Abstract
Human imaging studies show that psychostimulants such as cocaine produce functional changes in several areas of cortex and striatum. These may reflect neuronal changes related to addiction. We employed gene markers (zif 268, homer 1a) that offer a high anatomical resolution to map cocaine-induced changes in 22 cortical areas and 23 functionally related striatal sectors, in order to determine the corticostriatal circuits altered by repeated cocaine exposure (25 mg/kg, 5 days). Effects were investigated 1 day and 21 days after repeated treatment to assess their longevity. Repeated cocaine treatment increased basal expression of zif 268 predominantly in sensorimotor areas of the cortex. This effect endured for 3 weeks in some areas. These changes were accompanied by attenuated gene induction by a cocaine challenge. In the insular cortex, the cocaine challenge produced a decrease in zif 268 expression after the 21-day, but not 1-day, withdrawal period. In the striatum, cocaine also affected mostly sensorimotor sectors. Repeated cocaine resulted in blunted inducibility of both zif 268 and homer 1a, changes that were still very robust 3 weeks later. Thus, our findings demonstrate that cocaine produces robust and long-lasting changes in gene regulation predominantly in sensorimotor corticostriatal circuits. These neuronal changes were associated with behavioral stereotypies, which are thought to reflect dysfunction in sensorimotor corticostriatal circuits. Future studies will have to elucidate the role of such neuronal changes in psychostimulant addiction.
Keywords: cortex, striatum, psychostimulant, immediate-early gene, dopamine
INTRODUCTION
Drug addiction is associated with neuronal changes in specific parts of the brain. Imaging studies demonstrated that repeated exposure to psychostimulants produces functional changes in brain regions such as the cerebral cortex and the basal ganglia (e.g., London et al., 1990; Breiter et al., 1997; Beveridge et al., 2006; Porrino et al., 2007). Interactions between the cortex and the basal ganglia are critical for the organization of normal motivated behavior (Albin et al., 1989; DeLong, 1990; Robbins et al., 1998). These interactions are mediated by distinct anatomical loops that arise in all parts of the cortex, project in a topographical manner to specific functional domains of the striatum, and from there, via basal ganglia output nuclei and thalamus, back to the cortex (Alexander et al., 1986; 1990; Groenewegen et al., 1990). Identification of the functional loops and their neuronal processes that are altered by psychostimulants will further our understanding of the addiction process and guide treatment approaches.
Various studies identified psychostimulant-induced molecular changes in the basal ganglia, especially the striatum (e.g., Harlan & Garcia, 1998; Berke & Hyman, 2000; Yano & Steiner, 2007). Most previous work focused on limbic areas, which mediate motivational processes (Pierce & Kalivas, 1997) and are thus considered of central importance in early addiction stages. However, imaging studies indicate that, as the disease progresses, associational and sensorimotor domains of the striatum are increasingly affected (Porrino et al., 2007). These domains are implicated in habitual and compulsive aspects of drug taking (Berke & Hyman, 2000; Everitt & Robbins, 2005) and indeed display particularly robust psychostimulant-induced molecular changes (e.g., Steiner & Gerfen, 1993; Badiani et al., 1998; Willuhn et al., 2003; Yano & Steiner, 2005b).
Much less is known regarding molecular changes in the cortex after psychostimulant exposure. Several studies documented effects in limbic-related prefrontal areas (e.g., Freeman et al., 2002; Black et al., 2006), but there is evidence for changes in several other cortical areas as well. For example, we recently showed that the psychostimulant methylphenidate induces immediate-early genes (IEGs) such as zif 268 and homer 1a in a widespread but regionally selective manner, including many sensory and motor cortical areas (Yano & Steiner, 2005a; Cotterly et al., 2007). Cocaine and amphetamine have also been found to induce IEGs in the sensorimotor cortex (e.g., Curran et al., 1996; Badiani et al., 1998), but the regional specificity of these effects remains unclear.
In the present study, we mapped changes in IEG expression induced by acute and repeated cocaine treatment throughout the cortex (22 cortical areas on 4 rostrocaudal levels) and compared these effects with the distribution of striatal gene regulation (23 striatal sectors) to determine the corticostriatal circuits affected. For comparison with our previous studies (Yano & Steiner, 2005a; Cotterly et al., 2007), we assessed the IEGs zif 268 and homer 1a. These genes serve as functional markers (Sharp et al., 1993; Chaudhuri, 1997), but are also of interest because of their direct involvement in neuroplasticity; zif 268 encodes a transcription factor (Knapska & Kaczmarek, 2004) and homer 1a a synaptic plasticity regulator (Xiao et al., 2000).
MATERIALS AND METHODS
Subjects
Male Sprague–Dawley rats (175–200 g at the beginning of the experiment; Harlan, Madison, WI, USA) were housed 2 per cage under standard laboratory conditions (12:12-hr light/dark cycle; lights on at 0700 h) with food and water available ad libitum. The experiments were performed between 1300 and 1700 h. All procedures met the NIH guidelines for the care and use of laboratory animals and were approved by the Rosalind Franklin University Animal Care and Use Committee.
Drug treatments
Before the start of the pharmacological treatment, rats were repeatedly handled on three days. The animals then received an injection of vehicle or cocaine (cocaine hydrochloride; Sigma, St. Louis, MO, USA; 25 mg/kg, in 0.02% ascorbic acid, i. p.) once daily for 5 days, in their home cage. On days 6 (withdrawal day 1) or 26 (withdrawal day 21), the animals were transferred in their home cage to an adjacent room and, 3 hours later, received a challenge injection of cocaine (25 mg/kg) or vehicle (groups VV, CV, VC, CC; n=7–9). After the injection, the rat was placed in the arena of an activity monitoring system (43 × 43 cm; Truscan, Coulbourn Instruments, Allentown, PA, USA), and locomotion (ambulatory distance) and stereotypy (“stereotypy 2”) counts were measured for 30 min. These “stereotypy” counts reflect local, repetitive movements (e.g., head bobbing, focused sniffing). Animals of the 21-day withdrawal groups were handled every third day between repeated drug treatment and challenge injection.
Tissue preparation and in situ hybridization histochemistry
The rats were killed with CO2 30 min after the challenge injection. The brain was rapidly removed, frozen in isopentane cooled on dry ice and then stored at −30 °C until cryostat sectioning. Coronal sections (12 μm) were thaw-mounted onto glass slides (Superfrost/Plus, Daigger, Wheeling, IL, USA), dried on a slide warmer and stored at −30 °C. In preparation for the in situ hybridization histochemistry, the sections were fixed in 4% paraformaldehyde/0.9% saline for 10 min at room temperature, incubated in a fresh solution of 0.25% acetic anhydride in 0.1 M triethanolamine/0.9% saline (pH 8.0) for 10 min, dehydrated, defatted for 2 × 5 min in chloroform, rehydrated, and air-dried. The slides were then stored at −30 °C until hybridization.
Oligonucleotide probes (48-mers; Invitrogen, Rockville, MD, USA) were labeled with [35S]-dATP as described earlier (Steiner & Kitai, 2000). The probes had the following sequence: zif 268, complementary to bases 352–399, GenBank accession number M18416; homer 1a, bases 163–210, NM_031707. The latter targets the beginning of the homer 1 transcript, which yields a more robust signal at this timepoint (Bottai et al., 2002). Previous findings indicate that the cocaine-induced signal measured with this probe reflects homer 1a expression (Brakeman et al., 1997; Bottai et al., 2002; Zhang et al., 2007).
One hundred μl of hybridization buffer containing labeled probe (~3 x 106 cpm) was added to each slide. The sections were coverslipped and incubated at 37 °C overnight. After incubation, the slides were first rinsed in four washes of 1X saline citrate (150 mM sodium chloride, 15 mM sodium citrate), and then washed 3 times 20 min each in 2X saline citrate/50% formamide at 40 °C, followed by 2 washes of 30 min each in 1X saline citrate at room temperature. After a brief water rinse, the sections were air-dried and then apposed to X-ray film (BioMax MR-2, Kodak) for 4–10 days.
Analysis of autoradiograms
Gene expression in the cortex was assessed in sections from 4 rostrocaudal levels (see Fig. 4): frontal, approximately at +2.7 mm relative to bregma (Paxinos & Watson, 1998); rostral, +1.6; middle, +0.4; caudal, −0.8 (Yano & Steiner, 2005a). Levels of mRNA were measured in a total of 22 cortical regions (from medial to lateral; Paxinos & Watson, 1998): cingulate, medial agranular (M2), motor (M1), somatosensory and insular cortex on frontal to caudal levels, and infralimbic, prelimbic and lateral orbital cortex on the frontal level. Striatal gene expression was determined on rostral, middle and caudal levels in a total of 23 sectors mostly defined by their predominant cortical inputs (Fig. 8, Table 1; Willuhn et al., 2003). Eighteen of these sectors represented the caudate-putamen and 5 the nucleus accumbens (medial and lateral core, medial, ventral and lateral shell) (Yano & Steiner, 2005a).
Table 1.
