. doi: 10.1007/BF00769849. [DOI] [PubMed] [Google Scholar]; (b) Hardy MR, Townsend RR. Proc. Natl. Acad. Sci. USA. 1988;85:3289. doi: 10.1073/pnas.85.10.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson RM, Danishefsky SJ. Pure Appl. Chem. 2007;79:2189–2216. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures, NMR spectra, and characterization for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.