Functional domains: striatal sectors and their cortical inputs
| level | striatal sector | main cortical input regions |
|---|---|---|
| rostral | dorsolateral | motor (M1), somatosensory |
| dorsal | medial agranular (M2), motor (M1), somatosensory | |
| dorsomedial | cingulate | |
| medial | cingulate, prelimbic | |
| ventral | insular, lateral orbital | |
| NAC medial core | prelimbic | |
| NAC lateral core | insular | |
| NAC medial shell | prelimbic | |
| NAC ventral shell | infralimbic | |
| NAC lateral shell | insular, lateral orbital | |
| middle | medial | cingulate, prelimbic |
| dorsal | medial agranular (M2), motor (M1), somatosensory | |
| dorsolateral | motor (M1), somatosensory | |
| ventrolateral | motor (M1), somatosensory | |
| ventral | insular | |
| central | insular, lateral orbital | |
| caudal | medial | cingulate, prelimbic |
| dorsal | medial agranular (M2), motor (M1), somatosensory | |
| dorsolateral | motor (M1), somatosensory | |
| ventrolateral | motor (M1), somatosensory | |
| ventral | insular | |
| ventral central | insular | |
| dorsal central | insular, lateral orbital |
The function of the 23 striatal sectors (left) is mostly determined by their cortical inputs (simplified, right). These inputs are described and discussed in detail in Willuhn et al. (2003). NAC, nucleus accumbens.
Hybridization signals on film autoradiograms were measured by densitometry (NIH Image; Wayne Rasband, NIMH, Bethesda, MD, USA). The films were captured using a light table (Northern Light, Imaging Research, St. Catharines, Ontario, Canada) and a Sony CCD camera (Imaging Research). The “mean density” value of a region of interest was measured by placing a template over the captured image. Mean densities were corrected for background by subtracting mean density values measured over white matter (corpus callosum). Values from corresponding regions in the two hemispheres were then averaged.
Treatment effects were determined by two-factor ANOVA, followed by Newman-Keuls post hoc tests to describe differences between individual groups (Statistica, StatSoft, Tulsa, OK, USA). For illustrations of topographies (maps) and correlation analyses, the change in gene expression in a given region was expressed as the percentage of the maximal change (% of max.) observed for a particular probe. The illustrations of film autoradiograms displayed in Figure 2 are computer-generated images, and are contrast-enhanced where necessary. Maximal hybridization signal is black.
RESULTS
Behavioral effects
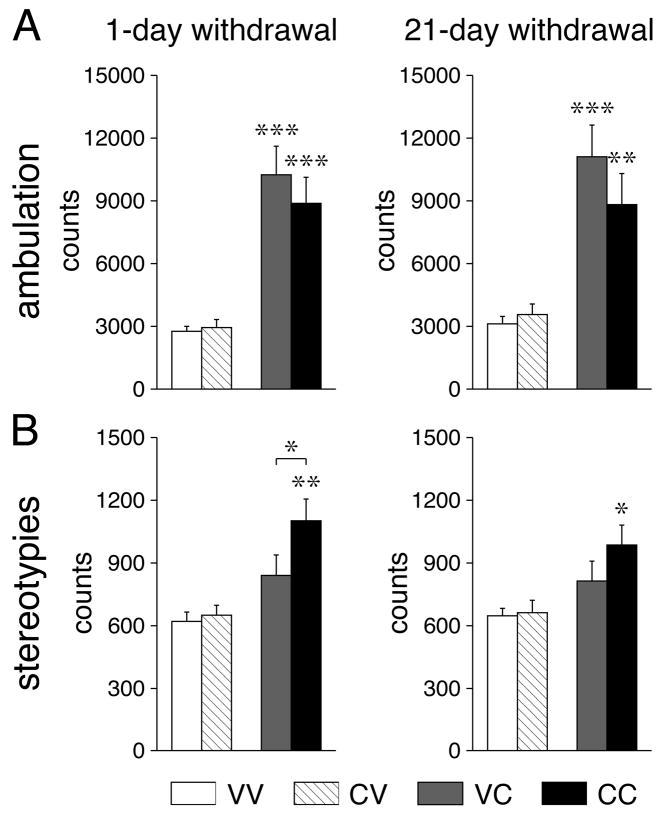
Figure 1 depicts behavioral effects of the cocaine challenge injection (or vehicle) given 1 or 21 days after repeated cocaine or vehicle treatment. Administration of cocaine increased ambulation counts in vehicle-pretreated (VC vs. VV, P<0.001) and in cocaine-pretreated animals (CC vs. CV, P<0.01), an effect that was similar in groups tested on withdrawal days 1 or 21 (Fig. 1A). The cocaine challenge produced significant increases in stereotypy counts in cocaine-pretreated animals (withdrawal day 1: CC vs. CV, P<0.01; CC vs. VC; P<0.05), but this effect was less robust (CC vs. CV, P<0.05) 21 days after repeated cocaine treatment.
Fig 1.
Repeated cocaine treatment increases levels of behavioral stereotypies. Ambulation (A) and stereotypy counts (B) (mean±SEM) are shown for animals that received 5 daily injections of vehicle or cocaine (25 mg/kg, i.p.) followed 1 day (left) or 21 days later (right) by a vehicle injection (groups VV and CV) or a cocaine challenge (25 mg/kg) (groups VC and CC). Ambulatory activity and stereotypies were assessed for 30 min in a novel openfield, immediately after the injection (see Materials and Methods). * P<0.05, ** P<0.01, *** P<0.001 vs. respective vehicle control or as indicated.
Cocaine effects on gene expression in the cortex
Zif 268 expression
Gene expression in the cortex was assessed in a total of 22 areas on four rostrocaudal levels. Absolute density values and challenge-induced values expressed in percentage of basal expression (i.e., in respective vehicle controls) are presented.
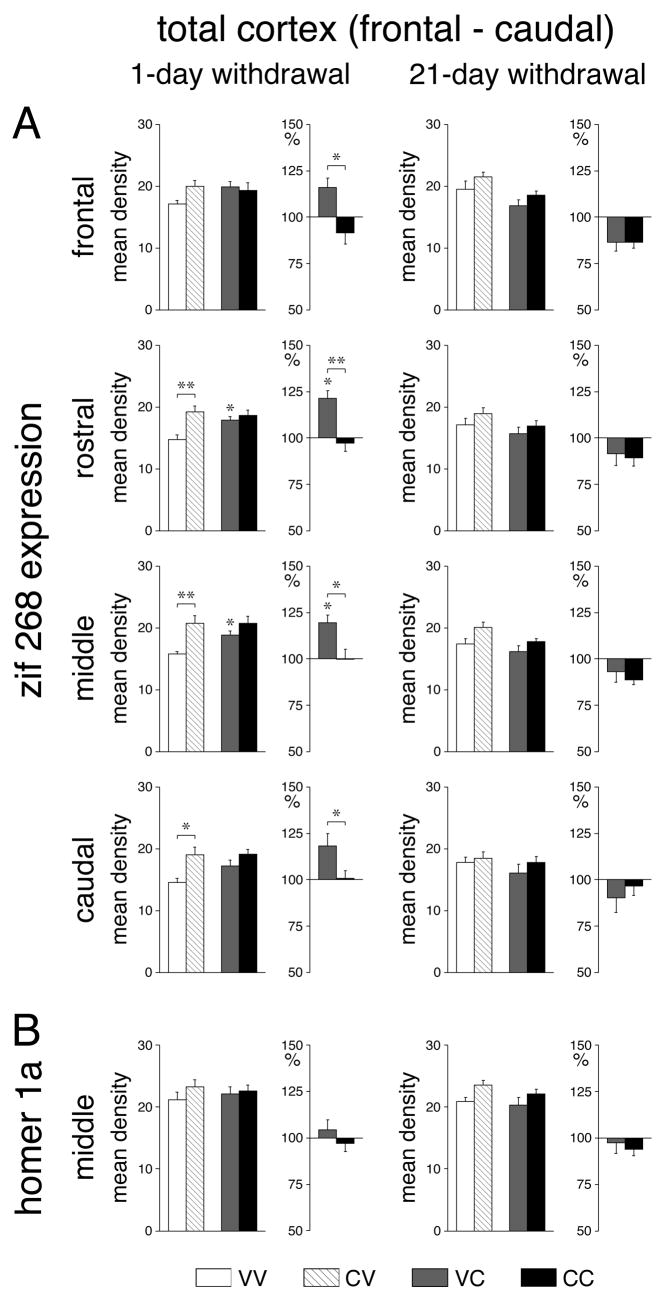
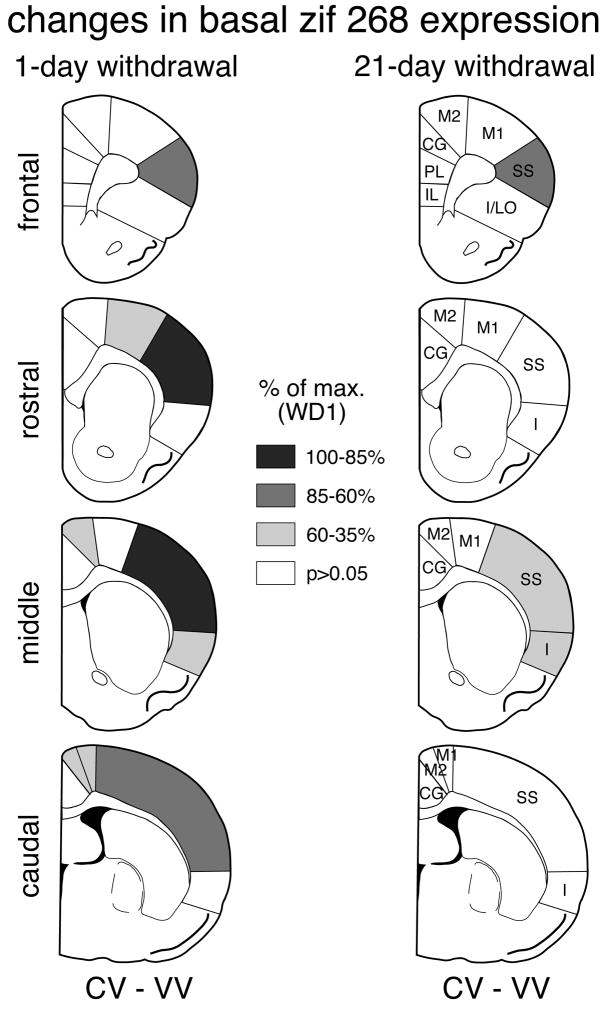
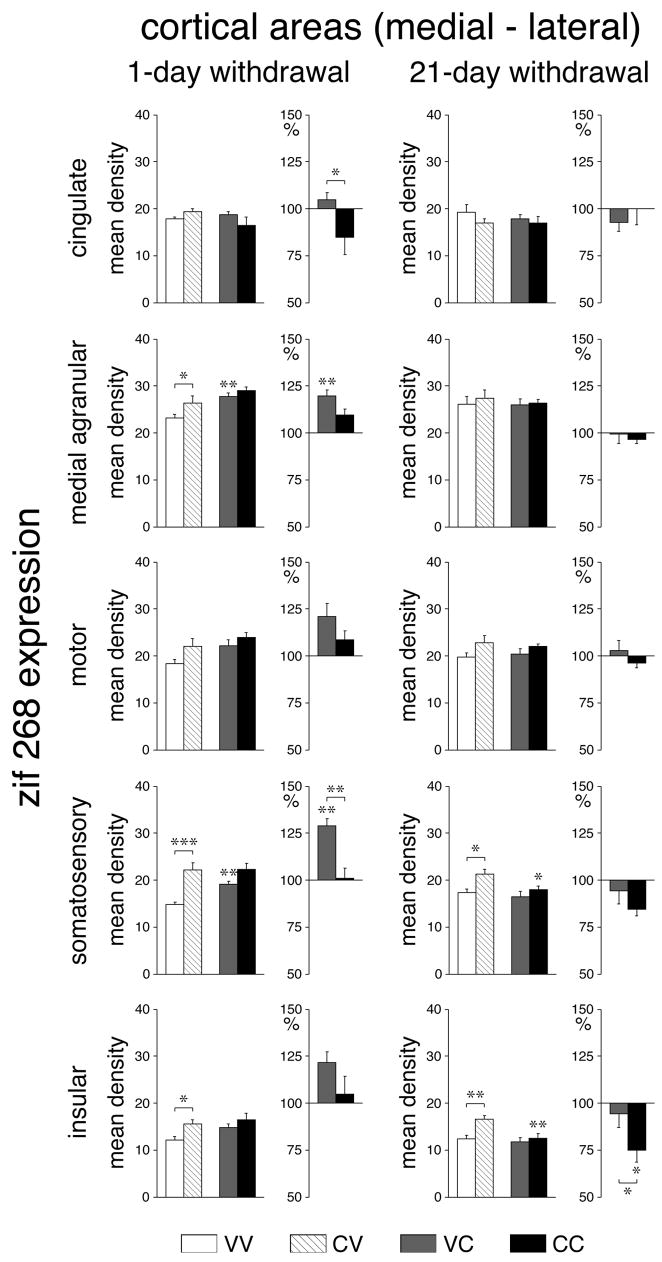
One day after repeated cocaine treatment, “basal” zif 268 expression (CV vs. VV) was significantly increased when measured across all cortical areas (total cortex) on rostral (P<0.01), middle (P<0.01) and caudal (P<0.05) levels (Figs. 2A, 3A). Regional analysis (Figs. 4, 6) showed that this effect was most pronounced in the somatosensory cortex on all 4 levels (frontal, P<0.05; rostral, P<0.001; middle, P<0.001; caudal, P<0.01). Weaker increases were seen in the motor (M1) (rostral, caudal, P<0.01), medial agranular (M2) (middle, caudal, P<0.05), and insular (middle, P<0.05) cortex. After the 21-day withdrawal period, a tendency for increased basal zif 268 expression was still widespread (Fig. 3A), but increased expression was statistically significant only in the somatosensory cortex on the frontal (P<0.01) and middle (P<0.05) levels and in the insular cortex on the middle level (P<0.01) (Figs. 4, 6).
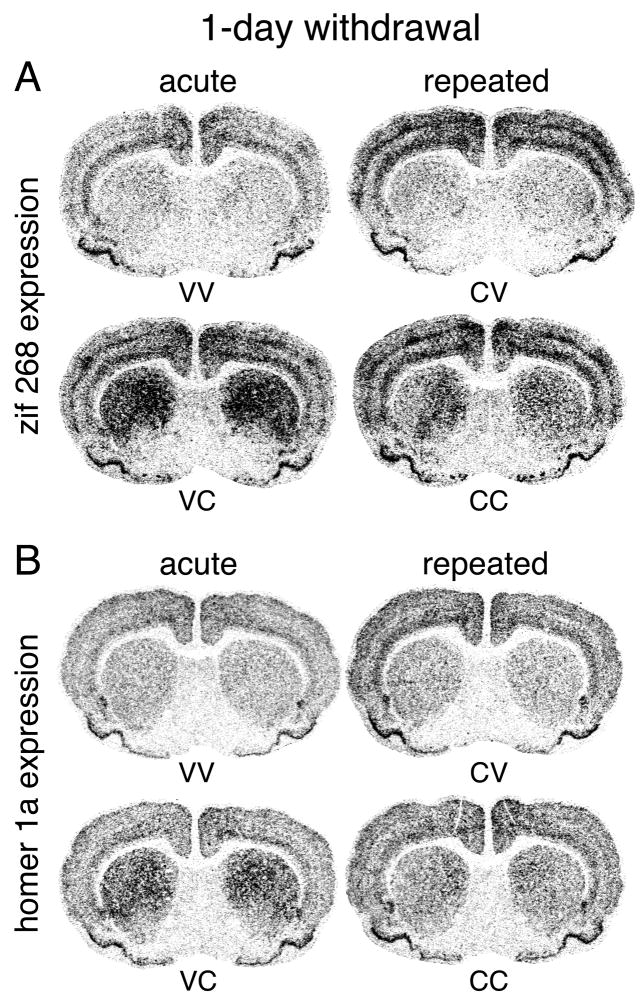
Fig. 2.
Effects of repeated cocaine treatment on IEG expression in the cortex and striatum after 1-day withdrawal. Illustrations of film autoradiograms depict expression of zif 268 (A) and homer 1a (B) in cortex and striatum on the mid-striatal level for rats that received 6 vehicle injections (VV), 5 vehicle injections followed one day later by a cocaine challenge (25 mg/kg) (VC) (acute cocaine), or 5 cocaine injections (25 mg/kg) followed by a vehicle (CV) or a cocaine injection (CC) (repeated cocaine, 1-day withdrawal). Repeated cocaine treatment significantly increased basal zif 268 expression in the cortex (CV vs. VV) (similar trend for homer 1a). In contrast, challenge-induced expression of zif 268 in the cortex and zif 268 and homer 1a in the striatum were attenuated (CC vs. CV compared with VC vs. VV).
Fig. 3.
Repeated cocaine treatment increases basal expression and attenuates challenge-induced expression of zif 268 in the cortex. Effects on zif 268 (A) and homer 1a (B) expression (mean density values; mean±SEM, arbitrary units) in the total cortex on frontal, rostral, middle and caudal levels are shown for rats that were treated with 6 vehicle injections (VV), 5 cocaine injections (25 mg/kg) followed 1 day later (left column) or 21 days later (right column) by a vehicle injection (CV), 5 vehicle injections followed by a cocaine challenge (25 mg/kg) (VC), or 5 cocaine injections followed by a cocaine challenge (CC). Absolute values (left) and relative values (% of vehicle controls, right) are depicted. * P<0.05, ** P<0.01 vs. vehicle controls or as indicated.
Fig. 4.
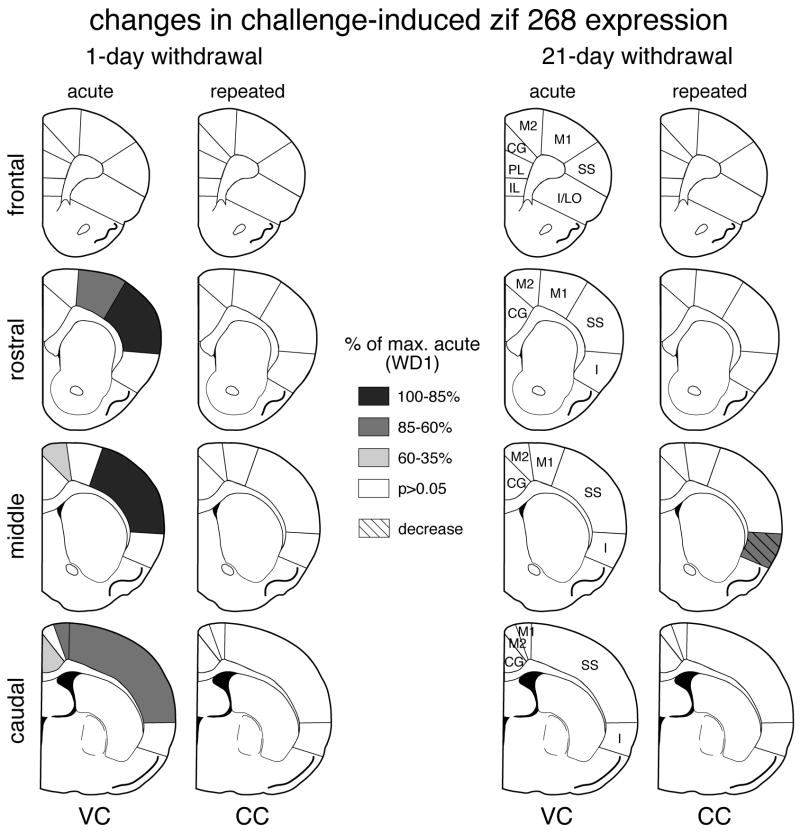
Distribution of increases in basal zif 268 expression in the cortex after repeated cocaine treatment: 1-day vs. 21-day withdrawal. The maps illustrate the differences in zif 268 expression between animals that received 5 cocaine injections followed by a vehicle injection (CV) and controls that received 6 vehicle injection (VV) on frontal, rostral, middle and caudal levels, 1 day (left column) and 21 days (right column) after repeated cocaine administration. The values are expressed relative to the maximal change on withdrawal day 1 (% of max. WD1). Areas with significant differences (P<0.05) are coded as indicated. Areas without significant effects are in white. IL, infralimbic; PL, prelimbic; CG, cingulate; M2, medial agranular; M1, motor; SS, somatosensory; I, insular; I/LO, insular/lateral orbital.
Fig. 6.
Regional specificity of repeated cocaine-induced changes in cortical zif 268 expression: 1-day vs. 21-day withdrawal. Effects on zif 268 expression (mean density values; mean±SEM) in the different cortical areas on the middle level are given for VV, CV, VC and CC groups, 1 day (left column) or 21 days (right column) after repeated treatment. Absolute values (left) and relative values (% of vehicle controls, right) are depicted. * P<0.05, ** P<0.01, *** P<0.001 vs. vehicle controls or as indicated.
Acute cocaine administration produced increased cortical zif 268 expression (VC vs. VV), but only when given 1 day after the repeated vehicle treatment. In this group, zif 268 expression across the total cortex was significantly elevated on the rostral and middle levels (Figs. 2, 3A); the greatest increases were present in the somatosensory cortex (rostral, middle, P<0.01; caudal, P<0.05), followed by the motor cortex (rostral, P<0.001; caudal, P<0.05), the medial agranular cortex (middle, P<0.01) and the cingulate cortex (caudal, P<0.05) (Figs. 5, 6). In contrast, no statistically significant changes in cortical zif 268 expression were observed when the acute cocaine challenge was administered 21 days after repeated vehicle treatment (Fig. 3A, 5, 6).
Fig. 5.
Distribution of changes in cocaine challenge-induced zif 268 expression in the cortex after repeated cocaine treatment: 1-day vs. 21-day withdrawal. The maps depict changes in zif 268 expression on frontal, rostral, middle and caudal levels after acute (VC) and repeated (CC) cocaine administration, 1 day (left column) and 21 days (right column) after repeated treatment. The value in a given region was first expressed as the percentage of that in vehicle controls, and was then normalized relative to the maximal change in the acute group on withdrawal day 1 (% of max. acute, WD1). Areas with significant differences (P<0.05) are coded as indicated. Areas without significant effects are in white. IL, infralimbic; PL, prelimbic; CG, cingulate; M2, medial agranular; M1, motor; SS, somatosensory; I, insular; I/LO, insular/lateral orbital.
The effects of repeated cocaine treatment on cortical zif 268 expression were also dependent on the withdrawal period. Animals that received the cocaine challenge injection 1 day after repeated cocaine treatment showed no significant changes in any cortical area (CC vs. CV) (Figs. 2A, 3A, 5, 6). When the zif 268 responses to acute (VC) and repeated (CC) cocaine treatments were expressed relative to their baseline controls (VV and CV, respectively), repeatedly treated animals (CC) displayed significantly reduced zif 268 responses compared with acute responses (VC) on all 4 rostrocaudal levels (total cortex, Fig. 3A). Significant decreases were found in 9 of the 22 cortical areas and were again most robust in the somatosensory (rostral, middle, P<0.01) and motor cortex (rostral, P<0.01) (Figs. 5, 6). When the cocaine challenge was given after the 21-day withdrawal period, 21 of the 22 cortical areas showed a tendency for lower zif 268 mRNA levels than baseline (CC vs. CV) (Fig. 3A), an effect that was statistically significant in the somatosensory cortex on the frontal and rostral levels (P<0.05, data not shown) and in the somatosensory cortex (P<0.05) and insular cortex (P<0.01) on the middle level (Fig. 6). When expressed relative to baseline, these animals (CC) displayed a significantly lower zif 268 response than acute animals (VC) in the middle insular cortex (P<0.05) (Fig. 6).
Homer 1a expression
Overall, these cocaine treatments had only modest effects on the expression of homer 1a in the cortex (Fig. 2B, 3B). Effects on basal homer 1a expression were similar in direction and location to effects on basal zif 268 expression. One day after repeated cocaine treatment, basal homer 1a expression was significantly increased (CV vs. VV) in the rostral somatosensory cortex (P<0.05, data not shown), the same area that showed the most robust increase in zif 268 expression (see above). After the 21-day withdrawal period, significant increases in basal homer 1a expression were found in the somatosensory and motor cortex on the middle level (P<0.05) (data not shown). Neither acute cocaine (VC vs. VV) nor a challenge after repeated cocaine treatment (CC vs. CV) produced consistent changes in homer 1a expression in these cortical areas, either 1 or 21 days after the repeated treatment (data not shown).
Cocaine effects on gene expression in the striatum
Zif 268 expression
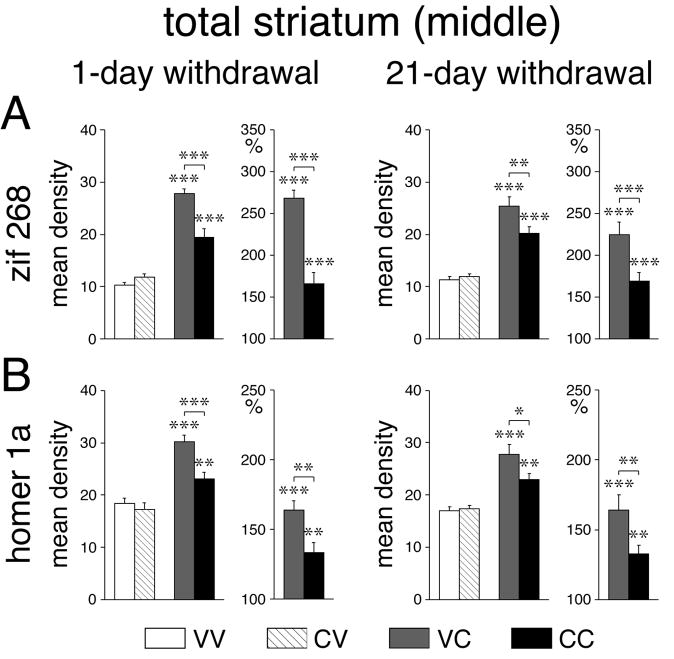
Gene expression in the striatum was measured in a total of 23 sectors on three rostrocaudal levels. While basal zif 268 expression in the striatum tended to be increased in a majority of sectors, this effect reached statistical significance only in the dorsolateral (sensorimotor) sector on the caudal level (CV vs. VV, P<0.05, data not shown), one day after repeated cocaine treatment.
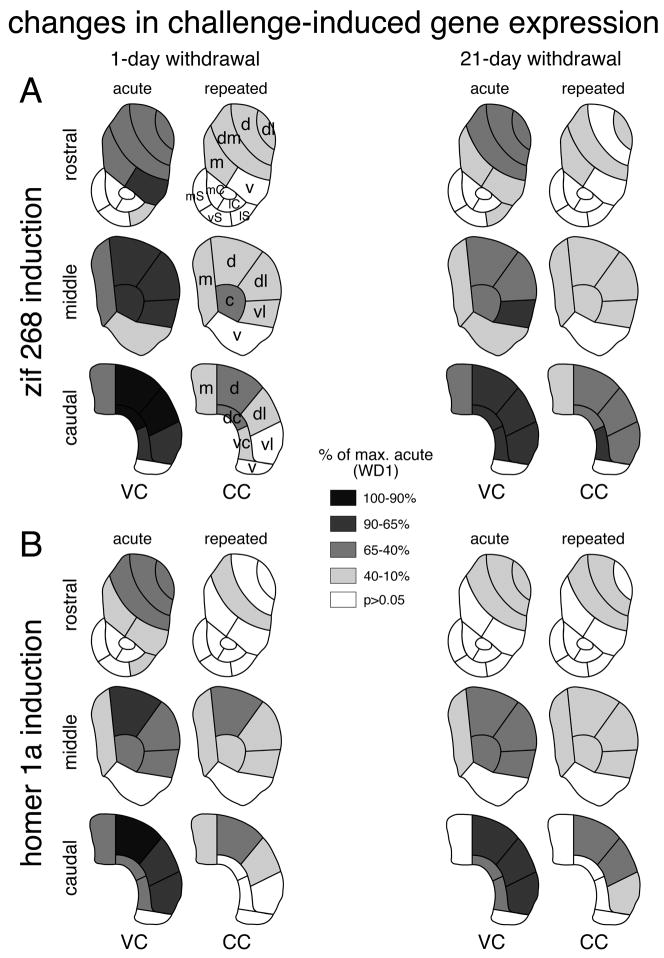
When given 1 day after the repeated vehicle treatment, acute cocaine (VC) induced highly significant (P<0.001) increases in zif 268 expression (VC vs. VV) in all but 2 of the 18 sectors representing the caudate-putamen (Figs. 2A, 7A, 8A), the exceptions being the ventral sectors on the middle (P<0.01) and caudal (P>0.05) levels (Fig. 8A). While these effects were present on all rostrocaudal levels, they tended to increase from rostral towards caudal and from medial towards lateral, and were most robust in the caudal sensorimotor striatum (Fig. 8A). In the 5 sectors representing the nucleus accumbens (rostral), a significant increase in zif 268 expression was only seen in the lateral shell (P<0.01) (Fig. 8A). The acute cocaine challenge administered 21 days after repeated vehicle treatment (VC) produced zif 268 induction with principally the same regional distribution (16/18 sectors, P<0.001; middle ventral, P<0.05; caudal ventral, P>0.05; lateral shell, P<0.05) (Fig. 8A), but the magnitude of the zif 268 response tended to be somewhat smaller (Figs. 7A, 8A).
Fig. 7.
Repeated cocaine treatment blunts inducibility of zif 268 and homer 1a in the striatum. Effects on zif 268 (A) and homer 1a (B) expression (mean density values; mean±SEM) in the total striatum on the middle level are shown for VV, CV, VC and CC groups, 1 day (left column) or 21 days (right column) after repeated treatment. Absolute values (left) and relative values (% of vehicle controls, right) are depicted. * P<0.05, ** P<0.01, *** P<0.001 vs. vehicle controls or as indicated.
Fig. 8.
Distribution of changes in cocaine challenge-induced zif 268 and homer 1a expression in the striatum after repeated cocaine treatment: 1-day vs. 21-day withdrawal. The maps illustrate changes in zif 268 (A) and homer 1a (B) expression in the rostral, middle and caudal striatum after acute (VC) and repeated (CC) cocaine administration, 1 day (left column) and 21 days (right column) after repeated treatment. The value in a given sector was first expressed as the percentage of that in vehicle controls, and was then normalized relative to the maximal change in the acute group on withdrawal day 1 (% of max. acute, WD1). Sectors with significant differences (P<0.05) are coded as indicated. Sectors without significant effects are in white. Caudate-putamen: m, medial; dm, dorsomedial; d, dorsal; dl, dorsolateral; vl, ventrolateral; v, ventral; c, central; vc, ventral central; dc, dorsal central; nucleus accumbens: mC, medial core; lC, lateral core; mS, medial shell; vS, ventral shell; lS, lateral shell.
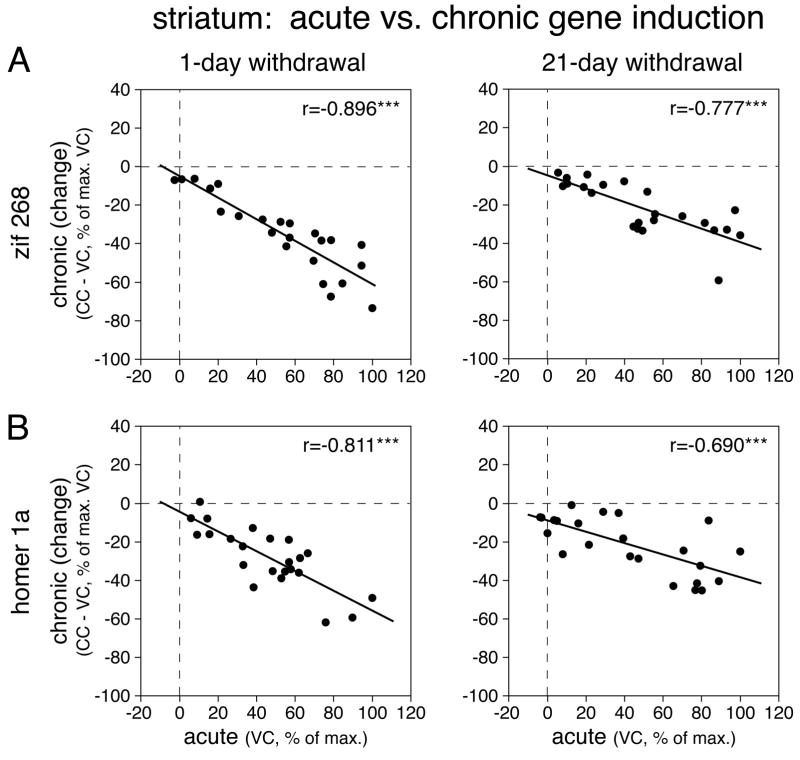
After repeated cocaine treatment, significant zif 268 induction by the cocaine challenge (CC vs. CV, P<0.05) occurred in 14 of the 23 striatal sectors (both 1-day and 21-day withdrawal) (Figs. 2A, 7A, 8A). While the overall topography of induction was similar to that after acute cocaine (CC vs. VC, 1-day withdrawal, r=0.843, P<0.001; 21-day withdrawal, r=0.919, P<0.001), “chronic” zif 268 induction was attenuated (blunted) throughout the striatum (Figs. 7A, 8A). Thus, a significant decrease in induction (CC vs. VC, P<0.05) was seen in 17 (1-day withdrawal) and 13 sectors (21-day withdrawal), including the nucleus accumbens lateral shell (1-day withdrawal, P<0.01). This blunting of chronic zif 268 induction was directly related to acute induction; the more robust the acute induction in a given sector, the more reduced the chronic response in that sector (CC-VC vs. VC, 1-day withdrawal, r=−0.896, P<0.001; 21-day withdrawal, r=−0.777, P<0.001; Fig. 9A).
Fig. 9.
Relationship between acute IEG induction and IEG induction after repeated (chronic) cocaine treatment in the striatum. Scatterplots show correlations between acute induction (VC, in % of max. VC) and changes in chronic induction (CC-VC, in % of max. VC) for zif 268 (A) and homer 1a expression (B) in the 23 striatal sectors. *** P<0.001.
Homer 1a expression
Overall, the topography of changes in cocaine-induced homer 1a expression in the striatum was similar to that for zif 268 expression. Indeed, regional effects on homer 1a and zif 268 expression were highly correlated for both induction after acute (VC vs. VC, 1-day withdrawal, r=0.907, P<0.001; 21-day withdrawal, r=0.910, P<0.001) and repeated treatments (CC vs. CC, 1-day withdrawal, r=0.937, P<0.001; 21-day withdrawal, r=0.828, P<0.001).
One day after the repeated vehicle treatment, acute cocaine (VC) induced highly significant (P<0.001) increases in homer 1a expression (VC vs. VV) in 13/18 sectors of the caudate-putamen (Figs. 2B, 7B, 8B). Induction was less robust or absent in the 3 ventral sectors (rostral, P<0.01; middle, caudal, P>0.05), as well as in the central sectors on the caudal level (P<0.05) (Fig. 8B). Again, these effects tended to increase towards lateral and caudal, and were most pronounced in the caudal sensorimotor sectors. In the nucleus accumbens, the only effect seen was a significant increase in expression in the lateral shell (P<0.01). When given 21 days after repeated vehicle treatment, the acute cocaine challenge (VC) produced homer 1a induction with a similar regional distribution (10/18 sectors, P<0.001; rostral medial, P>0.05; middle medial, P<0.01; caudal medial, P>0.05; rostral, middle, caudal ventral, P>0.05; caudal central, P<0.05; no effects in nucleus accumbens) (Fig. 8B), but the magnitude of induction again tended to be somewhat smaller in some sectors (Fig. 8B).
After repeated cocaine treatment, the cocaine challenge induced significant homer 1a expression (CC vs. CV, P<0.05) in 9 and 10 of the 23 striatal sectors (Figs. 2B, 7B, 8B). While the overall topography was also similar to that after acute cocaine (CC vs. VC, 1-day withdrawal, r=0.798, P<0.001; 21-day withdrawal, r=0.919, P<0.001), “chronic” homer 1a induction was blunted on all 3 rostrocaudal levels (Figs. 7B, 8B). Thus, significantly smaller induction (CC vs. VC, P<0.05) was seen in 13 (1-day withdrawal) and 8 sectors (21-day withdrawal), including the nucleus accumbens lateral shell (1-day withdrawal). Again, this blunting of chronic homer 1a induction was related to acute induction; the more robust the acute induction, the more blunted the chronic induction (CC-VC vs. VC, 1-day withdrawal, r=−0.811, P<0.001; 21-day withdrawal, r=−0.690, P<0.001; Fig. 9B).
DISCUSSION
In the present study, we used two functional markers, the IEGs zif 268 and homer 1a, to map neuronal changes in cortex and striatum after acute and repeated cocaine treatment. Moreover, by investigating these effects 1 versus 21 days after the repeated treatment, we determined whether such changes endure for a prolonged time period after cocaine exposure. Our findings show that repeated cocaine treatment produces dysregulated gene expression in specific functional domains of the cortex and striatum and that some of these effects last for at least 3 weeks. Our most important findings are summarized as follows. (1) Acute cocaine administration induced zif 268 expression predominantly in somatosensory and motor areas of the cortex. (2) Repeated cocaine treatment resulted in increased basal expression of zif 268 in the cortex on all rostrocaudal levels. This effect was most robust in sensorimotor areas one day after the treatment, but was still present 3 weeks later in some areas. (3) These increases in basal expression were accompanied by attenuated zif 268 inducibility by a cocaine challenge one day after the repeated treatment in all affected cortical regions. (4) After the 3-week withdrawal period, the cocaine challenge produced a decrease in zif 268 expression in the insular cortex. (5) Similar to the cortical effects, in the striatum, acute induction of zif 268 was also most robust in sensorimotor sectors. (6) After repeated cocaine treatment, zif 268 inducibility was blunted throughout the striatum, an effect that was proportional to the acute induction and lasted for at least 3 weeks. (7) Interestingly, unlike in the striatum, the acute zif 268 response in the cortex was dependent on the treatment context (handling history); acute cocaine given 1 day, but not 21 days, after repeated vehicle injections significantly enhanced zif 268 expression. (8) While cocaine had minimal or no effects on homer 1a expression in the cortex, cocaine-induced changes in homer 1a expression in the striatum were robust and displayed principally identical regional and temporal dynamics as those for zif 268. This study is the first to demonstrate long-lasting changes in gene regulation mostly in sensorimotor corticostriatal circuits after repeated cocaine treatment.
Repeated cocaine treatment produces long-lasting increases in basal zif 268 expression, but attenuates challenge-induced zif 268 responses, in the cortex
IEGs such as c-fos and zif 268 encode transcription factors that regulate other genes and are implicated in various forms of neuronal plasticity (Knapska & Kaczmarek, 2004; Valjent et al., 2006). Psychostimulant-induced changes in the expression of these IEGs thus suggests that repeated drug exposure produces local neuroadaptations or other neuroplastic changes. While imaging studies indeed indicate functional changes in various cortical regions in human or non-human drug users (London et al., 1990; Breiter et al., 1997; Beveridge et al., 2006; Porrino et al., 2007), only relatively few previous molecular studies investigated the effects of repeated psychostimulant exposure on cortical gene regulation, and these typically focused on the prefrontal cortex (e.g., Freeman et al., 2002; Black et al., 2006). Our study is, to our knowledge, the first to quantitatively map gene regulation effects of acute and repeated cocaine treatment throughout the main functional areas of the cerebral cortex.
Our results demonstrate that repeated cocaine treatment produces increased basal zif 268 expression in the cortex, an effect that occurred predominantly in somatosensory and motor areas, but spread across all rostrocaudal levels examined. Importantly, increased zif 268 mRNA levels persisted for at least 3 weeks after the treatment in some of the most affected areas. This increased basal zif 268 expression was accompanied by attenuated inducibility of zif 268 one day after the treatment. Thus, in these cocaine-pretreated animals, a cocaine challenge failed to further increase zif 268 expression. Future work will have to determine which neuroadaptations in cellular signaling pathways, or other neuronal changes (see below), produced by repeated cocaine treatment are responsible for these changes in zif 268 expression.
Regarding the underlying mechanisms, our most recent findings indicate that such psychostimulant-induced molecular changes in the cortex are not specific for cocaine, but may be age-dependent. Thus, similar to cocaine (present study), enhanced basal expression of zif 268, as well as attenuated zif 268 inducibility were also produced by repeated treatment with methylphenidate (Steiner et al., 2008). Cocaine blocks dopamine, norepinephrine and serotonin transporters, while methylphenidate only blocks reuptake of dopamine and norepinephrine, but not serotonin (c. f., Yano & Steiner, 2007). However, both the present findings with cocaine and the above methylphenidate-induced effects (Steiner et al., 2008) were obtained with repeated drug treatment in adult rats. Interestingly, these effects contrast with our prior findings in adolescent rats. Thus, repeated methylphenidate treatment in adolescents produced opposite effects on cortical IEG regulation, namely, reduced basal zif 268 expression and enhanced zif 268 and homer 1a induction by a (methylphenidate) challenge throughout the cortex (Cotterly et al., 2007). These age-dependent drug effects on cortical gene regulation are likely related to changes in cell-physiological (e.g., Tseng & O’Donnell, 2005; 2007) or other mechanisms (Andersen, 2005) that occur during normal maturation of cortical processes. It will be important to elucidate what the relevant age-dependent mechanisms are and their exact role in cortical gene regulation by psychostimulants.
Long-lasting blunting of gene induction in the striatum after repeated cocaine treatment
In contrast to the cortex, numerous studies have investigated psychostimulant effects on gene regulation in the striatum and the findings are very consistent (Harlan & Garcia, 1998; Torres & Horowitz, 1999; Yano & Steiner, 2007). For example, we previously described the topography of cocaine-induced gene regulation for c-fos and dynorphin in the striatum (Willuhn et al., 2003). Similar to that regional distribution, our present results for zif 268 and homer 1a demonstrate that acute gene induction by cocaine is most pronounced in dorsal and lateral (sensorimotor) sectors of the striatum, with lesser or minimal effects in the medial (associative) or ventral (limbic) striatum. Along the rostrocaudal axis, such gene regulation is moderate rostrally (associative/limbic striatum), increases towards caudal and peaks in the postcommissural striatum (corresponding to the putamen) (present study; Willuhn et al., 2003). While generally similar to gene regulation by amphetamine (e.g., Badiani et al., 1998) and methylphenidate (Yano & Steiner, 2005a; 2005b), differences in the distribution of cocaine effects were also noted. For example, our present study shows that cocaine induces more robust effects in the caudal striatum, compared with methylphenidate (Yano & Steiner, 2005a; 2005b; Cotterly et al., 2007). This difference may indicate a more pronounced role for serotonin in gene regulation in the caudal striatum (see Yano & Steiner, 2007, for discussion).
Repeated cocaine treatment has previously been shown to produce blunting of IEG induction in the striatum (Hope et al., 1992; Steiner & Gerfen, 1993; for reviews, see Harlan & Garcia, 1998; Yano & Steiner, 2007). Our present results demonstrate that this blunting is directly related to the magnitude of acute gene induction. This finding is consistent with the view that such blunting reflects drug-induced compensatory neuroadaptations, possibly both at the epigenetic level (Renthal & Nestler, 2008) and at the systems level (Steiner & Gerfen, 1998). Again, these adaptations appear to be long-lasting; our present results show for the first time that blunting of IEG induction is still very robust 3 weeks after repeated cocaine treatment.
While the regulation of IEGs such as c-fos and zif 268 is fairly well-known, ours is one of the first studies that assessed effects of repeated psychostimulant treatment on the regulation of homer1a in cortex and striatum. A few previous studies showed induction of homer 1 isoforms (homer 1a, ania-3) by acute cocaine in the striatum (Brakeman et al., 1997; Berke et al., 1998; Zhang et al., 2007), but little is known on the effects of repeated psychostimulant treatment on homer 1a expression (Szumlinski et al., 2008). In our previous study in adolescent rats, we found a marked dissociation between zif 268 and homer 1a regulation by repeated methylphenidate treatment (Cotterly et al., 2007). While the induction of zif 268 was significantly blunted in the striatum, that of homer 1a was modestly decreased in some areas but increased in others, after repeated treatment. The present study demonstrates that, in contrast, repeated cocaine treatment (in adults) produces identical blunting for both zif 268 and homer 1a, regionally and temporally. These findings extend our observations indicating that cocaine produces to some degree more severe (or other) neuroadaptations than methylphenidate (see Yano & Steiner, 2007, for discussion).
Homer/Vesl proteins are scaffolding proteins that anchor type I metabotropic glutamate receptors to the postsynaptic density and link them to IP3 receptors in the endoplasmatic reticulum (Brakeman et al., 1997; Kato et al., 1997; for reviews, see Xiao et al., 2000; Thomas, 2002). These proteins are implicated in calcium signaling, glutamate receptor clustering and trafficking, spine morphogenesis and other processes of synapse structuring (Xiao et al., 2000; Thomas, 2002). These and other findings suggest a role for homer 1a in activity-dependent synaptic plasticity (e.g., regulation of the signaling complex and synapse turnover; Xiao et al., 2000; Thomas, 2002). Our findings thus suggest that repeated cocaine treatment affects restructuring of synapses in the striatum. Interestingly, our results also indicate that this effect is much more prominent in striatal neurons than in the cortical neurons.
Context-dependent effects of acute cocaine on gene regulation in the cortex
A somewhat surprising finding of our study is that zif 268 induction in the cortex after acute cocaine administration was dependent on the handling history. Other studies have shown IEG induction in the cortex by acute psychostimulants (e.g., Graybiel et al., 1990; Johansson et al., 1994; Curran et al., 1996; Badiani et al., 1998; Yano & Steiner, 2005a). However, most of these previous studies apparently administered the psychostimulant to animals with limited preceding handling/habituation. Our present study underlines the importance of the handling context for cortical, but not striatal, IEG induction by acute cocaine. Thus, in contrast to the robust zif 268 response when acute cocaine was given 1 day after repeated vehicle treatment, rats that received acute cocaine 3 weeks after the repeated vehicle injections (plus further intermittent handling) did not show significantly increased zif 268 expression in the cortex. These results thus corroborate previous observations indicating a role of handling/arousal-related activities in cortical IEG induction by psychostimulants (Badiani et al., 1998; Yano & Steiner, 2005a; Conversi et al., 2006). Insofar as these genes are markers for neuroadaptations (see above), these findings highlight the importance of contextual variables for drug-induced neuroplasticity in the cortex, but not in the striatum.
Functional considerations
Our present results show extensive and long-lasting changes in gene regulation in cortex and striatum after repeated cocaine treatment. Most of these changes tended to be reduced after the 3-week withdrawal period, indicating that these are transient alterations produced by the drug exposure. Others, however, such as the decreased gene expression in the insular cortex, only emerged after 3 weeks. These latter changes are reminiscent of neurobehavioral changes that develop during drug withdrawal (Grimm et al., 2003; Conrad et al., 2008). These effects may thus reflect cortical alterations related to drug withdrawal-induced behavioral changes such as craving (Naqvi et al., 2007).
The finding that repeated cocaine treatment predominantly affects gene regulation in sensorimotor cortical and striatal domains suggests that especially sensorimotor functions are changed by exposure to such drugs, perhaps more so than is currently appreciated. The exact functional consequences of these molecular changes, however, remain to be determined. It has been shown that acute cocaine increases neuronal firing in cortex and striatum (e.g., Pederson et al., 1997; White et al., 1998; Drouin & Waterhouse, 2004; Devonshire et al., 2007), and such changes in neuronal activity are accompanied by changes in the expression of IEGs such as zif 268 (Chaudhuri et al., 1995; Melzer & Steiner, 1997). The following paragraphs speculate how neuronal functioning may be altered in these corticostriatal circuits after such treatments.
In the cortex, both (excitatory) projection neurons and (inhibitory) interneurons can upregulate IEG expression upon activation (e.g., Chaudhuri et al., 1995; Bertini et al., 2002; Staiger et al., 2002; Van der Gucht et al., 2002). However, by virtue of the relatively low interneuron numbers (<15–30%, depending on area and layer; Ren et al., 1992; Beaulieu, 1993), gene expression area measures as used here should mostly reflect activity changes in projection neurons. Thus, increased basal zif 268 expression may indicate chronically enhanced basal activity in (some of) these neurons (Drouin & Waterhouse, 2004). Alternatively, as zif 268 expression was measured following the open-field test, enhanced “basal” zif 268 expression in these cocaine-pretreated, vehicle-challenged animals (compared with the vehicle-pretreated, vehicle-challenged group) may reflect increased cortical responsiveness (Dong et al., 2005; Nasif et al., 2005) to arousal-enhancing situations such as the behavioral test. Either way, chronic overactivation of these neurons would be expected to cause adaptations in cellular signaling pathways (Hyman & Nestler, 1996), changes that could contribute to the here observed loss of (IEG) responsiveness after the cocaine challenge. It remains to be seen what the net effects of such complex cellular and molecular adaptations for behaviorally relevant cortical outputs are in such animals.
In the striatum, cocaine-induced IEG expression occurs mostly but not exclusively in neurons of the D1 receptor-regulated direct output pathway (Cenci et al., 1992; Johansson et al., 1994; Kosofsky et al., 1995; Badiani et al., 1999; Uslaner et al., 2001). A reduction in cortical input to the striatum has been shown to attenuate psychostimulant-induced IEG expression in striatal projection neurons (Cenci & Björklund, 1993; Vargo & Marshall, 1995; Ferguson & Robinson, 2004). Blunting of striatal IEG induction after repeated cocaine treatment could thus, at least in part, reflect dampened cortical input and/or output in direct pathway neurons. Activity in the direct pathway facilitates cortical activation (see Steiner, 2007, for review), which is reflected in increased cortical IEG expression (Steiner & Kitai, 2000). Reduced activity in the direct pathway would therefore be expected to result in a loss of basal ganglia-related input to the cortex (and IEG expression). Therefore, this scenario proposes chronically reduced/dampened activity or excitability in neurons of the affected cortico-basal ganglia-cortical circuits after repeated cocaine treatment.
Our results show that the most pronounced behavioral consequence of the repeated cocaine treatment was an increase in behavioral stereotypies (repetitive head bobbing, sniffing). Such stereotypies are typical behavioral correlates of repeated psychostimulant/dopamine agonist treatments and have been linked to drug-induced molecular changes in sensorimotor corticostriatal circuits before (Graybiel et al., 2000). Such motor stereotypies appear to reflect a dysfunction in selection or switching of motor actions, one of the main functions of basal ganglia circuits (c.f. Cotterly et al., 2007), and may be related to motor compulsions (Graybiel & Rauch, 2000). Drug-induced neuronal alterations in these circuits may thus play a role in compulsive drug taking (Berke & Hyman, 2000; Everitt & Robbins, 2005), a defining characteristic of drug addiction.
Acknowledgments
This work was supported by a grant from the National Institute on Drug Abuse (DA011261). We thank Dr. Kuei-Yuan Tseng for helpful discussions.
References
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Stimulants and the developing brain. Trends Pharmacol Sci. 2005;26:237–243. doi: 10.1016/j.tips.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Badiani A, Oates MM, Day HE, Watson SJ, Akil H, Robinson TE. Amphetamine-induced behavior, dopamine release, and c-fos mRNA expression: modulation by environmental novelty. J Neurosci. 1998;18:10579–10593. doi: 10.1523/JNEUROSCI.18-24-10579.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A, Oates MM, Day HE, Watson SJ, Akil H, Robinson TE. Environmental modulation of amphetamine-induced c-fos expression in D1 versus D2 striatal neurons. Behav Brain Res. 1999;103:203–209. doi: 10.1016/s0166-4328(99)00041-8. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. Numerical data on neocortical neurons in adult rat, with special reference to the GABA population. Brain Res. 1993;609:284–292. doi: 10.1016/0006-8993(93)90884-p. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Berke JD, Paletzki RF, Aronson GJ, Hyman SE, Gerfen CR. A complex program of striatal gene expression induced by dopaminergic stimulation. J Neurosci. 1998;18:5301–5310. doi: 10.1523/JNEUROSCI.18-14-05301.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertini G, Peng ZC, Fabene PF, Grassi-Zucconi G, Bentivoglio M. Fos induction in cortical interneurons during spontaneous wakefulness of rats in a familiar or enriched environment. Brain Res Bull. 2002;57:631–638. doi: 10.1016/s0361-9230(01)00757-2. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Daunais JB, Nader MA, Porrino LJ. Chronic cocaine self-administration is associated with altered functional activity in the temporal lobes of non human primates. Eur J Neurosci. 2006;23:3109–3118. doi: 10.1111/j.1460-9568.2006.04788.x. [DOI] [PubMed] [Google Scholar]
- Black YD, Maclaren FR, Naydenov AV, Carlezon WAJ, Baxter MG, Konradi C. Altered attention and prefrontal cortex gene expression in rats after binge-like exposure to cocaine during adolescence. J Neurosci. 2006;26:9656–9665. doi: 10.1523/JNEUROSCI.2391-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottai D, Guzowski JF, Schwarz MK, Kang SH, Xiao B, Lanahan A, Worley PF, Seeburg PH. Synaptic activity-induced conversion of intronic to exonic sequence in Homer 1 immediate early gene expression. J Neurosci. 2002;22:167–175. doi: 10.1523/JNEUROSCI.22-01-00167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O’Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Björklund A. Transection of corticostriatal afferents reduces amphetamine- and apomorphine-induced striatal Fos expression and turning behaviour in unilaterally 6-hydroxydopamine-lesioned rats. Eur J Neurosci. 1993;5:1062–1070. doi: 10.1111/j.1460-9568.1993.tb00959.x. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Campbell K, Wictorin K, Björklund A. Striatal c-fos induction by cocaine or apomorphine occurs preferentially in output neurons projecting to the substantia nigra in the rat. Eur J Neurosci. 1992;4:376–380. doi: 10.1111/j.1460-9568.1992.tb00885.x. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A. Neural activity mapping with inducible transcription factors. Neuroreport. 1997;8:v-ix. [PubMed] [Google Scholar]
- Chaudhuri A, Matsubara JA, Cynader MS. Neuronal activity in primate visual cortex assessed by immunostaining for the transcription factor Zif268. Vis Neurosci. 1995;12:35–50. doi: 10.1017/s095252380000729x. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conversi D, Bonito-Oliva A, Orsini C, Cabib S. Habituation to the test cage influences amphetamine-induced locomotion and Fos expression and increases FosB/DeltaFosB-like immunoreactivity in mice. Neuroscience. 2006;141:597–605. doi: 10.1016/j.neuroscience.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Cotterly L, Beverley JA, Yano M, Steiner H. Dysregulation of gene induction in corticostriatal circuits after repeated methylphenidate treatment in adolescent rats: Differential effects on zif 268 and homer 1a. Eur J Neurosci. 2007;25:3617–3628. doi: 10.1111/j.1460-9568.2007.05570.x. [DOI] [PubMed] [Google Scholar]
- Curran EJ, Akil H, Watson SJ. Psychomotor stimulant- and opiate-induced c-fos mRNA expression patterns in the rat forebrain: comparisons between acute drug treatment and a drug challenge in sensitized animals. Neurochem Res. 1996;21:1425–1435. doi: 10.1007/BF02532384. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Devonshire IM, Mayhew JE, Overton PG. Cocaine preferentially enhances sensory processing in the upper layers of the primary sensory cortex. Neuroscience. 2007;146:841–851. doi: 10.1016/j.neuroscience.2007.01.070. [DOI] [PubMed] [Google Scholar]
- Dong Y, Nasif FJ, Tsui JJ, Ju WY, Cooper DC, Hu XT, Malenka RC, White FJ. Cocaine-induced plasticity of intrinsic membrane properties in prefrontal cortex pyramidal neurons: adaptations in potassium currents. J Neurosci. 2005;25:936–940. doi: 10.1523/JNEUROSCI.4715-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin C, Waterhouse BD. Cocaine-induced vs. behaviour-related alterations of spontaneous and evoked discharge of somatosensory cortical neurons. Eur J Neurosci. 2004;19:1016–1026. doi: 10.1111/j.0953-816x.2004.03186.x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Robinson TE. Amphetamine-evoked gene expression in striatopallidal neurons: regulation by corticostriatal afferents and the ERK/MAPK signaling cascade. J Neurochem. 2004;91:337–348. doi: 10.1111/j.1471-4159.2004.02712.x. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Brebner K, Lynch WJ, Patel KM, Robertson DJ, Roberts DC, Vrana KE. Changes in rat frontal cortex gene expression following chronic cocaine. Mol Brain Res. 2002;104:11–20. doi: 10.1016/s0169-328x(02)00197-3. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Canales JJ, Capper-Loup C. Levodopa-induced dyskinesias and dopamine-dependent stereotypies: a new hypothesis. Trends Neurosci. 2000;23:S71–S77. doi: 10.1016/s1471-1931(00)00027-6. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci USA. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28:343–347. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Wolters JG, Lohman AH. The anatomical relationship of the prefrontal cortex with the striatopallidal system, the thalamus and the amygdala: evidence for a parallel organization. Prog Brain Res. 1990;85:95–116. doi: 10.1016/s0079-6123(08)62677-1. [DOI] [PubMed] [Google Scholar]
- Harlan RE, Garcia MM. Drugs of abuse and immediate-early genes in the forebrain. Mol Neurobiol. 1998;16:221–267. doi: 10.1007/BF02741385. [DOI] [PubMed] [Google Scholar]
- Hope B, Kosofsky B, Hyman SE, Nestler EJ. Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc Natl Acad Sci USA. 1992;89:5764–5768. doi: 10.1073/pnas.89.13.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Nestler EJ. Initiation and adaptation: a paradigm for understanding psychotropic drug action. Am J Psychiatry. 1996;153:151–162. doi: 10.1176/ajp.153.2.151. [DOI] [PubMed] [Google Scholar]
- Johansson B, Lindström K, Fredholm BB. Differences in the regional and cellular localization of c-fos messenger RNA induced by amphetamine, cocaine and caffeine in the rat. Neuroscience. 1994;59:837–849. doi: 10.1016/0306-4522(94)90288-7. [DOI] [PubMed] [Google Scholar]
- Kato A, Ozawa F, Saitoh Y, Hirai K, Inokuchi K. vesl, a gene encoding VASP/Ena family related protein, is upregulated during seizure, long-term potentiation and synaptogenesis. FEBS Lett. 1997;412:183–189. doi: 10.1016/s0014-5793(97)00775-8. [DOI] [PubMed] [Google Scholar]
- Knapska E, Kaczmarek L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog Neurobiol. 2004;74:183–211. doi: 10.1016/j.pneurobio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Kosofsky BE, Genova LM, Hyman SE. Substance P phenotype defines specificity of c-fos induction by cocaine in developing rat striatum. J Comp Neurol. 1995;351:41–50. doi: 10.1002/cne.903510105. [DOI] [PubMed] [Google Scholar]
- London ED, Cascella NG, Wong DF, Phillips RL, Dannals RF, Links JM, Herning R, Grayson R, Jaffe JH, Wagner HNJ. Cocaine-induced reduction of glucose utilization in human brain. A study using positron emission tomography and [fluorine 18]-fluorodeoxyglucose. Arch Gen Psychiatry. 1990;47:567–574. doi: 10.1001/archpsyc.1990.01810180067010. [DOI] [PubMed] [Google Scholar]
- Melzer P, Steiner H. Stimulus-dependent expression of immediate-early genes in rat somatosensory cortex. J Comp Neurol. 1997;380:145–153. doi: 10.1002/(sici)1096-9861(19970331)380:1<145::aid-cne11>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasif FJ, Hu XT, White FJ. Repeated cocaine administration increases voltage-sensitive calcium currents in response to membrane depolarization in medial prefrontal cortex pyramidal neurons. J Neurosci. 2005;25:3674–3679. doi: 10.1523/JNEUROSCI.0010-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1998. [Google Scholar]
- Pederson CL, Wolske M, Peoples LL, West MO. Firing rate dependent effect of cocaine on single neurons of the rat lateral striatum. Brain Res. 1997;760:261–265. doi: 10.1016/s0006-8993(97)00395-8. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Smith HR, Nader MA, Beveridge TJ. The effects of cocaine: a shifting target over the course of addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1593–1600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren JQ, Aika Y, Heizmann CW, Kosaka T. Quantitative analysis of neurons and glial cells in the rat somatosensory cortex, with special reference to GABAergic neurons and parvalbumin-containing neurons. Exp Brain Res. 1992;92:1–14. doi: 10.1007/BF00230378. [DOI] [PubMed] [Google Scholar]
- Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med. 2008;14:341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Granon S, Muir JL, Durantou F, Harrison A, Everitt BJ. Neural systems underlying arousal and attention. Implications for drug abuse. Ann N Y Acad Sci. 1998;846:222–237. [PubMed] [Google Scholar]
- Sharp FR, Sagar SM, Swanson RA. Metabolic mapping with cellular resolution: c-fos vs. 2-deoxyglucose. Crit Rev Neurobiol. 1993;7:205–228. [PubMed] [Google Scholar]
- Staiger JF, Masanneck C, Bisler S, Schleicher A, Zuschratter W, Zilles K. Excitatory and inhibitory neurons express c-Fos in barrel-related columns after exploration of a novel environment. Neuroscience. 2002;109:687–699. doi: 10.1016/s0306-4522(01)00501-2. [DOI] [PubMed] [Google Scholar]
- Steiner H. Basal ganglia – cortex interactions: Regulation of cortical function by D1 dopamine receptors in the striatum. In: Tseng KY, Atzori M, editors. Monoaminergic Modulation of Cortical Excitability. Springer; Berlin: 2007. pp. 265–285. [Google Scholar]
- Steiner H, Gerfen CR. Cocaine-induced c-fos messenger RNA is inversely related to dynorphin expression in striatum. J Neurosci. 1993;13:5066–5081. doi: 10.1523/JNEUROSCI.13-12-05066.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Role of dynorphin and enkephalin in the regulation of striatal output pathways and behavior. Exp Brain Res. 1998;123:60–76. doi: 10.1007/s002210050545. [DOI] [PubMed] [Google Scholar]
- Steiner H, Kitai ST. Regulation of rat cortex function by D1 dopamine receptors in the striatum. J Neurosci. 2000;20:5449–5460. doi: 10.1523/JNEUROSCI.20-14-05449.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Lim SAO, Beverley JA. Repeated methylphenidate treatment: Age-dependent effects on gene regulation in the cortex. Soc Neurosci Abstr. 2008;34:59.29. [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD. Homers regulate drug-induced neuroplasticity: implications for addiction. Biochem Pharmacol. 2008;75:112–133. doi: 10.1016/j.bcp.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas U. Modulation of synaptic signalling complexes by Homer proteins. J Neurochem. 2002;81:407–413. doi: 10.1046/j.1471-4159.2002.00869.x. [DOI] [PubMed] [Google Scholar]
- Torres G, Horowitz JM. Drugs of abuse and brain gene expression. Psychosom Med. 1999;61:630–650. doi: 10.1097/00006842-199909000-00007. [DOI] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Post-pubertal emergence of prefrontal cortical up states induced by D1-NMDA co-activation. Cereb Cortex. 2005;15:49–57. doi: 10.1093/cercor/bhh107. [DOI] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex. 2007;17:1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslaner J, Badiani A, Norton CS, Day HE, Watson SJ, Akil H, Robinson TE. Amphetamine and cocaine induce different patterns of c-fos mRNA expression in the striatum and subthalamic nucleus depending on environmental context. Eur J Neurosci. 2001;13:1977–1983. doi: 10.1046/j.0953-816x.2001.01574.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Aubier B, Corbillé AG, Brami-Cherrier K, Caboche J, Topilko P, Girault JA, Hervé D. Plasticity-associated gene Krox24/Zif268 is required for long-lasting behavioral effects of cocaine. J Neurosci. 2006;26:4956–4960. doi: 10.1523/JNEUROSCI.4601-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Gucht E, Clerens S, Cromphout K, Vandesande F, Arckens L. Differential expression of c-fos in subtypes of GABAergic cells following sensory stimulation in the cat primary visual cortex. Eur J Neurosci. 2002;16:1620–1626. doi: 10.1046/j.1460-9568.2002.02226.x. [DOI] [PubMed] [Google Scholar]
- Vargo JM, Marshall JF. Time-dependent changes in dopamine agonist-induced striatal Fos immunoreactivity are related to sensory neglect and its recovery after unilateral prefrontal cortex injury. Synapse. 1995;20:305–315. doi: 10.1002/syn.890200404. [DOI] [PubMed] [Google Scholar]
- White IM, Doubles L, Rebec GV. Cocaine-induced activation of striatal neurons during focused stereotypy in rats. Brain Res. 1998;810:146–152. doi: 10.1016/s0006-8993(98)00905-6. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Sun W, Steiner H. Topography of cocaine-induced gene regulation in the rat striatum: Relationship to cortical inputs and role of behavioural context. Eur J Neurosci. 2003;17:1053–1066. doi: 10.1046/j.1460-9568.2003.02525.x. [DOI] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Worley PF. Homer: a link between neural activity and glutamate receptor function. Curr Opin Neurobiol. 2000;10:370–374. doi: 10.1016/s0959-4388(00)00087-8. [DOI] [PubMed] [Google Scholar]
- Yano M, Steiner H. Methylphenidate (Ritalin) induces Homer 1a and zif 268 expression in specific corticostriatal circuits. Neuroscience. 2005a;132:855–865. doi: 10.1016/j.neuroscience.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Yano M, Steiner H. Topography of methylphenidate (Ritalin)-induced gene regulation in the striatum: differential effects on c-fos, substance P and opioid peptides. Neuropsychopharmacology. 2005b;30:901–915. doi: 10.1038/sj.npp.1300613. [DOI] [PubMed] [Google Scholar]
- Yano M, Steiner H. Methylphenidate and cocaine: the same effects on gene regulation? Trends Pharmacol Sci. 2007;28:588–596. doi: 10.1016/j.tips.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Zhang GC, Mao LM, Liu XY, Parelkar NK, Arora A, Yang L, Hains M, Fibuch EE, Wang JQ. In vivo regulation of Homer1a expression in the striatum by cocaine. Mol Pharmacol. 2007;71:1148–1158. doi: 10.1124/mol.106.028399. [DOI] [PubMed] [Google Scholar]